Abstract
Rationale
Although leptin receptors are found in hypothalamic nuclei classically associated with homeostatic feeding mechanisms, they are also present in brain regions known to regulate hedonic-based feeding, natural reward processing, and responses to drugs of abuse. The ob/ob mouse is deficient in leptin signaling, and previous work has found altered mesolimbic dopamine signaling and sensitivity to the locomotor activating effects of amphetamine in these mice.
Objectives
We directly assessed responses to three drugs of abuse and non-drug rewards in the leptin-deficient ob/ob mouse.
Methods
Ob/ob mice were tested in assays of sweet preference, novelty seeking, and drug reward/reinforcement.
Results
In assays of novelty seeking, novel open field activity and operant sensation seeking were reduced in ob/ob mice, although novel object interaction and novel environment preference were comparable to wild types. We also found that ob/ob mice had specific phenotypes in regard to cocaine: conditioned place preference for 2.5 mg/kg was increased, while the locomotor response to 10 mg/kg was reduced, and cocaine self-administration was the same as wild types. Ob/ob mice also acquired self-administration of the potent opioid remifentanil, but breakpoints for the drug were significantly reduced. Finally, we found significant differences in ethanol drinking in ob/ob mice that correlated negatively with body weight and positively with operant sensation seeking.
Conclusions
In conclusion, ob/ob mice displayed task-specific deficits in novelty seeking and dissociable differences in reward/reinforcement associated with cocaine, remifentanil, and ethanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptin is a peripherally released peptide that powerfully modulates food intake (Friedman and Halaas 1998). The ob/ob mouse is homozygous for a spontaneous mutation in the leptin gene (lep), which disrupts leptin signaling, resulting in excessive food intake. This phenotype is largely due to a lack of leptin signaling in neurons of the arcuate nucleus of the hypothalamus (Davis et al. 2011; Friedman 1998; Houseknecht et al. 1998). While the leptin receptors found in this area are classically associated with homeostatic feeding mechanisms, leptin receptors are also present in brain regions known to regulate hedonic-based feeding, natural reward processing, and responses to drugs of abuse (Leinninger 2009; Narayanan et al. 2010). Specifically, the leptin receptor (LepRb) is found in the ventral tegmental area (VTA), nucleus tractis solitari, and lateral hypothalamus (Caron et al. 2010; Figlewicz et al. 2003; Scott et al. 2009). Consistent with the hypothesis that leptin can modulate non-homeostatic feeding, sucrose self-administration (in non-food restricted animals) is reduced by both i.c.v. and intra-VTA infusions of leptin (Davis et al. 2011; Figlewicz et al. 2006), and VTA knockdown of leptin receptors increases consumption of palatable foods (Hommel et al. 2006).
There is also evidence that changes in leptin signaling can alter responses to drugs of abuse. Leptin-deficient ob/ob mice have a reduced locomotor response to amphetamine, and administration of leptin increases amphetamine locomotor responses in ob/ob and WT mice (Fulton et al. 2006). A more recent study demonstrated that intra-VTA leptin reduced conditioned place preference for cocaine and cocaine seeking in rats that had previously self-administered cocaine, but had no acute effect on cocaine self-administration (You et al. 2015). Together, these data suggest that leptin signaling may play different roles in locomotor and reward-related responses to psychostimulants, although differences may also be attributable to differences between the chronic leptin deficiency in the ob/ob mouse and acute modulation of leptin signaling in rats. Based on the location of leptin receptors and reported phenotypes of ob/ob mice, we hypothesized that reinforcement of non-calorie-containing rewards would be reduced in these mice. To test this hypothesis and to determine if psychostimulant phenotypes following chronic disruption of leptin signaling would be congruent with data from acute modulation, we examined responses to a variety of drugs of abuse and non-drug rewards in the leptin-deficient ob/ob mouse.
Materials and methods
Animals
Age-matched male ob/ob and wild-type (WT) mice (6–20 weeks old) were bred from heterozygous breeders generated by mating male congenic C57BL/6J ob/ob (Jackson Labs, stock #000632) and female C57BL/6J mice. Mice were housed in MCW animal care facilities on a reverse light cycle (lights off 0800–2000 hours) and experiments were performed between 0900 and 1600 hours. Genotyping was performed according to the PCR method available from Jackson Labs. Food and water was provided ad libitum during all experiments. Mice were housed in groups of two to five with the exception of animals in sweet preference, ethanol drinking, and intravenous drug self-administration experiments, which were single housed prior to experiments. All mice were handled for 3 days prior to experiments as described (Olsen and Winder 2010). All experiments were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin and were performed within the guidelines of the Guide for the Care and Use of Laboratory Animals.
Jugular catheterization
Jugular catheterization was similar to that described (Grueter et al. 2006; Olsen and Winder 2006; Schramm-Sapyta et al. 2006). Mice were anesthetized with isoflurane (3–5 % induction, 1–3 % maintenance) and were implanted with a silicone catheter (0.2 mm ID, 0.4 mm OD, Access Technologies, Skokie, IL) into the right jugular vein, which exited through the intrascapular region and was connected to a cannula assembly. The cannula assembly consisted of a 26 gauge stainless steel cannula (Plastics One, Roanoke, VA) mounted on a silicone base with nylon mesh (similar to that described in Thomsen and Caine 2007), which was implanted subcutaneously. Rimadyl (carprofen, 5 mg/kg s.c.) was administered immediately following surgery and 24 h later. Mice were allowed to recover ≥5 days before experiments.
Apparatus
Novel open field (NOF)/novel object interaction (NOI)
A plastic gray circular chamber (45.5 cm diameter, 32.8 cm height) was placed on a smooth white floor with overhead lighting (150 ± 20 lx). Custom novel objects were made using various Lego pieces. Two variations were used (object 1: 8.9 × 6.4 × 6.2 cm (l × w × h); object 2: 8.9 × 3.9 × 6.6 cm). Mice were exposed to one object in each of two separate NOI tests (order counterbalanced). Data was recorded by an overhead video camera and analyzed by ANY-maze Video Tracking System (Stoelting, Wood Dale, IL).
Novel environment preference (NEP)
A custom two-chamber clear acrylic apparatus (32.9 × 21.7 × 30.4 cm (l × w × h)) was housed inside a sound-attenuating chamber (Med Associates, St. Albans, VT) with overhead lighting (105 ± 5 lx) and a fan that provided background noise. The apparatus was divided into two equal chambers (15.3 × 20.5 cm) by a black plastic divider with an opening at the base to allow access between chambers. A removable black plastic insert was used to block opening. The two chambers differed only in B/W wall pattern (checkerboard vs. diagonal stripes) that was affixed to the outside of the clear walls. Video was acquired from overhead cameras and stored and analyzed by ANY-maze.
Conditioned place preference (CPP) for cocaine
CPP took place in three-chamber apparatuses (Med Associates) in a room with a white noise generator (70 dB). The two main chambers (16.9 × 12.7 cm) differed in wall color (black or white), floor texture, and illumination (black 14 lx, white 4 lx), and were separated by a center gray compartment (10.0 × 12.7 cm) with a smooth plastic floor. Animal location and locomotor activity were determined using photobeam detectors within the chambers and data were analyzed by Med-PC IV software. There was no consistent pre-conditioning bias between the main chambers, and assignment of the cocaine-paired chamber was counterbalanced within each genotype based on pre-conditioning preference test.
Operant conditioning chambers
Operant conditioning studies were performed in self-administration chambers (Med Associates, 21.6 × 17.8 × 12.7 cm) similar to those described (Olsen and Winder 2009), with the exception that the left wall was equipped with two retractable levers and the right wall was equipped with nosepokes with a liquid receptacle between them. Each manipulandum had a yellow LED mounted above it.
Drugs
Cocaine-HCl (generously provided by the NIDA Drug Supply Program), remifentanil-HCl (Ultiva, Mylan Pharmaceuticals, Morgantown, WV), and methohexital sodium (Brevital, JHP Pharmaceuticals, Rochester, MI) were diluted in 0.9 % saline (Hospira, Lake Forest, IL). Sucrose and saccharin-sodium (Sigma-Aldrich, St. Louis, MO) and USP-grade ethanol (Decon Labs, King of Prussia, PA) were diluted in drinking water (hyperchlorinated) obtained from the Medical College of Wisconsin vivarium.
Behavioral procedures
Sweet preference
Mice were singly housed with ad libitum food and given access to sweet solutions under a standard two-bottle choice paradigm: one containing water, the other a sweet (saccharin or sucrose) solution. Bottles were available 24 h/day, and mice had access to each concentration for 48 h, with the bottle side switched each 24 h. All concentrations were given in ascending order, and saccharin doses (0.001, 0.003, 0.01, 0.03, 0.1, 0.3 %) were completed before animals were advanced to sucrose (0.03, 0.01, 0.03, 1, 1.8, 3, 10 %).
Novelty seeking assays
Mice (n = 16 WT, n = 23 ob/ob) underwent a series of novelty seeking assays: novel open field/novel object interaction (NOF/NOI), novel environment preference (NEP), and operant sensation seeking (OSS (Olsen and Winder 2009)). Each novelty test was separated by ≥48 h. In the NOF test, mice were placed in the center of the open field and monitored for 60 min and analyzed for distance traveled and center time (center 1/3 of the apparatus). After 60 min, a novel object was placed in the center of the apparatus and interaction was measured for 30 min. Interaction was calculated in ANY-Maze and defined as the head of the mouse being within 2 cm of any edge of the object. Mice were then tested for NEP using similar methods to those described for rats (Belin et al. 2011; Meyer et al. 2010). Mice were habituated to one of the two chambers (counterbalanced within genotype) for 30 min, then allowed access to both chambers for the 20-min test. The preference ratio was calculated as time in the novel chamber/test time. Next, mice were tested for OSS in 2-h sessions as described (Olsen and Winder 2009), with the exception that each light flash was mirrored on the opposite wall, resulting in either two front or two rear lights being simultaneously illuminated. FR-1 sessions continued until criteria were met (three consecutive sessions of ≥30 reinforcers and ≥2:1 ratio of active to inactive lever presses, minimum 8 sessions) or 12 sessions had been completed. After completion of OSS, mice underwent a second NOF/NOI test. To avoid potential confounding effects of the novel object being located in the center, a second NOI test was conducted where the novel object was placed in the perimeter of the open field. Mice were placed into the NOF apparatus for 1 h, then a novel object (different than the one previously used) was placed in the perimeter of the apparatus and NOI was measured for 30 min. Data from one subject in the NOI and NEP were lost due to a software error.
Cocaine conditioned place preference (CPP)
Mice (n = 47 WT, n = 44 ob/ob) were tested for cocaine CPP similar to described methods (Vialou et al. 2012). Day 1 consisted of a pre-conditioning test where mice were placed into the center chamber and had access to all three chambers for 15 min. Mice were then assigned one of the two main chambers to be paired with cocaine (counterbalanced, the alternate main chamber was assigned to saline), and over the next 4 days, mice underwent one conditioning session per day. During conditioning, mice received either saline (10 ml/kg) or cocaine (2.5, 5, or 10 mg/kg, i.p.) immediately prior to being confined to their assigned conditioning chamber for 20 min. Cocaine and saline conditioning sessions alternated daily, resulting in two conditioning sessions per treatment. The order of cocaine/saline sessions was counterbalanced within genotype. On the final day, a post-conditioning test was performed under the same conditions as the pre-conditioning test. Data were analyzed by calculating the time spent on the cocaine-paired chamber in the pre-test to the post-test.
Cocaine self-administration
Mice (n = 11 WT, n = 17 ob/ob) were implanted with jugular catheters and underwent cocaine self-administration without prior training or food restriction. Sessions began with turning on the fan (houselight remained off), extension of the two levers, and a single non-contingent dose of cocaine (0.5 mg/kg, 40 μl infusion over 2.3 s) with presentation of the cue light above the active lever (5 s). Each mouse was assigned either the left or right lever as the active lever (counterbalanced within genotype). Mice first underwent fixed ratio-1 (FR-1) sessions, where a single press on the active lever resulted in delivery of cocaine and cue light, followed by a 10-s timeout. An additional non-contingent dose of cocaine (with cue light) was delivered if no active lever presses occurred within 30 min of the session start. FR-1 sessions were 3 h in length, but terminated early if 64 infusions were earned. FR-1 sessions continued until criteria were met (three consecutive sessions of ≥15 infusions and ≥2:1 ratio of active to inactive lever presses, minimum 7 sessions) or 12 sessions had been completed. Next, the reinforcement schedule was advanced to a progressive ratio (PR). PR sessions were 4 h in length unless no reinforcer was earned within a 1-h period, in which case the session terminated early. During PR sessions, only the active lever was extended and each ratio was calculated based on the formula described by (Richardson and Roberts 1996), using j = 0.18. PR sessions were conducted for 3 days using 0.5 mg/kg cocaine, then for 3 days using 1.0 mg/kg cocaine. Catheter patency was determined at the end of FR-1 and PR sessions using Brevital (9 mg/kg, i.v.) and any mouse not meeting criteria for patency (sedation within 5 s) was removed from the study. Four WT and five ob/ob mice were removed due to non-patent catheters, resulting in n = 7 WT and n = 12 ob/ob mice in FR-1 analysis. In some cases, catheter patency was verified after the FR-1, but not the PR stage and thus only FR-1 data from those animals was included in analysis.
Remifentanil self-administration
Remifentanil self-administration was conducted in the same manner as cocaine self-administration, with the exception of drug and dose. Mice (n = 10 WT, n = 13 ob/ob) self-administered 0.003 mg/kg remifentanil under an FR-1 schedule of reinforcement. Mice were next tested under PR conditions (as described for cocaine) for 0.003 mg/kg, then 0.01 mg/kg remifentanil. Catheter patency was also verified after FR-1 and PR stages of self-administration as described for cocaine. Two WT and three ob/ob mice were removed due to non-patent catheters, resulting in n = 8 WT and n = 10 ob/ob mice in FR-1 analysis.
Ethanol two-bottle choice
In order to determine if any of the novelty seeking measures correlated with ethanol drinking (Manzo et al. 2014; Parkitna et al. 2013), mice from the first two (of four) cohorts that underwent the novelty seeking battery (n = 8 WT, n = 9 ob/ob) were singly housed, then exposed to ethanol in a continuous access two-bottle choice paradigm as described (Lee et al. 2013). Mice had access to food at all times and were exposed to each concentration of ethanol (3, 6, 10, 14, and 20 %) in ascending order for 4 days each. Every 2 days, bottles and animals were weighed and the positions of the two bottles were switched to prevent any side bias. To determine consumption, the weight of 1 ml of solution was determined for each concentration of ethanol using the same balance as that used throughout the experiment. Additionally, fluid loss (estimated by placing bottles into empty cages along the same schedule) was subtracted from measurements.
Data analysis
Data were analyzed by two-tailed Student’s t test, Fisher’s exact test, and ANOVA (repeated measures when appropriate) followed by Holm-Sidak multiple comparisons using Prism 6.0 (t tests), SPSS 21 (three-way repeated measures ANOVA), or SigmaPlot 11.0 (other tests). In the case of unequal variances in ANOVA, statistics were performed on ranked values and denoted with asterisk (*). In the case of unequal variances in t tests, Welch’s correction was applied, and adjusted degrees of freedom are reported and denoted with asterisk (*). Significance was set at p = 0.05.
Results
Sweet preference
Despite a previous report of enhanced sweet preference in db/db mice (mice that lack a functional leptin receptor) (Ninomiya et al. 1995), and the ability of leptin to directly regulate signaling in sweet-responsive taste bud cells (Yoshida et al. 2013), we found no group differences between WT and ob/ob mice in preference for either saccharin or sucrose under continuous access conditions (Fig. 1a, b). Although the preference was comparable, ob/ob mice had significantly higher fluid intake during saccharin (F(1,45)* = 52.8, p < 0.001) and sucrose (F(1,45)* = 34.5, p < 0.001) preference tests (Fig. 1c, d).
Sweet preference and fluid intake using continuous access two-bottle choice procedure. Mice had continuous access to chow during preference tests for saccharin (a) and sucrose (b). Total fluid intake during preference tests for saccharin (c) and sucrose (d). Symbols represent mean ± SEM. n = 5–6/group. *p < 0.05, **p < 0.01, ***p < 0.001 compared to lowest concentration
Novelty seeking assays
To examine novelty seeking, we tested mice in a battery of novelty seeking assays that included tests of forced exposure, free choice, and self-administration of novelty (Bardo et al. 1996; Olsen and Winder 2009). Activity in the novel open field was significantly reduced in ob/ob mice (t(37) = 11.2, p < 0.0001; Fig. 2a), as previously described (Pelleymounter et al. 1995), but center time was not significantly different between genotypes (Fig. 2c). Our pilot data indicated a possible difference between WT and ob/ob mice in center time, so NOI was analyzed using side placement of the novel object. There were no significant group differences in NOI (Fig. 2d) or novel environment preference (Fig. 2f). Contrary to results from the first three tests, ob/ob mice had significantly reduced novelty seeking in the OSS task. In the initial 8 days of OSS, there were main effects of genotype (F(1,74)* = 95.4, p < 0.001), lever (F(1,74)* = 32.7, p < 0.001), and session (F(7, 518)* = 10.7, p < 0.001) (Fig. 3a). There was also a significant interaction of session and lever (F(7,518)* = 7.9, p < 0.001), and genotype and lever (F(1,74)* = 4.1, p < 0.05), but not session and genotype or three-way interaction. When the final 3 days of OSS were aligned for animals that met criteria (Fig. 3b), there were significant effects of session (F(2,100)* = 4.1, p < 0.05), genotype (F(1,50)* = 16.9, p < 0.001), and lever (F(1,50)* = 234.9, p < 0.001), but no significant interactions. However, there were no effects of genotype or session on the number of reinforcers earned during these sessions (Fig. 3c). Despite the similar OSS performance among mice that met criteria, a significantly smaller proportion of ob/ob mice met criteria than WT (52 vs. 94 %, p < 0.05, Fisher’s exact test, Fig. 3d), and among mice that met OSS criteria, ob/ob required significantly more sessions (t(14.7)* = 3.7, p < 0.01; Fig. 3e).
Operant sensation seeking. a FR-1 responding during the first eight sessions in all mice. b Nosepokes and c reinforcers earned during the final three sessions in mice that acquired. d Proportion of mice that met acquisition criteria. e Number of days taken to reach criteria for mice that acquired OSS. n = 16–23/group *p < 0.05, **p < 0.01, ***p < 0.001
Cocaine conditioned place preference (CPP)
Comparison of WT and ob/ob mice in cocaine CPP revealed a significant main effect of dose (F(1,83)* = 7.1, p < 0.001), but not genotype or interaction (Fig. 4a). Both genotypes had a significant CPP for the 10 mg/kg dose, and ob/ob had a significantly greater difference score than WT at the 2.5 mg/kg dose. Locomotor responding during conditioning sessions for the highest dose of cocaine was also different between WT and ob/ob mice. There was a significant effect of genotype (F(1,19) = 30.0, p < 0.001) and session (F(2,38) = 8.2, p < 0.01) on locomotor activity during the first saline and the two cocaine conditioning sessions, and when locomotor activity during the first saline conditioning session was compared to the activity during cocaine conditioning, only WT mice showed elevated cocaine-associated locomotor activity (p < 0.01; Fig. 4b).
Conditioned place preference for cocaine. a Difference score (post-conditioning minus pre-conditioning time in cocaine-paired side), n = 7–15/group. b Locomotor counts during saline (Sal) and the 2 cocaine (C1, C2) conditioning sessions (n = 10–11/group). Bars represent mean + SEM. #p < 0.05, ##p < 0.01 compared to 0 mg/kg, *p < 0.05, **p < 0.01
Cocaine self-administration
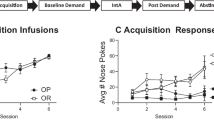
Both WT and ob/ob mice had significant cocaine CPP, although we observed a reduced locomotor effect of the drug as has been previously reported for amphetamine (Fulton et al. 2006). There is substantial evidence for distinctive neural mechanisms mediating Pavlovian and instrumental conditioning. Therefore, we also tested self-administration of cocaine in WT and ob/ob mice. Comparison of the initial 7 days of cocaine self-administration revealed significant main effects of lever (F(1,32)* = 19.8, p < 0.001; Fig. 5a) and session (F(6,192)* = 12.1, p < 0.001), but not genotype. Analysis of lever pressing (Fig. 5b) and reinforcers earned (Fig. 5c) during the last 3 days of FR-1 sessions revealed no significant differences in genotype. Furthermore, there was no significant difference between WT and ob/ob mice in the number of days or the proportion of mice to meet acquisition criteria (Fig. 5d, e). Under a progressive ratio, a main effect of dose (F(1,9) = 7.1, p < 0.05) was observed, but not genotype or interaction (Fig. 5f).
Intravenous cocaine self-administration. a FR-1 responding during the first seven sessions in all mice for cocaine (0.5 mg/kg/infusion). b Lever presses and c reinforcers earned and corresponding cocaine intake during the final three sessions in mice that acquired. d Proportion of mice that met acquisition criteria. e Number of days taken to reach criteria for mice that acquired cocaine self-administration. f Number of infusions earned and corresponding breakpoint during progressive ratio responding for 0.5 and 1.0 mg/kg/infusion cocaine. Bars represent mean + SEM. n = 8–12/group (FR-1), n = 4–7/group (PR)
Remifentanil self-administration
To determine if opioid reinforcement is altered in ob/ob mice, we performed intravenous remifentanil self-administration. Our initial studies using 0.01 mg/kg remifentanil yielded variable results during acquisition, so the training dose was reduced to 0.003 mg/kg. Thus, FR-1 acquisition includes animals (n = 1 WT, n = 3 ob/ob) that had up to 5 days of previous self-administration for 0.01 mg/kg, although this did not have a significant impact on the number of days to reach criteria. Comparison of the initial 7 days of remifentanil self-administration (Fig. 6a) revealed significant main effects of genotype (F(1,32)* = 5.6, p < 0.05), session (F(6,192)* = 12.3, p < 0.001), and lever (F(1, 32)* = 21.2, p < 0.001), with only the interaction of session and lever being significant (F(6,192)* = 4.4, p < 0.001). Analysis of lever pressing (Fig. 6b) and reinforcers earned (Fig. 6c) during the last 3 days of FR-1 sessions revealed a significant genotype difference only in reinforcers earned (p < 0.05)*. There was no significant difference between WT and ob/ob mice in the number of days or the proportion of mice to meet acquisition criteria (Fig. 6d, e). Under progressive ratio remifentanil self-administration, there was a significant main effect of genotype (F(1,10)* = 8.7, p < 0.05), where ob/ob mice earned significantly fewer infusions at both doses (Fig. 6f).
Intravenous Remifentanil self-administration. a FR-1 responding during the first seven sessions in all mice for remifentanil (0.003 mg/kg/infusion). b Lever presses and c reinforcers earned and corresponding remifentanil intake during the final three sessions in mice that acquired. d Proportion of mice that met acquisition criteria. e Number of days taken to reach criteria for mice that acquired remifentanil self-administration. f Number of infusions earned and corresponding breakpoint during progressive ratio responding for 0.003 and 0.01 mg/kg/infusion remifentanil. Bars represent mean + SEM. n = 8–10/group (FR-1), n = 5–7/group (PR). *p < 0.05 WT compared to ob/ob mice
Ethanol two-bottle choice
Alcohol intake was compared under continuous access two-bottle choice conditions for 4 days at each concentration as described (Lee et al. 2013). Analysis of ethanol consumption revealed a main effect of concentration (F(4,76)* = 18.6, p < 0.001) and interaction (F(4,84)* = 6.9, p < 0.001), but no significant main effect of genotype (Fig. 7a). Differences between WT and ob/ob mice became larger with higher concentrations of ethanol, and ob/ob mice had significantly lower intake of 20 % ethanol than WT (p < 0.05).
Ethanol drinking under continuous access two-bottle choice conditions. a Average amount consumed per day and b corresponding ethanol preference for each of the ethanol concentrations. c Correlation of body weight and total ethanol intake during the 20-day experiment. d Correlation of age and total ethanol intake during the 20-day experiment. n = 8–9/group. **p < 0.01 between genotypes
Analysis of ethanol preferences also revealed a main effect of concentration without an effect of genotype or interaction (Fig. 7b). When total intake across all concentrations was analyzed, ob/ob mice had greater variability compared to WT (F(8,7) = 11.8, p < 0.01), and further analysis revealed a significant inverse correlation between body weight and ethanol intake in ob/ob (F(1,7) = 15.3, p < 0.01, r 2 = 0.69), but not WT mice (Fig. 7c). This is not explained by age differences, as there was no significant correlation between age and ethanol intake in either ob/ob mice or WT mice (Fig. 7d).
To determine if this relationship extended to the other measures, and to examine relationships between the novelty seeking measures and ethanol intake, we performed correlations between body weight and each of the behavioral outcomes (Table 1). In ob/ob mice, significant inverse relationships were found between body weight and novel open field distance (r 2 = 0.20, p < 0.05) and novel environment preference (r 2 = 0.22, p < 0.05), although age was also negatively correlated these measures (r 2 = 0.24, p < 0.05 and r 2 = 0.30, p < 0.01, respectively). In WT mice, we observed a significant correlation between weight and novel object interaction (r 2 = 0.34, p < 0.05), which also generalized to age (r 2 = 0.27, p < 0.05). Among the novelty seeking measures, OSS was most highly correlated with ethanol intake in ob/ob mice (r 2 = 0.49, p < 0.05), but this relationship was not significant in WT mice. Thus, while correlations were observed between weight and several measures in ob/ob mice, only the relationship with ethanol intake was unique to weight. Weight differences did not explain the variance observed in remifentanil self-administration, as correlations between weight and self-administration were not significant on either the FR-1 or PR schedule.
Discussion
To gain insight into how leptin deficiency may alter behavioral responses to natural and drug rewards, we screened ob/ob mice in a variety of assays. Surprisingly, we found no significant differences between ob/ob and WT mice in many of the assays performed, despite expected differences in locomotor activity. Differences were, however revealed in cocaine CPP, OSS, and opioid self-administration.
In our screen of sweet preference, we found no difference between genotypes for either a non-caloric (saccharin) or a calorie-containing (sucrose) sweetener, although overall fluid consumption in ob/ob mice was approximately double that of WT throughout the experiment. A previous study found enhanced sucrose preference in the leptin receptor-deficient db/db mouse (Ninomiya et al. 1995). One potential reason for this difference in findings is that there may have been a ceiling effect in our study, where WT mice in Ninomiya et al. (1995) did not reach a maximum preference level for any concentrations tested. Another potential reason is that the lowest concentration of sucrose used in the current study produced greater than 50 % preference in both genotypes, which could have masked an increase in preference for lower sucrose concentrations in ob/ob mice.
We also performed a series of novelty seeking assays. Significant evidence suggests that novelty seeking is associated with mesolimbic dopamine transmission (Gjedde et al. 2010; Olsen and Winder 2009; Rebec et al. 1997). Leptin receptors are found within dopaminergic and non-dopaminergic neurons of the VTA, and leptin can regulate activity of VTA dopamine neurons both directly and indirectly (Hommel et al. 2006; Leinninger 2009; Thompson and Borgland 2013). Ob/ob mice appear to have reduced mesolimbic dopamine signaling, as tyrosine hydroxylase (the rate limiting enzyme in catecholamine synthesis) and evoked dopamine release are reduced in these mice (Fulton et al. 2006). Although we observed the expected reduction in novel open field activity (Sakkou et al. 2007) in ob/ob mice, we observed no differences in novel object interaction or novel environment preference. This is in contrast to reports of novelty-associated anxiety-like phenotypes in ob/ob mice (Asakawa et al. 2003; Finger et al. 2010). Reasons for this discrepancy are unclear, although it should be noted that mice in the current study were tested during the dark phase of their light cycle and handled prior to testing. Ob/ob mice in the present study did not show decreased center time in the novel open field, suggesting that under the experimental conditions employed here, novelty did not provoke anxiogenic responses.
In contrast to these free choice novelty seeking assays (NOI and NEP), we found that only 52 % of ob/ob mice acquired OSS. This was not a result of impaired learning, as ob/ob mice readily acquired self-administration of cocaine and remifentanil. Thus, although novelty promoted exploration in the NOI and NEP tests in ob/ob mice, the OSS stimuli (which novelty represents a significant component of (Olsen and Winder 2012)) failed to reinforce instrumental responding in nearly half of these mice.
We found that cocaine established CPP in ob/ob mice, despite a lack of significant locomotor activating effects of the psychostimulant in this assay. Furthermore, we found that ob/ob mice had a greater preference score for 2.5 mg/kg cocaine than WT mice. Our finding that CPP for the lowest dose of cocaine tested was surprising, but is consistent with a recent study showing a reduction in cocaine CPP following intra-VTA leptin (You et al. 2015). This is unlikely to be related to the increase in food intake/body weight, as diet-induced obese mice were found to have reduced cocaine CPP (Morales et al. 2012). Similarly, increased food intake/body weight likely does not explain the lack of locomotor effects of cocaine, as cocaine-induced locomotor activity is increased in mice fed high fat and/or high sugar diets (Collins et al. 2015; Olsen 2011).
As a complementary approach, we performed an experiment to determine if ob/ob mice would self-administer intravenous cocaine. We found that neither acquisition of cocaine self-administration nor motivation to obtain it (as measured by progressive ratio responding) was altered in ob/ob mice. While this may seem inconsistent, CPP and self-administration do not measure the same constructs (conditioned reward and reinforcement, respectively (Bardo and Bevins 2000; Mackintosh 1974; Tzschentke 2007)). Considering reports that ob/ob mice have reductions in basal locomotor activity (Goulding et al. 2008), psychostimulant-induced locomotor activity (Fulton et al. 2006), and mesolimbic dopamine levels (Fulton et al. 2006) (although see Roseberry et al. 2007), our finding that ob/ob mice achieved similar breakpoints for cocaine is especially noteworthy, as dopamine signaling is critically important for effort based responding in operant conditioning tasks (Salamone and Correa 2012).
Similar to cocaine, we found that ob/ob mice acquired intravenous self-administration of remifentanil without prior training. Despite similar levels of intake under FR-1 conditions, PR responding was significantly reduced in ob/ob mice. This is consistent with remifentanil not being as effective as a reinforcer in ob/ob mice (Richardson and Roberts 1996). Cocaine was able to support high levels of PR responding in ob/ob mice, indicating that task performance is not compromised in these mice. Instead, these differences may reflect a reported reduction of brain mu opioid receptors (MORs) in ob/ob mice (Khawaja et al. 1989).
Finally, we found that ob/ob mice had significant differences in ethanol consumption that were associated with body weight. A possibility is that the aforementioned reduction in mu opioid receptors (Khawaja et al. 1989) reduced drinking in ob/ob mice. Ethanol intake is significantly reduced by knockout or blockade of the mu opioid receptor (Hall et al. 2001; Le et al. 1993). Another possibility is that heavier ob/ob mice may have a greater severity of metabolic disturbances than those with lower body weight. Blednov et al. found that the pharmacokinetic profile of ethanol is markedly different in ob/ob mice (Blednov et al. 2004). A possible consequence of this is excessive accumulation of acetaldehyde (an intermediary of ethanol metabolism), resulting in dysphoria and subsequent ethanol avoidance. Consistent with this idea, Blednov et al. (2004) also reported decreased ethanol consumption in ob/ob mice.
It is difficult to identify a specific mechanism for chronic leptin deficiency that explains our pattern of findings, although several lines of evidence suggest that altered opioid signaling may be involved. Ob/ob mice have approximately 40 % fewer MOR binding sites than wild types (Khawaja et al. 1989). Furthermore, genetic deletion of MORs results in strikingly similar phenotypes to those reported here. Similar to our results from ob/ob mice, MOR knockout mice have reduced novelty seeking (Yoo et al. 2004), opioid self-administration (Becker et al. 2000), and alcohol intake (Hall et al. 2001; Roberts et al. 2000). The similarities also extend to reward-related behaviors that are not altered by MOR deletion: MOR knockout mice do not have significant differences in cocaine self-administration (Gutierrez-Cuesta et al. 2014) or sucrose consumption when measured under the conditions used in the present study (Kas et al. 2004; Ostlund et al. 2013). Alterations in MOR signaling may also explain the negative association between body weight and alcohol intake we observed. Diet-induced obesity has been shown to reduce MOR mRNA in the VTA and nucleus accumbens (Vucetic et al. 2011), and diet-induced obesity results in a reduction in alcohol consumption (Thanos et al. 2012). Despite these similarities, there are also distinct differences between some of the observed phenotypes in ob/ob and MOR knockout mice. MOR knockout mice do not have altered novel open field activity (Hall et al. 2004), and the locomotor response to cocaine has been reported to either be elevated (Hall et al. 2004; Hummel et al. 2004), unchanged (Lesscher et al. 2005), or reduced (Chefer et al. 2004). Cocaine CPP is also reduced in MOR knockout mice (Hall et al. 2004), contrary to our finding of elevated CPP at low doses of cocaine.
The putative nature of these hypotheses and the fact that some of our findings are consistent with results from acute leptin inhibition (e.g., cocaine CPP), while others are consistent with a reduction in MOR signaling highlight the fact that the ob/ob mouse is a chronic model of leptin deficiency. Thus, as with other chronic models of gene disruption, phenotypes observed in ob/ob mice may be a direct result of leptin deficiency or secondary to chronic deficiency. In particular, chronic deficiency could influence the development of circuitry that is involved in reward-related behaviors. Ob/ob mice have reduced brain weight and myelination (Bereiter and Jeanrenaud 1979; Hashimoto et al. 2013), and basal and evoked dopamine in the nucleus accumbens are reduced in these mice (Fulton et al. 2006). This reduction is contrary to what would be predicted by experiments demonstrating that acute leptin administration depresses mesolimbic dopamine function (Hommel et al. 2006; Thompson and Borgland 2013; You et al. 2015).
It should also be noted that all of the current studies were performed without food restriction. Food restriction increases performance on food and drug-associated behaviors (Baldo et al. 2013; Carr 2007), and the influence of leptin signaling on reward-related behaviors is modulated by the macronutrient content and availability of food (Figlewicz et al. 2006; Fulton et al. 2000), even in a chronic model of disrupted leptin signaling (Fulton et al. 2004).
Our data indicate that ob/ob mice do not have overarching differences in behavioral responses to rewards, and differences may be most apparent under conditions that require effort. For example, normal performance in the novel open field requires sustained activity, and OSS requires instrumental responding to obtain novel audiovisual stimuli. On the contrary, novelty seeking in the NOI and NEP tasks does not require additional activity or effort. Similarly, remifentanil self-administration was only reduced in ob/ob mice under a progressive ratio schedule—a schedule that requires increasing effort to maintain drug intake.
References
Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M (2003) Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications 17:105–107
Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M (2013) Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev 37:1985–1998
Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153:31–43
Bardo MT, Donohew RL, Harrington NG (1996) Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res 77:23–43
Becker A, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh HH, Hollt V (2000) Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 361:584–589
Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36:569–579
Bereiter DA, Jeanrenaud B (1979) Altered neuroanatomical organization in the central nervous system of the genetically obese (ob/ob) mouse. Brain Res 165:249–260
Blednov YA, Walker D, Harris RA (2004) Blockade of the leptin-sensitive pathway markedly reduces alcohol consumption in mice. Alcohol Clin Exp Res 28:1683–1692
Caron E, Sachot C, Prevot V, Bouret SG (2010) Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518:459–476
Carr KD (2007) Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav 91:459–472
Chefer VI, Kieffer BL, Shippenberg TS (2004) Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience 127:497–503
Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP (2015) Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice. Exp Clin Psychopharmacol 23:228–237
Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC (2011) Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 69:668–674
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115
Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW (2006) Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav 89:611–616
Finger BC, Dinan TG, Cryan JF (2010) Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 210:559–568
Friedman JM (1998) Leptin, leptin receptors, and the control of body weight. Nutr Rev 56:s38–s46, discussion s54-75
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770
Fulton S, Woodside B, Shizgal P (2000) Modulation of brain reward circuitry by leptin. Science 287:125–128
Fulton S, Richard D, Woodside B, Shizgal P (2004) Food restriction and leptin impact brain reward circuitry in lean and obese Zucker rats. Behav Brain Res 155:319–329
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822
Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A (2010) Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A 107:3870–3875
Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH (2008) A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci U S A 105:20575–20582
Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG (2006) Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci 26:3210–3219
Gutierrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martin-Garcia E, Maldonado R (2014) Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology 39:2974–2988
Hall FS, Sora I, Uhl GR (2001) Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 154:43–49
Hall FS, Goeb M, Li XF, Sora I, Uhl GR (2004) mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res Mol Brain Res 121:123–130
Hashimoto R, Matsumoto A, Udagawa J, Hioki K, Otani H (2013) Effect of leptin administration on myelination in ob/ob mouse cerebrum after birth. Neuroreport 24:22–29
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810
Houseknecht KL, Baile CA, Matteri RL, Spurlock ME (1998) The biology of leptin: a review. J Anim Sci 76:1405–1420
Hummel M, Ansonoff MA, Pintar JE, Unterwald EM (2004) Genetic and pharmacological manipulation of mu opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neuroscience 125:211–220
Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, Spruijt BM (2004) Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci 20:1624–1632
Khawaja XZ, Bailey CJ, Green IC (1989) Central mu, delta, and kappa opioid binding sites, and brain and pituitary beta-endorphin and met-enkephalin in genetically obese (ob/ob) and lean mice. Life Sci 44:1097–1105
Le AD, Poulos CX, Quan B, Chow S (1993) The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res 630:330–332
Lee AM, Zou ME, Lim JP, Stecher J, McMahon T, Messing RO (2013) Deletion of Prkcz increases intermittent ethanol consumption in mice. Alcohol Clin Exp Res 38(1):170−178
Leinninger GM (2009) Location, location, location: the CNS sites of leptin action dictate its regulation of homeostatic and hedonic pathways. Int J Obes (Lond) 33(2):S14–S17
Lesscher HM, Hordijk M, Bondar NP, Alekseyenko OV, Burbach JP, van Ree JM, Gerrits MA (2005) Mu-opioid receptors are not involved in acute cocaine-induced locomotor activity nor in development of cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology 30:278–285
Mackintosh NJ (1974) The psychology of animal learning. Academic, London
Manzo L, Gomez MJ, Callejas-Aguilera JE, Donaire R, Sabariego M, Fernandez-Teruel A, Canete A, Blazquez G, Papini MR, Torres C (2014) Relationship between ethanol preference and sensation/novelty seeking. Physiol Behav 133:53–60
Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT (2010) Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav 9(7): 790−798
Morales L, Del Olmo N, Valladolid-Acebes I, Fole A, Cano V, Merino B, Stucchi P, Ruggieri D, Lopez L, Alguacil LF, Ruiz-Gayo M (2012) Shift of circadian feeding pattern by high-fat diets is coincident with reward deficits in obese mice. PLoS One 7, e36139
Narayanan NS, Guarnieri DJ, DiLeone RJ (2010) Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol 31:104–112
Ninomiya Y, Sako N, Imai Y (1995) Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol 269:R930–R937
Olsen CM (2011) Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61:1109–1122
Olsen CM, Winder DG (2006) A method for single-session cocaine self-administration in the mouse. Psychopharmacology (Berl) 187:13–21
Olsen CM, Winder DG (2009) Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology 34:1685–1694
Olsen CM, Winder DG (2010) Operant sensation seeking in the mouse. J Vis Exp. doi: 10.3791/2292
Olsen CM, Winder DG (2012) Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett 511:8–11
Ostlund SB, Kosheleff A, Maidment NT, Murphy NP (2013) Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology (Berl) 229:105–113
Parkitna JR, Sikora M, Golda S, Golembiowska K, Bystrowska B, Engblom D, Bilbao A, Przewlocki R (2013) Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biol Psychiatry 73:263–270
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Rebec GV, Christensen JR, Guerra C, Bardo MT (1997) Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res 776:61–67
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH (2000) mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther 293:1002–1008
Roseberry AG, Painter T, Mark GP, Williams JT (2007) Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci 27:7021–7027
Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschop MH, Treier M (2007) A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell metabolism 5:450–463
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485
Schramm-Sapyta NL, Olsen CM, Winder DG (2006) Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology 31:1444–1451
Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK (2009) Leptin targets in the mouse brain. J Comp Neurol 514:518–532
Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, Wang GJ, Volkow ND, Hajnal A (2012) Gastric bypass increases ethanol and water consumption in diet-induced obese rats. Obes Surg 22:1884–1892
Thompson JL, Borgland SL (2013) Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons. Biol Psychiatry 73:860–868
Thomsen M, Caine SB (2007) Intravenous drug self-administration in mice: practical considerations. Behav Genet 37:101–118, Epub 2006 Aug 2
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Vialou V, Feng J, Robison AJ, Ku SM, Ferguson D, Scobie KN, Mazei-Robison MS, Mouzon E, Nestler EJ (2012) Serum response factor and cAMP response element binding protein are both required for cocaine induction of DeltaFosB. J Neurosci 32:7577–7584
Vucetic Z, Kimmel J, Reyes TM (2011) Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology 36(6):1199–1206
Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG (2004) Altered emotional behaviors and the expression of 5-HT1A and M1 muscarinic receptors in micro-opioid receptor knockout mice. Synapse 54:72–82
Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, Ninomiya Y (2013) Modulation of sweet responses of taste receptor cells. Semin Cell Dev Biol 24:226–231
You ZB, Wang B, Liu QR, Wu Y, Otvos L, Wise RA (2015) Reciprocal inhibitory interactions between the reward-related effects of leptin and cocaine. Neuropsychopharmacology 41(4):1024–1033
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number K99/R00 DA026994. Research was also supported by the Medical College of Wisconsin Research Affairs Committee and the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin. We gratefully acknowledge the NIDA Drug Supply Program for providing cocaine. The authors wish to thank Cassie Arthur for helping with data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Fluid intake during ethanol intake tests. Fluid intake of ethanol (A) and total fluid intake (B) during two-bottle choice tests. Bar: median, box: quartiles, whiskers: range. (DOC 65 kb)
Rights and permissions
About this article
Cite this article
Muelbl, M.J., Nawarawong, N.N., Clancy, P.T. et al. Responses to drugs of abuse and non-drug rewards in leptin deficient ob/ob mice. Psychopharmacology 233, 2799–2811 (2016). https://doi.org/10.1007/s00213-016-4323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4323-9