Abstract
Rationale
There has been an explosion of research on the potential benefits of the social neuropeptide oxytocin for a number of mental disorders including substance use disorders. Recent evidence suggests that intranasal oxytocin has both direct anti-addiction effects and pro-social effects that may facilitate engagement in psychosocial treatment for substance use disorders.
Objectives
We aimed to assess the tolerability of intranasal oxytocin and its effects on heroin craving, implicit association with heroin and social perceptual ability in opioid-dependent patients receiving opioid replacement therapy (ORT) and healthy control participants.
Methods
We performed a randomized, double-blind, placebo-controlled, within- and between-subjects, crossover, proof-of-concept trial to examine the effects of oxytocin (40 international units) on a cue-induced craving task (ORT patients only), an Implicit Association Task (IAT), and two social perception tasks: the Reading the Mind in the Eyes Task (RMET) and The Awareness of Social Inference Test (TASIT).
Results
Oxytocin was well tolerated by patients receiving ORT but had no significant effects on craving or IAT scores. There was a significant reduction in RMET performance after oxytocin administration versus placebo in the patient group only, and a significant reduction in TASIT performance after oxytocin in both the patient and healthy control groups.
Conclusions
A single dose of intranasal oxytocin is well tolerated by patients receiving ORT, paving the way for future investigations. Despite no significant improvement in craving or IAT scores after a single dose of oxytocin and some evidence that social perception was worsened, further investigation is required to determine the role oxytocin may play in the treatment of opioid use disorder.
Clinical Trial Registration
Methadone Oxytocin Option. ClinicalTrials.gov identifier: NCT01728909
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Opioid use disorder is an increasingly prevalent condition characterized by aversive withdrawal symptoms, the inability to control intake, intense cravings triggered by environmental cues, and social dysfunction and isolation (Koob and Le Moal 1997; De Vries and Shippenberg 2002; Schulteis et al. 2000). In the USA alone, over two million people abuse opioids, with over 450,000 addicted to heroin, and heroin use has nearly doubled over the past 10 years as prescription opioid abusers move toward street heroin (NIDA 2012). Further, currently available pharmacological and psychosocial treatments are marked by high relapse rates (Gossop et al. 1989, 2002). Given the prevalence and morbidity of opioid use disorder, new treatment options are desperately needed (Veilleux et al. 2010).
Emerging evidence supports the potential benefit of acute administration of intranasal oxytocin (a naturally occurring hypothalamic neuropeptide) in the treatment of psychiatric illness, including autism (Hollander et al. 2007), social anxiety disorder (Guastella et al. 2009), schizophrenia (Feifel et al. 2010), and substance abuse (Sarnyai and Kovács 2014). However, no published study has examined the effects of oxytocin in opioid-dependent individuals. We aimed to target patients receiving opioid replacement therapy (ORT) with methadone or buprenorphine. These long-acting opioid agonists reduce withdrawal symptoms and craving without producing the intense euphoria of short-acting opioids such as heroin (Raisch et al. 2002). As oxytocin can decrease opioid tolerance in animals (Kovács et al. 1985a; Kovacs et al. 1985; Kriván et al. 1992), we must first determine if oxytocin is safe to administer concurrently with ORT in humans. If oxytocin is safe when given with ORT, oxytocin’s ability to block opioid tolerance may actually increase the therapeutic effects of ORT, and ultimately reduce ORT dosages and the negative side effects they induce, which include fatal respiratory depression, bradycardia and arrhythmias, and hypotension.
A growing body of preclinical and clinical evidence suggests that oxytocin also has direct anti-addiction effects in both animal models and humans with substance use disorders. For example, central oxytocin infusion reduces heroin self-administration (Kovács et al. 1985b) and opioid withdrawal (Sarnyai and Kovács 1994) in rodents. Oxytocin also has inhibitory action on dopamine signaling in addiction-relevant brain regions such as the nucleus accumbens, suggesting that oxytocin has the potential to reduce the rewarding properties of drugs of abuse (Sarnyai and Kovács 1994; Kovács et al. 1998). Given the increasing evidence that substance abuse “hijacks” the brain’s reward system responsible for adaptive social behaviors (Liu et al. 2010), oxytocin may put a “brake” on some of the long-term habit forming properties of drugs of abuse and instead promote the formation of adaptive social relationships (Sarnyai and Kovács 2014; Carson et al. 2013; McGregor et al. 2008). As previous studies have linked implicit associations (i.e., unconscious and automatic) with opioids to relapse (Marhe et al. 2013; Robinson and Berridge 2001), it would be useful to investigate if oxytocin shifts associations away from opioid-related stimuli, in addition to more direct measures of tolerance, withdrawal, and craving. Taken together, these studies suggest that oxytocin administration may have the potential to reduce opioid tolerance, withdrawal symptoms, and the hardwired associations with opioids that contribute to the persistent craving and susceptibility to relapse experienced by opioid-dependent individuals.
In addition to anti-addiction effects, oxytocin has been widely studied for its beneficial role in social perception (Gordon et al. 2011; Meyer-Lindenberg et al. 2011), which is impaired in individuals with substance use disorders and is a key target for psychosocial intervention (Mueller et al. 2009). For example, individuals with opioid use disorder have difficulty reading emotional facial expressions (Kornreich et al. 2003) and are more inclined to perceive others as less friendly (Haertzen and Hooks Jr 1969) using laboratory measures. During a 60-day inpatient trial, heroin self-administration over 10 days was related to significant reductions in social interaction with other participants, which returned to baseline after detoxification (Babor et al. 1976). This is clinically important because, for individuals in recovery, social isolation directly increases craving for heroin (Stein et al. 2007). Furthermore, family conflict significantly predicted drug use during treatment for patients entering a rehabilitation program and reduction in family conflict was associated with a reduction in drug use; however, upon admission, 54 % of patients reported having no social contacts at all and, thus, were not included in this analysis (Knight and Simpson 1996). Thus, social deficits are a risk factor for, as well as a consequence of, substance use disorders. It is not surprising then that the treatment of disrupted social networks is an important mechanism of action of 12-step programs and is often integrated into ORT treatment programs through individual and group psychotherapy (Kelly et al. 2009). However, there are currently no pharmacological interventions to improve social perception in patients with substance use disorders. As oxytocin has been shown to improve multiple aspects of social functioning, including emotion recognition (Domes et al. 2007), oxytocin may improve social abilities in patients with opioid use disorder. This could facilitate their engagement in psychosocial treatments and help them develop social support systems critical to maintaining sobriety.
In the current study, we conducted a proof-of-concept pilot study to examine the effects of a single dose of intranasal oxytocin versus placebo in individuals with a history of heroin addiction currently receiving ORT and demographically matched healthy participants. The aims of the study were as follows: (a) to investigate the tolerability of administering a single dose of oxytocin concurrently with ORT, and (b) to explore the effects of intranasal oxytocin on our a priori endpoints of opioid craving, implicit associations with opioids, and social perception (ClinicalTrials.gov, NCT01728909). Our ultimate goal was to determine if intranasal oxytocin has potential as an adjunct treatment for opioid use disorder.
Methods
Subjects
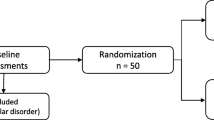
Thirty-six male patients with a history of intravenous heroin use were recruited from the San Francisco Veterans Affairs Medical Center (SFVAMC) ORT clinic, and 34 healthy control participants were recruited from the San Francisco Bay Area via Craigslist. The University of California, San Francisco Committee on Human Research approved all protocols, and informed written consent was obtained from each participant. Patients were on a stable dose of methadone or buprenorphine and were otherwise abstinent from illicit drugs and alcohol for a minimum of 2 weeks prior to study enrollment. Sobriety was assessed with a 30-day Timeline Follow Back (TLFB) procedure for drug and alcohol use (Fals-Stewart et al. 2000) and confirmed with routine clinic urine toxicology screens, administered randomly 1–4 times per month. Healthy control participants were excluded if they reported a history of an alcohol or substance use disorder, or indicated illicit drug use or heavy alcohol use during the 30-day TLFB procedure. Individuals with a history of serious mental illness, including chronic psychotic disorders, bipolar disorder, or major depression with suicidality were excluded from the study. However, given the comorbidity of substance use disorders and other mental health symptoms, taking psychiatric medications was not an exclusion criterion for the patient group provided the patient did not currently have a primary diagnosis of a nonsubstance-related Axis I mental disorder. Healthy control participants currently taking psychiatric medications were excluded from the study. Diagnoses were confirmed with medical records at the SFVAMC for patients and by the Structured Clinical Interview for DSM-IV (SCID) for healthy control participants. All participants had negative urine toxicology screens for cocaine, amphetamine, methamphetamine, and opioids (excluding ORT medications for patients) (Innovacon, San Diego, CA), and 0.00 Blood Alcohol Level (BACtrack, San Francisco, CA) at the time of each testing session. We administered the Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991), and participants with nicotine dependence were allowed to smoke if they wished to during designated breaks throughout the testing session in order to avoid nicotine withdrawal. Only male participants were recruited in order to minimize intersubject variation, as oxytocin administration may have sexually dimorphic effects (Meyer-Lindenberg et al. 2011).
Study design and drug administration
The study used a randomized, double-blind, placebo-controlled, crossover design, with the two testing days separated by at least 1 week. For standardization purposes, all appointments occurred at 1 p.m., which was approximately 4 h following ORT dosing. Peak methadone plasma levels occur 2.5 to 4 h after oral dosage (Inturrisi and Verebely 1971). On each testing visit, baseline safety assessments for withdrawal symptoms, subjective drug effects, and heroin craving were administered. Following baseline assessments and toxicology screening, 40 IU of intranasal oxytocin (Abbots Compounding Pharmacy, Berkeley, CA) or matched placebo were administered via nasal spray. Trained staff assisted with drug administration by instructing subjects to alternate insufflations every 15 s between nostrils over a 5-min time period (Feifel et al. 2010). The 40 IU dose has been used safely and is well tolerated in healthy populations (MacDonald et al. 2011). Previous studies have shown that intranasal oxytocin enters the CSF in animals (Neumann et al. 2013) and humans (Striepens et al. 2013) and begins to have physiological effects within 30 min, which last for a minimum of 90 min (Norman et al. 2011). In the current study, behavioral testing began 45 min post-administration to ensure the onset of oxytocin effects and continued for roughly 90 min thereafter. Following drug administration, subjects watched one of two 45-min banal nature documentaries, counterbalanced across visits. Such “vanilla baselines” are associated with greater between- and within-subject baseline stability (Jennings et al. 1992). Participants then completed a series of behavioral tasks measuring cue-induced drug craving, implicit association with drug stimuli, and social perception followed by repeated tolerability and craving assessments (Fig. 1).
Tolerability assessments
Opioid agonist and withdrawal scale
Prior to study drug administration and again 90 min following drug administration, patients were administered the opioid agonist and withdrawal scale (OAWS) (Stein et al. 2007; Stoller et al. 2001) to evaluate opioid agonist and withdrawal symptoms and oxytocin’s effects on these symptoms in the presence of ORT. Positive change scores indicate that a subject experienced greater agonist or withdrawal symptoms 90 min following nasal spray administration. The OAWS consists of a series of 38 adjectives, each either an opioid agonist or withdrawal symptom. Subjects were asked to rate how much they were currently experiencing each symptom on a five-point Likert scale. Composite scores for both agonist and withdrawal symptoms were calculated. Control participants were also given the OAWS to evaluate whether oxytocin administration has effects on these symptoms in the absence of opioid use disorder or ORT.
Visual analog scale for drug effects
Prior to study drug administration and again 90 min following drug administration, patients marked on an unnumbered 10-cm line the extent to which they were experiencing various drug effects (Bond and Lader 1974): (1) “do you feel any drug effects?”, (2) “does the drug have any good effects?”, (3) “does the drug have any bad effects?”, (4) “do you like the drug?”, (5) “does the drug make you feel sick?”, (6) “how high are you?”. For these questions, participants were told that “drug” included any medications recently taken, including the following: ORT (patients only), over-the-counter or prescription drugs, or the study drug. If participants were not experiencing any subjective drug effects, they were instructed to indicate this by marking their response at the left edge of the scale to indicate no effect. Comparisons between effects before, and 90 min after, study drug administration were made to assess if oxytocin alters sensitivity to ORT (i.e., acute reduction in opioid tolerance) or leads to other significant subjective effects. Positive change scores indicate that a subject experienced greater drug effects 90 min following nasal spray administration.
Craving
Cue-induced craving (patients only)
This task assesses heroin craving in response to a 1-min video of documentary footage from “Black Tar Heroin: The Dark End of the Street” (Okazaki 1999) depicting the use of black tar heroin (needle preparation, cooking, intravenous injection), as exposure to drug-related cues have been shown to trigger craving in individuals with heroin, cocaine, and alcohol use disorders (Shi et al. 2008; Bonson et al. 2002; Monti et al. 1987). Participants completed a two question visual analog craving scale to evaluate both “craving” for heroin and “urge to use” heroin at five time points: before study drug administration, 45 min following study drug administration (i.e., directly before cue exposure), directly after cue exposure, 3 min following cue exposure, and again at the end of the study (∼135 min following study drug administration). We measured change in craving in response to study drug administration and video cue exposure. Positive “after cue–before cue” scores indicate cue-induced craving.
Implicit association
Implicit Association Test
A modified Implicit Association Test (IAT) was used to assess participants’ implicit self-identification with opioids (Greenwald et al. 2009). Participants categorized pictures and words as fast as possible using the e and i keys on a standard PC keyboard, which corresponded to category labels depicted on the upper left (for e) and upper right (for i) portion of the screen. Pictures were either opioid-related images or neutral tools, and words were either self-identifying (e.g., “me,” “my”) or other-identifying (e.g., “other,” “their”). Scores of implicit associations between words and images are calculated with the assumption that participants respond more quickly and with fewer mistakes when the picture and word sharing the same response key are more strongly associated (Greenwald et al. 2009). Negative IAT scores indicate that participants self-identify with opioid imagery, and positive scores indicate that opioid imagery is associated with “others.” Implicit self-identification with alcohol cues has been shown to predict alcohol craving (Lindgren et al. 2014), and positive implicit associations with heroin cues have been shown to predict heroin relapse (Marhe et al. 2013). Furthermore, implicit associations may be a more reliable measure of attentional bias toward drugs than self-reports (utilized elsewhere in our protocol), which are vulnerable to reporting biases (Lane et al. 2007).
Social perception testing
Reading the Mind in the Eyes Test
The Reading the Mind in the Eyes Test (RMET) measures the ability to label the mental state of others based on viewing the eye region of faces making subtle affective expressions and choosing from four options the best word or phrase to describe what each person is thinking or feeling (Baron‐Cohen et al. 2001; Bora et al. 2009). All participants completed the 28-item child version of the RMET in which the language used in multiple-choice options has been simplified compared with the standard version to minimize IQ effects on performance. Studies have shown that oxytocin administration can improve RMET performance in both healthy (Domes et al. 2007) and autistic individuals (Guastella et al. 2010). Identical stimuli were used on both testing days.
The Awareness of Social Inference Test III
In The Awareness of Social Inference Test (TASIT) (Pearson Education, New York, NY), participants make social inferences after viewing video clips of actors engaging in social scenarios of various types and complexity. TASIT-III is composed of 16 video clips, all of which entail a message that is literally untrue. Half of the clips depict a “white lie,” where the main speaker attempts to hide information from another character, and the other half depict a sarcastic exchange, where the literal message is contrary to the actual message emphasized by the main speaker through paralinguistic and contextual cues. At the end of each clip, participants answer four yes/no questions regarding the speaker’s true beliefs, intentions, and emotional state. TASIT-III requires complex processing to decode speakers’ thoughts and intentions and thus measures high-level social cognition and theory of mind (Shany-Ur et al. 2012). Scores range from 0 to 64 questions correct. Two validated alternate forms were used, and order was randomized between test days.
Data analysis
GEE analyses
For tasks that measured outcomes repeatedly within participants (i.e., RMET and TASIT), we used generalized estimating equations (GEEs) to accommodate for correlations among the observations originating from the same participant. Binomial family GEEs were used to account for dichotomous (correct/incorrect) responding of the outcome variables, and unstructured correlation matrices were used for all analyses. For RMET, we included group (patient vs. control) as a between-subjects factor and drug (oxytocin vs. placebo) as a within-subjects factor. For TASIT-III, we included group (patient vs. control) as a between-subjects factor, and drug (oxytocin vs. placebo) and inference (lie vs. sarcasm) as within-subjects factors. To account for order and practice/fatigue effects, we included trial number and drug administration day as covariates in both models.
Parametric analyses
For other tasks and self-report measures that met assumptions for normality (i.e., OAWS and IAT), we used repeated-measures ANOVAs to compare differences in oxytocin effects within and between groups. For the OAWS, we ran separate repeated-measures ANOVAs for withdrawal and agonist scores with drug (oxytocin vs. placebo) as the within-subjects factor and group (patient vs. control) as the between-subjects factor, and also used one-way ANOVAs to compare group differences at baseline. Drug administration day was included as a covariate in both models.
Nonparametric analyses
Because the scales for craving and subjective drug effects violated assumptions of normality, we used nonparametric analyses. We used the Wilcoxon signed-rank test to examine within-subject differences in craving and subjective drug effects and the Mann–Whitney U test to examine between-group differences in subjective drug effects.
Additionally, chi-squared tests were performed to determine if participants could determine which drug (oxytocin or placebo) they received on each day, with expected values set to a chance level of 50 % (MacDonald et al. 2011). Participants were asked which drug they believed they received at the end of each test day.
All statistical tests were performed using SPSS Statistics version 22 (IBM, Armonk, NY).
Results
Demographics
For demographic information, see Table 1. Of the 36 recruited patients, two were removed from the study because they stopped receiving ORT after the first testing session, and one was excluded because he received placebo on both test days due to a pharmacy error. Of the 34 healthy control participants recruited, one was excluded because he tested positive for methamphetamine after his initial screen.
Tolerability assessments
Opioid agonist and withdrawal scale
There were no main effects of group [withdrawal F(1, 60) = 1.04, p = 0.31; agonist F(1, 60) = 0.56, p = 0.46] or drug [withdrawal F(1, 60) = 0.98, p = 0.33; agonist F(1, 60) = 0.02, p = 0.89] on OAWS change scores (i.e., the change in score from before to 90 min after study drug administration) (Table 2). There was also no group by drug interaction. Prior to study drug administration, patients experienced significantly greater withdrawal [F(1,62) = 12.64, p = 0.001], but not agonist [F(1,62) = 0.009, p = 0.92], symptoms compared to healthy controls when averaging ratings from both test days.
Visual analog scale for drug effects
In patients, there were significant increases in perceived “bad” [Z = −2.19, p = 0.029] and “high” [Z = −1.97, p = 0.049] drug effects following oxytocin administration compared with placebo (Table 2). For healthy controls, changes in perceived drug effects did not differ between oxytocin and placebo test day. Before study drug administration, patients experienced greater subjective drug effects compared with healthy controls for all items on the visual analog scale for drug effects (VASD) scale when averaging ratings from both test days: overall “drug effects” [Z = −2.92, p = 0.004], “good effects” [Z = −8.87, p < 0.001], “bad effects” [Z = −1.96, p = 0.050], “like” [Z = −4.32, p < 0.001], “sick” [Z = −2.12, p = 0.034], and “high” [Z = −2.38, p = 0.017].
Craving
Cue-induced craving (patients only)
Our video cue reliably induced craving on both placebo [craving: Z = −2.76, p = 0.006; urge: Z = −3.25, p = 0.001] and oxytocin [craving: Z = −3.48, p = 0.001; urge: Z = −3.25, p = 0.001] test days. Oxytocin had no effects on cue-induced craving when compared with placebo [“after cue”–“before cue” craving ratings: craving: Z = −0.55, p = 0.58; urge: Z = −0.58, p = 0.56; “before spray”–“pre-video” craving ratings: [craving: Z = −0.99, p = 0.32; urge: Z = −0.49, p = 0.63] (Fig. 2).
Mean heroin craving at five study time points (patients only). Craving level is percentage of 10 cm visual analog scale. The video cue significantly increased cravings for both oxytocin and placebo test days. Oxytocin had no significant effects on craving at any time point. Error bars indicate standard error
Implicit association
Implicit Association Test
On the placebo test day, patients showed no association in either direction [mean IAT score = 0.034, SE = 0.072], whereas healthy control participants showed a slight association between heroin imagery and other-identifying words [mean IAT score = 0.22, SE = 0.058]. However, there were no significant effects of group [F(1, 63) = 2.57, p = 0.11] or drug [F(1, 63) = 2.66, p = 0.11], nor any group by drug interaction [F(1, 64) = 0.98, p = 0.33].
Social perception testing
Reading the Mind in the Eyes Test
There was a main effect of group such that healthy controls performed significantly more accurately on the task than did patients [β = 0.39, SE = 0.04, p < 0.001]. There was also a main effect of drug [β = 0.20, SE = 0.04, p < 0.001] and a group by drug interaction [β = −0.17, SE = 0.05, p = 0.001], indicating that oxytocin significantly decreased RMET performance, though only among patients. Pairwise comparisons confirmed that patients performed significantly less accurately following oxytocin administration than they did following placebo administration [β = −0.05, SE = 0.01, p < 0.001]. Patients: [mean RMET % correct(SD)], placebo [65.96(10.81)] versus oxytocin [60.71(11.44)], p=0.005. Healthy controls: placebo [69.91(12.69)] versus oxytocin [69.26(12.11)], p=0.72.
The Awareness of Social Inference Test
Analysis of TASIT-III revealed no main effect of group [β = −0.04, SE = 0.13, p = 0.744]. There was a main effect of drug [β = 0.13, SE = 0.07, p = 0.049] with oxytocin decreasing TASIT-III performance (Table 3). There was a trend for a group by drug interaction [β = 0.23, SE = 0.14, p = 0.092]. Although both groups performed significantly worse on oxytocin than on placebo, this trend was more pronounced for healthy controls (80 % accuracy on placebo, 72 % on oxytocin) than for patients (76 % on placebo, 72 % on oxytocin). There was a main effect of inference type across groups, such that participants identified white lies significantly more accurately than sarcasm [β = 0.25, SE = 0.10, p = 0.015]. There were no drug by inference [β = 0.13, SE = 0.08, p = 0.132] or group by inference [β = 0.08, SE = 0.15, p = 0.606] interactions.
Additional analyses
Chi-squared tests revealed that both patients and healthy controls were at chance level when guessing whether they received oxytocin or placebo on a given test day [patients: 48.5 % accuracy, χ 2(1, N = 66) = 0.030, p = 0.86; healthy controls: 45.5 % accuracy, χ 2(1, N = 66) = 0.12, p = 0.73]. We also examined whether age correlated with performance on our behavior tasks for both patients and healthy controls. As age was not correlated with outcomes for either group, age was excluded from all analyses.
Discussion
A single 40 IU dose of intranasal oxytocin is tolerable when administered to patients receiving ORT for opioid use disorder. We did not detect any effects of oxytocin on opioid craving or drug-related implicit association. Patients did not show a significant implicit association with heroin cues on the placebo day, limiting our ability to detect any oxytocin-induced changes. Additionally, we found that oxytocin may paradoxically worsen performance on measures of social perceptual ability in patients receiving ORT.
Participants reported no difference in opioid agonist or withdrawal symptoms on the OAWS, which is reassuring regarding the tolerability of oxytocin in patients receiving ORT. However, patients did report more “bad effects” and feeling more “high” on the VASD after receiving oxytocin. Based on the lack of changes in the OAWS, which specifically identifies opioid withdrawal symptoms, this is unlikely to be a sign of reduction in opioid tolerance. It may be related to differences in tobacco use, age, or education between the two groups or some unmeasured factor. Participants guessed based on chance when asked if they had received oxytocin or placebo, which further suggests that there were no detectable subjective effects of oxytocin and is consistent with prior literature (MacDonald et al. 2011). Most importantly, patients did not report experiencing symptoms of opioid withdrawal after receiving a single dose of oxytocin, which was a concern based on preclinical studies of rodents (Sarnyai and Kovács 1994). This is reassuring given that such a reduction in opioid tolerance could lead to dangerous levels of opioid toxicity in patients receiving daily ORT. Because we only used a single dose of oxytocin and measured tolerability at only two time points (baseline and 90 min post-administration), we are limited in our assessment of tolerability. Nonetheless, this is a first step toward assessing the safety of oxytocin administration to patients receiving ORT. Future studies should include repeated oxytocin dosing in this population as well as frequent physiological measurements of safety related to opioid toxicity (e.g., respiration rate or oxygen saturation).
Oxytocin had no obvious effects on the addiction-related measures used in our study. Previous studies of intranasal oxytocin’s effects in substance use disorders demonstrated a reduction in alcohol withdrawal and craving (Pedersen et al. 2011, 2013), a reduction in stress-induced marijuana craving (McRae-Clark et al. 2013), and mitigation of the effect of state anger on cocaine cue-reactivity (Lee et al. 2014) in dependent individuals. Given that McRae-Clark et al. (2013) found a significant effect of oxytocin on marijuana craving following a reliable social stressor, the participants in Pedersen et al.’s (2011) study were undergoing acute alcohol withdrawal, and Lee et al. (2014) found no effect on cue-reactivity when anger was not factored in, it is possible that intranasal oxytocin primarily reduces acute stress-induced craving. If so, the cue-induced craving paradigm used in the current study may not be sensitive to oxytocin-related effects. Given that the evidence reviewed by Bartz et al. (2011) indicates that there are substantial individual differences in oxytocin response, it is possible that trait-level individual differences may mask a significant drug effect in some subpopulations when analyzing aggregate data. If so, future analyses and clinical trials might assess trait-level sources of oxytocin response variability. On the other hand, another way to interpret the current null findings is that intranasal oxytocin may not be a viable treatment for opioid use disorder in humans.
Despite a growing body of preclinical evidence demonstrating oxytocin’s effects on opioid tolerance, withdrawal, and self-administration in rodents (Sarnyai and Kovács 2014), no studies have previously investigated oxytocin’s effects in humans with opioid use disorder. There are many factors to consider when translating preclinical research to clinical populations. The relative complexity of human neurobiology and behavior compared to animal models likely limits translatability. Our pilot study was small and limited in its generalizability to males with a history of intravenous heroin use currently receiving ORT. Moreover, knowledge of optimal oxytocin drug delivery and dosing for humans remains unknown. Studies of the effects of repeated oxytocin dosing on the symptoms of schizophrenia often required several weeks before symptom reduction was observed (Feifel et al. 2010; Pedersen et al. 2011; Modabbernia et al. 2013). Given strong preclinical evidence for oxytocin’s effects on opioid use disorders and the preliminary nature of our study, future studies of oxytocin administration in this clinical population are warranted and should include dose-tiered and repeated-dosing protocols.
Patients’ significantly lower scores on the RMET compared to healthy controls verified that our study participants experienced social deficits consistent with existing knowledge of populations with substance use disorders. Our finding of an oxytocin-induced reduction in overall RMET score is consistent with Lee et al. (2014), who demonstrated worsened social cognition in participants with a history of cocaine use disorder after receiving oxytocin. On the other hand, we did not replicate the oxytocin-induced enhancement of RMET performance demonstrated by Domes et al. (2007), even when questions were divided by difficulty (Domes showed a particular enhancement with oxytocin on difficult compared with easy questions). Several factors may have contributed to this, including differing dosages of intranasal oxytocin (40 versus 24 IU) and different versions of the RMET (28-item child version versus 36-item standard version). However, others, including our group (Woolley et al. 2014), have also failed to replicate this finding in healthy populations (Radke and de Bruijn 2015). Additionally, our healthy control participants demonstrated worse performance on TASIT-III after receiving oxytocin. This is partly consistent with our previous study, in which we saw oxytocin-induced worse performance on aspects of TASIT-III in healthy participants (Woolley et al. 2014). Future studies should continue to explore oxytocin’s complex effects on social perception in patients with substance use disorders.
While our single-dose study of oxytocin’s effects on the symptoms of opioid use disorder did not find any effects on opioid craving or implicit association, the safety and tolerability of single-dose intranasal oxytocin demonstrated in our study of patients receiving ORT paves the way for studies examining tiered and repeated dosing in this population. Given the risk for the “file drawer” problem of publication bias, it is important to report clinical trials that do not find an effect of a pharmacological agent. Further research is warranted to determine oxytocin’s effects in opioid use disorder.
References
Babor TF, Meyer RE, Mirin SM, Mcnamee HB, Davies M (1976) Behavioral and social effects of heroin self-administration and withdrawal. Arch Gen Psychiatry 33:363–367
Baron‐Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I (2001) The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 42:241–251
Bartz JA, Zaki J, Bolger N, Ochsner KN (2011) Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 15(7):301–319
Black Tar Heroin: The Dark End of the Street (1999) Directed by OKAZAKI, S. United States: Farallon Films
Bond A, Lader M (1974) The use of analogue scales in rating subjective feelings. Br J Med Psychol 47:211–218
Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED (2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26(3):376–86
Bora E, Yücel M, Pantelis C (2009) Theory of mind impairment: a distinct trait-marker for schizophrenia spectrum disorders and bipolar disorder? Acta Psychiatr Scand 120:253–264
Carson DS, Guastella AJ, Taylor ER, Mcgregor IS (2013) A brief history of oxytocin and its role in modulating psychostimulant effects. J Psychopharmacol 27(3):231–247
De Vries TJ, Shippenberg TS (2002) Neural systems underlying opiate addiction. J Neurosci 22:3321–3325
Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC (2007) Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61:731–733
Fals-Stewart W, O’Farrell TJ, Freitas TT, Mcfarlin SK, Rutigliano P (2000) The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol 68:134
Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W (2010) Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 68:678–680
Gordon I, Martin C, Feldman R, Leckman JF (2011) Oxytocin and social motivation. Dev Cogn Neurosci 1:471–493
Gossop M, Green L, Phillips G, Bradley B (1989) Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry 154:348–353
Gossop M, Stewart D, Browne N, Marsden J (2002) Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction 97:1259–1267
Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR (2009) Understanding and using the implicit association test: III. Meta-analysis of predictive validity. J Pers Soc Psychol 97:17
Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS (2009) A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 34:917–923
Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB (2010) Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67:692–694
Haertzen CA, Hooks NT Jr (1969) Changes in personality and subjective experience associated with the chronic adminstration and withdrawal of opiates. J Nerv Ment Dis 148:606–614
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The fagerstrom test for nicotine. Br J Addict 86:1119–27
Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S (2007) Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61:498–503
Inturrisi CE, Verebely K (1971) Disposition of methadone in man after a single oral dose. Clin Pharmacol Ther 13:923–930
Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P (1992) Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology 29:742–50
Kelly JF, Magill M, Stout RL (2009) How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in Alcoholics Anonymous. Addict Res Theory 17:236–259
Knight DK, Simpson DD (1996) Influences of family and friends on client progress during drug abuse treatment. J Subst Abus 8:417–429
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Kornreich C, Foisy M-L, Philippot P, Dan B, Tecco J, Noël X, Hess U, Pelc I, Verbanck P (2003) Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Res 119:251–260
Kovács G, Horváth Z, Sarnyai Z, Faludi M, Telegdy G (1985a) Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology 24:413–419
Kovács GL, Borthaiser Z, Telegdy G (1985b) Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci 37:17–26
Kovacs GL, Vecsernyes M, Laczi F, Faludi M, Telegdy G, Laszlo FA (1985) Acute morphine treatment and morphine tolerance/dependence alter immunoreactive oxytocin levels in the mouse hippocampus. Brain Res 328:158–60
Kovács GL, Sarnyai Z, Szabó G (1998) Oxytocin and addiction: a review. Psychoneuroendocrinology 23:945–962
Kriván M, Szabó G, Sarnyai Z, Kovács GL, Telegdy G (1992) Oxytocin blocks the development of heroin-enkephalin cross-tolerance in mice. Pharmacol Biochem Behav 43:187–192
Lane KA, Banaji MR, Nosek BA, Greenwald AG (2007) Understanding and using the implicit association test: IV. In: Wittenbrink B, Schwarz N (eds) Implicit measures of attitudes. New York, The Guilford Press, pp 59–102
Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, Salmeron BJ (2014) Complexity of oxytocin׳ s effects in a chronic cocaine dependent population. Eur Neuropsychopharmacol 24:1483–1491
Lindgren KP, Neighbors C, Westgate E, Salemink E (2014) Self-control and implicit drinking identity as predictors of alcohol consumption, problems, and cravings. J Stud Alcohol Drugs 75:290
Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z (2010) Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci 107:1217–1222
Macdonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ (2011) A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 36:1114–1126
Marhe R, Waters AJ, Van De Wetering BJM, Franken IHA (2013) Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol 81:1
Mcgregor IS, Callaghan PD, Hunt GE (2008) From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long‐term adverse consequences of drug use? Br J Pharmacol 154:358–368
Mcrae-Clark AL, Baker NL, Moran-Santa Maria M, Brady KT (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology 228:623–631
Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011) Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–538
Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SMR, Tajdini M, Ghaleiha A, Akhondzadeh S (2013) Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia. CNS Drugs 27:57–65
Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR (1987) Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96:122
Mueller SE, Degen B, Petitjean S, Wiesbeck GA, Walter M (2009) Gender differences in interpersonal problems of alcohol-dependent patients and healthy controls. Int J Environ Res Public Health 6:3010–3022
Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R (2013) Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38:1985–1993
NIDA 2012. Mental Health Services Administration (2012) Results from the 2011 national survey on drug use and health: summary of national findings. NSDUH Series H-44, HHS Publication No (SMA), 12–4713
Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, Devries AC, Berntson GG (2011) Selective influences of oxytocin on the evaluative processing of social stimuli. J Psychopharmacol 25(10):1313–1319
Pedersen CA, Boccia ML, Kampov-Polevoi A (2011) Methods and formulations for oxytocin treatment of substance use, psychiatric and other disorders. Google Patents
Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov‐Polevoi A, Casey RL, Fender T, Garbutt JC (2013) Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res 37:484–489
Radke S, De Bruijn ERA (2015) Does oxytocin affect mind-reading? A replication study. Psychoneuroendocrinology 60:75–81
Raisch DW, Fye CL, Boardman KD, Sather MR (2002) Opioid dependence treatment, including buprenorphine/naloxone. Ann Pharmacother 36:312–321
Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Sarnyai Z, Kovács GL (1994) Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology 19:85–117
Sarnyai Z, Kovács GL (2014) Oxytocin in learning and addiction: from early discoveries to the present. Pharmacol Biochem Behav 119:3–9
Schulteis G, Ahmed SH, Morse AC, Koob GF, Everitt BJ (2000) Conditioning and opiate withdrawal. Nature 405:1013–4
Shany-Ur T, Poorzand P, Grossman SN, Growdon ME, Jang JY, Ketelle RS, Miller BL, Rankin KP (2012) Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex 48:1329–1341
Shi J, Zhao L-Y, Copersino ML, Fang Y-X, Chen Y, Tian J, Deng Y, Shuai Y, Jin J, Lu L (2008) PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol 579:160–166
Stein DJ, Van Honk J, Ipser J, Solms M, Panksepp J (2007) Opioids: from physical pain to the pain of social isolation. CNS Spectr 12:669–674
Stoller KB, Bigelow GE, Walsh SL, Strain EC (2001) Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology 154:230–242
Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R (2013) Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 3:3440
Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ (2010) A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev 30:155–166
Woolley JD, Chuang B, Lam O, Lai W, O’Donovan A, Rankin KP, Mathalon DH, Vinogradov S (2014) Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology 47:116–25
Acknowledgments
This project was supported by the San Francisco Treatment Research Center at the University of California, San Francisco, via grant number P50 DA009253 from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JDW, PAA, CSS, DF, SB, and SV have no conflicts of interest to report. DSC is a full-time employee of Trigemina, Inc. The opinions expressed here do not necessarily reflect the views or positions of Trigemina, Inc.
Rights and permissions
About this article
Cite this article
Woolley, J.D., Arcuni, P.A., Stauffer, C.S. et al. The effects of intranasal oxytocin in opioid-dependent individuals and healthy control subjects: a pilot study. Psychopharmacology 233, 2571–2580 (2016). https://doi.org/10.1007/s00213-016-4308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4308-8