Abstract
Background
Olfactory bulbectomy (OBX) in rodents is considered a putative animal model of depression. It has been reported that some abnormal behaviors observed in this animal model of depression involve dopaminergic neurons of the mesolimbic pathway. Therefore, we examined changes in the dopaminergic system in the caudate putamen (CPu), nucleus accumbens core (NAcC), and shell (NAcSh) of OBX mice and whether or not these alterations were reversed by chronic administration of imipramine.
Methods
We observed climbing behavior, which is a dopamine (DA) receptor-associated behavior, to demonstrate changes in the dopaminergic system of the mesolimbic pathway, when mice were administrated either the nonselective DA agonist apomorphine only or were pre-treated with the selective D1 antagonist SCH23390, with the selective D2 antagonist sulpiride, or with the D2/D3 partial agonist aripiprazole (ARI). Moreover, we examined tyrosine hydroxylase (TH) and D1- and D2-like receptor levels in the CPu, NAcC, and NAcSh using immunohistochemistry and autoradiography.
Results
The OBX group exhibited significantly enhanced apomorphine-induced climbing behavior, and this enhanced behavior was reversed by administration of sulpiride, ARI, and imipramine but not SCH23390. Moreover, we found a reduction in TH levels in the CPu, NAcC, and NAcSh of OBX mice and an increase in D2 receptor densities in the NAcC of OBX mice. The increased D2 receptor density observed in OBX mice was reversed by imipramine administration.
Conclusions
These findings reveal that OBX mice display enhanced DA receptor responsiveness, which may relate to some of the behavioral abnormalities reported in this animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olfactory bulbectomized (OBX) rodents are one of the most commonly used animal models of depression. OBX in rodents leads to various abnormal behaviors, such as aggressive behavior (Cain 1974), reduced sexual activity (Larsson 1971; Lumia et al. 1992; Sato et al. 2010b), increased exploratory behavior (Sieck 1972), memory dysfunction (Hozumi et al. 2003; Nakagawasai et al. 2003a), anhedonia (Calcagnetti et al. 1996), and depressive behavior (Kelly et al. 1997; Sato et al. 2010a). These behaviors are observed 1–3 weeks after the OBX. The abnormal behaviors in OBX rodents are reported to be ameliorated by chronic, but not acute, administration of anti-depressants (Breuer et al. 2009a, b). Moreover, there are also alterations in neurotransmitter and receptor function associated with these behavioral effects in OBX rodents. Specifically, there are many reports concerning an altered serotonergic or noradrenergic system (Jancsàr and Leonard 1984; Nakagawasai et al. 2003b; Song and Leonard 1995). We have previously reported changes in the mesolimbic dopaminergic system associated with maternal behavior in female OBX mice (Sato et al. 2010a, b). The dopaminergic system is strongly involved in emotion-related behaviors in OBX rodents, especially in the nucleus accumbens (NAc) which is associated with memory, pleasure, and motivation (Mele et al. 2004; Ploeger et al. 1994; Setlow and McGaugh 1998). The dopaminergic system in the NAc is known to play an important role in motivational processes, and dysfunctions of the mesolimbic dopaminergic system may contribute to the motivational symptoms of depression and other disorders, as well as features of substance abuse (Salamone and Correa 2012). It has been proposed that the NAc and ventral tegmental area (VTA) contribute importantly to the pathophysiology and symptomatology of depression and may even be involved in its etiology (Nestler and Carlezon 2006; Randrup and Mogilnicka 1976; Willner 1983). Electroconvulsive therapy (ECT) is used to treat drug-resistant depressive disorders. ECT decreases the autoreceptor functions in noradrenergic and dopaminergic neurons in the locus coeruleus and substantia nigra, respectively, resulting in an increase in release of noradrenaline (NA) and dopamine (DA) (Jancsár and Leonard 1983). It is thought that normalization of the dopaminergic system has a beneficial effect on drug-resistant depressive disorders. Thus, decoding the functions of the mesolimbic dopaminergic system may help in the development of future anti-depressant drugs.
In the present study, we examined the climbing behavior of mice, which is a DA receptor-associated behavior, to demonstrate changes in the dopaminergic system in the brain. Moreover, we examined tyrosine hydroxylase (TH) levels, a biosynthetic rate-controlling enzyme of catecholamines such as DA, in the caudate putamen (CPu), NAc core (NAcC), and shell (NAcSh) using a brain mapping analyzer system to investigate pre-synaptic function of dopaminergic nerves and quantify D1- and D2-like receptors in these areas using autoradiography and the mapping analyzer system to demonstrate an association between behavior and DA receptors. Then, we examined whether OBX-induced alterations of behavior and the dopaminergic system were reversed by chronic administration of the tricyclic anti-depressant imipramine.
Method
Efforts were made to minimize suffering and to reduce the number of animals used.
Animals
Male ddY strain mice (weighing 28–32 g; Japan SLC, Japan) were used for all experiments (total n = 560, behavioral tests n = 371, autoradiography n = 108, and immunohistochemical experiment n = 81). Mice were housed in cages with free access to food and water under conditions of constant temperature (22 ± 2 °C) and humidity (55 ± 5 %), on a 12-h light–dark cycle (lights on 07:00 to 19:00). Mice were housed in groups in cages (cage dimensions: height 12 cm, width 17 cm, and length 27 cm).
Olfactory bulbectomized mice
Mice anesthetized with pentobarbital Na (50 mg/kg, intraperitoneally (i.p.); Dainippon Sumitomo Pharma, Osaka, Japan) were placed in a stereotaxic frame. The scalp was incised; two holes were drilled to expose the olfactory bulb (OB) (2 mm anterior to bregma and 1 mm lateral to the midbrain), which was then bilaterally aspirated using a suction pump. All animals were sacrificed at the end of the experiment and the lesions were verified visually. It was confirmed that at least two thirds of the OB had been removed and that some parts of the olfactory nuclei also had been lesioned. If the lesion was either not extensive enough or extended to the cortex, data from these animals were excluded (the OBX operation was successful for approximately 90 % of the operated mice). Sham operations were performed in the same manner but without the removal of the OB.
Drugs
Apomorphine hydrochloride (0.33, 1.0, and 3.0 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline containing 0.1 % ascorbic acid. SCH23390 (Sigma-Aldrich) and imipramine (Sigma-Aldrich) were dissolved in saline. Aripiprazole (ARI; 0.1, 0.03, and 0.01 mg/kg; Wako Pure Chemical Industries Ltd., Osaka, Japan) and sulpiride (Sigma-Aldrich) were suspended in 0.5 % Tween 80. Apomorphine hydrochloride was administered subcutaneously (s.c.) 10 min before behavioral observation. SCH23390, sulpiride, and ARI were i.p. administered 30 min before behavioral observation. Imipramine was i.p. administered for 2 weeks from the 14th day after operation, and behavioral observations were performed 24 h after the last drug injection. These drugs were administered at a dose of 0.1 mL/10 g of mouse body weight.
Climbing behavior measurement
Climbing behavior is defined as tending to adopt a vertical position either by rearing with their forepaws against the wall of their cage or by climbing toward its top, where they stay motionless, grabbing the wire mesh with all four paws (Protais et al. 1976). The climbing behavior measurement was conducted in steel cylinders (height 14 cm and diameter 11.5 cm) which have walls of vertical bars (1 cm apart and 2 mm diameter). After administration of drug, a mouse was placed in the instrument. Measurement of the climbing behavior time was conducted for 2 min at 10–15-min intervals from 10 to 60 min (10, 20, 30, 45, and 60 min) after drug administration by a blinded observer. Climbing behavior times represent the total of five 2-min tests (600 s). Measurements were performed between 11:00 and 17:00. Each mouse was tested for climbing behavior only once, on day 3, 7, or 14 postoperation.
Quantitative receptor autoradiography
Sham and OBX mice were sacrificed by decapitation on the 14th day after surgery, and their brains were removed and frozen in isopentane at −40 °C and stored at −80 °C. Using a cryostat (Leitz, Stuttgart, Germany), 20-μm-thick coronal sections including both NAc and CPu (Bregma from +0.86 to +1.10 mm) were collected on gelatin-coated slides and stored at −80 °C until use. D1- and D2-like receptor binding sites were visualized using [3H] SCH23390 (85.0 Ci/mmol) and [3H] raclopride (85.0 Ci/mmol), respectively (PerkinElmer Life Analytical Science, Boston, MA, USA). The protocol was carried out as previously described (Sato et al. 2010b). Briefly, sections were pre-incubated for 15 min in Tris–HCl buffer [Tris 50 (for D1) or 170 (for D2) mM, NaCl 120 mM, KCl 5 mM, CaCl2 2 mM, and MgCl2 1 mM, pH 7.4] at room temperature prior to a 60-min incubation in the same buffer containing either 1 nM [3H] SCH23390 or 3 nM [3H] raclopride. Nonspecific binding was determined by incubation of 1 μM SCH23390 (Sigma-Aldrich) or 300 μM sulpiride (Sigma-Aldrich) in the assay buffer containing 1 nM [3H] SCH23390 or 3 nM [3H] raclopride, respectively. At the end of the incubation, sections were rinsed four times, 2 min each in ice-cold buffer, then dipped in deionized water to remove salts, and rapidly dried. All slides were exposed to a tritium-sensitive imaging plate (Fujifilm Corporation, Tokyo, Japan) together with [3H] microscale standards (GE Healthcare UK Ltd., Buckinghamshire, UK) for 10 days for both bindings. Specific labeling was quantified (nCi/tissue) using a computer-assisted Image Reader BAS 5000 version 1.12 (Fujifilm Corporation).
Immunohistochemical procedure
The preparation of brains for the measurement of TH levels was conducted on the 14th or 28th day after surgery. Moreover, D1- and D2-like receptor distribution was measured on the 28th day after surgery. To investigate the effect of chronic administration of drugs on TH levels and D1- and D2-like receptor distribution, the animals were sacrificed 24 h after the last drug injection (28th day after surgery). Mice used for the immunohistochemical experiments had not been previously used for behavioral tests. The mice were anesthetized with pentobarbital Na (50 mg/kg, i.p.) and perfused through the heart with ice-cold phosphate-buffered saline (PBS; pH 7.4), immediately followed by a fixative containing 4 % paraformaldehyde (Sigma-Aldrich) and 0.2 % glutaraldehyde (Nacalai Tesque, Osaka, Japan) in PBS. The brain was then postfixed with the same fixative solution at 4 °C for 1 h and then placed in a 20 % sucrose-buffered solution at 4 °C for 12 h. The tissue was frozen on dry ice and cut into 20-μm-thick coronal sections (Bregma from +0.86 to +1.10 mm) on a cryostat (Leiz). The immunohistochemical staining procedure was carried out as previously described (Nakagawasai et al. 2007; Sutoo et al. 2001). Briefly, the rabbit anti-TH antibody (diluted 1:100 with PBS including 0.1 % Triton X-100 and 0.05 % normal goat serum (NGS); Millipore Corporation, Billerica, MA, USA), the rabbit anti-D1 receptor antibody (diluted 1:200 with PBS including 0.05 % NGS; Abcam Ltd., UK), or the rabbit anti-D2 receptor antibody (diluted 1:100 with PBS including 0.05 % NGS; Millipore Corporation) was applied to each brain slice, which was then incubated at 4 °C for 12 h. The TH antibody cross-reacted immunohistochemically with TH from the brains of several species including mice, rats, ferrets, and feline. The D1 receptor antibody cross-reacted immunohistochemically with D1 receptors from the brains of several species including mice, rats, human, and aplysia. The D2 receptor antibody cross-reacted immunohistochemically with D2 receptors from the brains of several species including mice, rats, human, and monkey. The secondary antibody consisted of FITC-labeled anti-rabbit IgG goat serum (diluted 1:200 with PBS; Millipore Corporation) and was allowed to react in the dark at room temperature for 3 h. The stained sections were mounted in 10 % glycerin-PBS and kept at 4 °C in a dark room until measurements were carried out. The distribution of TH, D1, or D2 receptor immunofluorescence intensities were quantitatively analyzed using a brain mapping analyzer system (Yamato Science Co., Inc., Tokyo, Japan). The background value, including nonspecific fluorescence originating from glutaraldehyde, was subtracted photometrically from the total fluorescence intensity value at each point measured. Immunohistochemical fluorescence intensities obtained for the various regions are relative to that of standard 1 mM quinine sulfate.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). The significance of differences was determined by the Student’s t test for two-group comparison, and by a two-way analysis of variance (ANOVA), followed by Fisher’s PLSD test for multigroup comparisons. p < 0.05 represents a significant difference. For the saturation-binding experiments, size effects were calculated using Cohen’s d for within- and between-group comparisons, with the pooled standard deviation of the compared groups as denominator.
Results
Change in apomorphine-induced climbing behavior after OBX
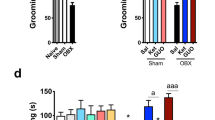
Apomorphine-induced climbing behavior (3.0 mg/kg) on the 14th day after OBX was significantly increased compared to the sham group (0.33 mg/kg p > 0.05, 1.0 mg/kg p > 0.05, and 3.0 mg/kg p < 0.01; Fig. 1a). A two-way ANOVA revealed a significant effect of group and dose but not group × dose (group F (1, 66) = 11.04, p < 0.01; dose F (2, 66) = 42.50, p < 0.01; and group × dose F (2,66) = 2.70, p = 0.075; Fig. 1a). Next, we observed time-dependent changes in apomorphine-induced climbing behavior after OBX. Apomorphine-induced climbing behavior on the third and seventh days after OBX was not significantly different from the sham group (third p > 0.05 and seventh p > 0.05; Fig. 1b), but the climbing behavior on the 14th day after OBX was significantly increased compared to the sham group (p < 0.01; Fig. 1b). A two-way ANOVA revealed a significant effect of group, time course, and group × time course (group F (1, 68) = 8.27, p < 0.01; time course F (2, 68) = 3.59, p < 0.05; and group × time course F (2, 68) = 3.00, p < 0.05; Fig. 1b). Based on this result, the 14th day after OBX was the time point used to investigate changes in DA neuronal function in the brain following OBX.
Changes in apomorphine (3 mg/kg)-induced climbing behavior after OBX in mice. a Dose-dependent change (on the 14th day after operation). b Time-dependent change. Each column shows the total climbing behavior. Vertical bars represent mean ± standard errors of mean (SEM). **p < 0.01 significant difference between sham and OBX groups (n = 10–18 per group)
Effects of DA antagonists on apomorphine-induced climbing behavior on the 14th day after OBX
Pre-treatment with the D1 antagonist SCH23390 (0.1 mg/kg, i.p.) significantly inhibited apomorphine-induced climbing behavior in both the sham and OBX groups (p < 0.01; Fig. 2a). Moreover, post hoc analysis revealed that pre-treatment with the D2 antagonist sulpiride (5.0 mg/kg, i.p.) significantly inhibited apomorphine-induced climbing behavior in the OBX group (p < 0.01; Fig. 2b). A two-way ANOVA revealed a significant effect of group and treatment but not group × treatment for the group pre-treated with the D1 antagonist SCH23390 group (group F (1, 66) = 20.07, p < 0.01; treatment F (2, 66) = 8.76, p < 0.01; and group × treatment F (2, 66) = 0.93, p = 0.93; Fig. 2a) and for the D2 antagonist sulpiride group (group F (1, 84) = 17.06, p < 0.01; treatment F (3, 84) = 3.35, p < 0.05; and group × treatment F (3, 84) = 1.78, p = 0.16; Fig. 2b).
Effects of a SCH23390 or b sulpiride on apomorphine-induced climbing behavior after OBX in mice. Each column shows the total climbing behavior. Vertical bars represent mean ± SEM. **p < 0.01 compared with apomorphine (3.0 mg/kg)-treated sham group, ## p < 0.01 compared with apomorphine (3.0 mg/kg)-treated OBX group (n = 10–18 per group)
Effects of ARI on apomorphine-induced climbing behavior on the 14th day after OBX
Pre-treatment with ARI (0.1 mg/kg, i.p.) significantly inhibited apomorphine-induced climbing behavior in the OBX group (p < 0.01; Fig. 3). A two-way ANOVA revealed a significant effect of treatment and group × treatment but not group (group F (1, 34) = 1.0, p = 0.33; treatment F (1, 34) = 155.5, p < 0.01; and group × treatment F (1, 34) = 86.56, p < 0.01; Fig. 3).
Effects of aripiprazole on apomorphine-induced climbing behavior after OBX in mice. Each column shows the total climbing behavior. Vertical bars represent mean ± SEM. **p < 0.01 compared with apomorphine (3.0 mg/kg)-treated sham group, ## p < 0.01 compared with apomorphine (3.0 mg/kg)-treated OBX group (n = 8–10 per group)
Quantitative autoradiography of [3H] SCH23390 and [3H] raclopride binding sites in mice
We conducted autoradiography to investigate the binding of DA receptors in the CPu, NAcC, and NAcSh. There was on overall tendency for D1 and D2 receptor binding levels to be higher in the OBX group compared to the sham. However, only the D2 receptor binding in the NAcC was significantly increased compared to the sham group (Cohen’s d = 0.49 for CPu t = 1.00, p > 0.05; d = 1.0 for NAcC t = 2.06, p > 0.05; and d = 0.53 for NAcSh t = 1.08, p > 0.05 (Fig. 4a) and d = 0.47 for CPu t = 1.02, p > 0.05; d = 0.99 for NAcC t = 2.17, p < 0.05; and d = 0.77 for NAcSh t = 1.71, p > 0.05 (Fig. 4b)). A two-way ANOVA revealed a significant effect of group but not region or group × region in the D1 receptor binding levels (group F (1, 45) = 5.41, p < 0.05; region F (2, 45) = 0.78, p = 0.46; and group × region F(2, 45) = 0.29, p = 0.75; Fig. 4a). But, the D2 receptor binding levels revealed a significant effect of group and region but not group × region (group F (1, 51) = 6.42, p < 0.05; region F (2, 51) = 24.4, p < 0.01; and group × region F (2, 51) = 0.02, p = 0.98; Fig. 4b).
Quantitative autoradiography of [3H] SCH23390 and [3H] raclopride binding sites in mice. Nonspecific binding was defined in the presence of 1 μM SCH23390 (D1 binding) and 300 μM sulpiride (D2 binding). Specific binding is expressed as nCi/tissue. Vertical bars represent mean ± SEM. *p < 0.05 significant difference between the sham group and the OBX group (n = 8–10 per group)
Distribution of TH fluorescence intensity in the striatum
The distribution of TH fluorescence intensity in each mouse brain section was determined by microphotometry and classified into 18 levels (shown as different colors in Fig. 5, with the lowest concentration shown as black and the highest concentration represented by white). There was a significant decrease in TH levels in the CPu, NAcC, and NAcSh in OBX mice compared to the sham controls (CPu p < 0.01, NAcC p < 0.01, and NAcSh p < 0.01; Fig. 5d). A two-way ANOVA revealed a significant effect of group and region but not group × region (group F (1, 30) = 28.16, p < 0.01; region F (2, 30) = 55.65, p < 0.01; and group × region F (2, 30) = 0.20, p = 0.82; Fig. 5).
Distribution of the immunohistochemical fluorescence intensity for TH in mouse brain (Bregma from +0.86 to +1.10 mm), including NAcC, NAcSh, and CPu. a Sham mouse; b OBX mouse; c diagram showing the NAcC, NAcSh, and CPu; and d values for the immunofluorescence intensity of TH in CPu, NAcC, and NAcSh of the mouse brain. Vertical bars represent mean ± SEM. **p < 0.01 significant difference between the sham group and the OBX group (n = 6 per group)
Alteration of apomorphine-induced climbing behavior after chronic administration of imipramine in OBX mice
Chronic administration of imipramine, a tricyclic anti-depressant, altered apomorphine-induced climbing behavior. Post hoc analysis revealed that the climbing behavior on the 28th day after OBX was significantly increased compared to the sham group (p < 0.01; Fig. 6) and chronic administration of imipramine (20 mg/kg, i.p.) in OBX mice significantly inhibited apomorphine-induced climbing behavior compared to the OBX controls (p < 0.01; Fig. 6). In contrast, chronic administration of imipramine in sham mice significantly increased apomorphine-induced climbing behavior compared to the sham controls (p < 0.05; Fig. 6). A two-way ANOVA revealed a significant effect of group and group × treatment but not treatment (group F (1, 18) = 79.78, p < 0.01; treatment F (1, 18) = 2.79, p = 0.11; and group × treatment F (1, 18) = 64.48, p < 0.01; Fig. 6).
Effects of chronic administration of imipramine on apomorphine-induced climbing behavior after OBX in mice. Each column shows the total climbing behavior. Vertical bars represent mean ± SEM. *p < 0.05 and **p < 0.01 compared with apomorphine (3.0 mg/kg)-treated sham group, ## p < 0.01 compared with apomorphine (3.0 mg/kg)-treated OBX group (n = 5–6 per group)
Distribution of D1 and D2 receptors in the striatum after chronic administration of imipramine in OBX mice
We observed changes in D1 and D2 receptor fluorescence intensity distribution in the CPu, NAcC, and NAcSh in OBX mice after chronic administration of imipramine. Regarding D1 receptor distribution, post hoc analysis revealed a significant increase in D1 receptor density in the CPu, NAcC, and NAcSh in OBX controls (CPu p < 0.01, NAcC p < 0.01, and NAcSh p < 0.01; Fig. 7f–h) and in the NAcC and NAcSh of imipramine-treated sham mice (CPu p > 0.05, NAcC p < 0.05, and NAcSh p < 0.05; Fig. 7f–h) compared to the sham controls. Moreover, chronic administration of imipramine in OBX mice significantly decreased D1 receptor density in the CPu, NAcC, and NAcSh compared to OBX controls (CPu p < 0.05, NAcC p < 0.01, and NAcSh p < 0.01; Fig. 7f–h). A two-way ANOVA revealed a significant effect of group and group × treatment but not treatment in both CPu and NAcSh [(CPu group F (1, 11) = 10.91, p < 0.01; treatment F (1, 11) = 4.10, p = 0.068; and group × treatment F (1, 11) = 30.61, p < 0.01; Fig. 7f), (NAcSh (group F (1, 11) = 24.21, p < 0.01; treatment F (1, 11) = 1.88, p = 0.20; and group × treatment F (1, 11) = 82.65, p < 0.01; Fig. 7h), and (of group, treatment, and group × treatment in NAcC (group F (1, 11) = 23.50, p < 0.01; treatment F (1, 11) = 7.15, p < 0.05; and group × treatment F (1, 11) = 60.53, p < 0.01; Fig. 7g)].
Distribution of the immunohistochemical fluorescence intensity for D1 receptors in mouse brain, including NAcC, NAcSh, and CPu. a Sham treated with saline; b sham treated with imipramine; c OBX treated with saline; d OBX treated with imipramine; e diagram showing the NAcC, NAcSh, and CPu; and values for the immunofluorescence intensity of TH in CPu (f), NAcC (g), and NAcSh (h) of the mouse brain. Vertical bars represent mean ± SEM. *p < 0.05 and **p < 0.01 compared with sham control group, # p < 0.05 and ## p < 0.01 compared with OBX control group (n = 3–4 per group)
Regarding D2 receptor distribution, post hoc analysis revealed a significant increase in D2 receptor density in the CPu, NAcC, and NAcSh in OBX controls (CPu p < 0.01, NAcC p < 0.01, and NAcSh p < 0.01; Fig. 8e–g) and in imipramine-treated sham mice (CPu p < 0.01, NAcC p < 0.05, and NAcSh p < 0.01; Fig. 8e–g) compared to the sham controls. Moreover, chronic administration of imipramine in OBX mice significantly decreased D2 receptor density in the CPu, NAcC, and NAcSh compared to the OBX controls (CPu p < 0.01, NAcC p < 0.01, and NAcSh p < 0.01; Fig. 8e–g). A two-way ANOVA revealed a significant effect of group, treatment and group × treatment in all regions [(CPu group F (1, 11) = 36.95, p < 0.01; treatment F (1, 11) = 5.28, p < 0.05; and group × treatment F (1, 11) = 126.8, p < 0.01; Fig. 8e), (NAcC group F (1, 11) = 81.93, p < 0.01; treatment F (1, 11) = 21.57, p < 0.01; and group × treatment F(1, 11) = 143.00, p < 0.01; Fig. 8f), and (NAcSh group F (1, 11) = 44.07, p < 0.01; treatment F (1, 11) = 17.78, p < 0.01; and group × treatment F (1, 11) = 191.5, p < 0.01; Fig. 8g)].
Distribution of the immunohistochemical fluorescence intensity for D2 receptors in mouse brain, including NAcC, NAcSh, and CPu. a Sham treated with saline; b sham treated with imipramine; c OBX treated with saline; d OBX treated with imipramine; and values for the immunofluorescence intensity of TH in CPu (e), NAcC (f), and NAcSh (g) of the mouse brain. Vertical bars represent mean ± SEM. *p < 0.05 and **p < 0.01 compared with sham control group, ## p < 0.01 compared with OBX control group (n = 3–4 per group)
Distribution of TH fluorescence intensity in the striatum following chronic administration of imipramine in OBX mice
We observed alterations in TH fluorescence intensity in the CPu, NAcC, and NAcSh after chronic administration of imipramine in OBX mice. Post hoc analysis revealed a significant decrease in TH levels in the CPu, NAcC, and NAcSh in OBX controls (CPu p < 0.01, NAcC p < 0.05, and NAcSh p < 0.01; Fig. 9e–g) and imipramine-treated sham mice (CPu p < 0.05, NAcC p < 0.05, and NAcSh p < 0.05; Fig. 9e–g) compared to the sham controls. Moreover, chronic administration of imipramine in OBX mice was not significantly different from OBX controls (CPu p > 0.05, NAcC p > 0.05, and NAcSh p > 0.05; Fig. 9e–g). A two-way ANOVA revealed a significant effect of group and group × treatment but not treatment in the CPu (group F (1, 11) = 22.47, p < 0.01; treatment F (1, 11) = 1.30, p = 0.28; and group × treatment F (1, 11) = 23.07, p < 0.01; Fig. 9e) and of group, treatment, and group × treatment in both NAcC and NAcSh [NAcC group F (1, 11) = 25.00, p < 0.01; treatment F (1, 11) = 6.07, p < 0.05; and group × treatment F (1, 11) = 12.12, p < 0.01, Fig. 9f) and (NacSh group F (1, 11) = 69.00, p < 0.01; treatment F (1, 11) = 5.57, p < 0.05; and group × treatment F (1, 11) = 27.77, p < 0.01; Fig. 9g)].
Distribution of the immunohistochemical fluorescence intensity for TH in mouse brain, including NAcC, NAcSh, and CPu. a Sham treated with saline; b sham treated with imipramine; c OBX treated with saline; d OBX treated with imipramine; and values for the immunofluorescence intensity of TH in CPu (e), NAcC (f), and NAcSh (g) of the mouse brain. Vertical bars represent mean ± SEM. *p < 0.05 and **p < 0.01 compared with sham control group (n = 3–4 per group)
Discussion
Climbing behavior represents a convenient means to assess the stimulation of cerebral dopaminergic area DA receptors in mice (Protais et al. 1976). In the present study, apomorphine-induced climbing behavior in OBX showed a significant enhancement compared to the sham group. It has been reported that D1 and D2 receptor messenger RNA (mRNA) levels and DA-regulated adenylate cyclase activity are significantly increased in the olfactory tubercle on the 14th day after OBX (Holmes 1999). Interestingly, our study also demonstrated that DA receptor levels increased and that the enhanced effect of apomorphine is observed on the 14th day after OBX. This is consistent with other neurochemical and behavioral changes that are primarily observed 1~3 weeks after OBX (Jancsàr and Leonard 1984; Mucignat-Caretta et al. 2006; Nakagawasai et al. 2003b; Song and Leonard 1995). Thus, this data suggest that OBX results in plastic alternations in central dopaminergic neurotransmission.
As apomorphine is a nonselective dopaminergic receptor agonist, we decided to try to determine which receptor subtype is involved in the hyperresponsiveness to this drug in OBX mice. Pre-treatment with SCH23390 or sulpiride showed a significant decrease in apomorphine-induced climbing behavior. Apomorphine-induced climbing behavior in both sham and OBX groups was inhibited by pre-treatment with SCH23390. Climbing behavior is generally considered as a measure of stereotypy not locomotion. However, it has been reported that stereotypic behavior is an obvious interaction with locomotion behavior (Segal 1976; Todzy et al. 1978). Thereby, climbing behavior could indirectly reflect locomotor behavior. We propose that SCH23390 (0.1 mg/kg) indirectly suppressed climbing behavior by decreasing locomotor activity. Moreover, pre-treatment with sulpiride at doses that did not effect locomotor activity in the sham group showed a significant decrease in apomorphine-induced climbing behavior in OBX mice. It has been reported that treatment with the indirect DA agonist amphetamine, the direct DA agonist apomorphine, or a combination of D1 and D2 agonists elicited intense climbing behavior in wild-type mice (which predominantly express D2L in the striatum), but this behavior was absent or reduced in mice lacking D2L receptors (Fetsko et al. 2003). Interestingly, it has been reported that OBX rats learned how to self-administer amphetamine (a behavior associated with the dopaminergic system of the NAc) faster than sham rats, and this effect was reversed by sulpiride coinfusion (Holmes et al. 2002). Thus, we hypothesize that OBX causes a supersensitivity of DA receptors in the striatum, especially the D2 receptor.
ARI is a partial agonist at D2, D3, and 5-HT1A receptors and behaves as an antagonist in hyperdopaminergic or serotonergic states and as an agonist in hypodopaminergic or serotonergic states (Feltenstein et al. 2007; Greenaway and Elbe 2009). Because of its activity, ARI has been described as a dopamine-serotonin system stabilizer. In the present study, administration of ARI (0.1 mg/kg) showed a significant decrease in apomorphine-induced climbing behavior in OBX mice only. But, it has been reported that ARI (0.1 mg/kg) attenuated apomorphine-induced climbing behavior (scores for each animal were totaled every 5 min during the 30-min observation period) in undisposed CF-1 mice (Brennan et al. 2010). We propose that strain differences or variation in the assessment of climbing behavior may be responsible for these differing results. It is possible that supersensitivity of DA receptors in the striatum and enhanced functional responses are involved in apomorphine-induced climbing behavior in OBX mice and that ARI can normalize this behavior by decreasing the activity of DA receptors.
The abnormalities seen in the OBX rodents result from dysfunctional changes in many brain regions as a consequence of disrupted neuronal connections between the OB and other brain regions. OBX results in a retrograde degeneration of the neurons that project to and from the main and accessory OBs (Jancsár and Leonard 1983; Song and Leonard 2005). The neuronal degeneration by OBX also occurs in the main and accessory projection areas to the cortex, hippocampus, NAc, CPu, and more (Jancsár and Leonard 1983; Song and Leonard 2005). Previous experiments reveal that OBX increases D1 and D2 receptor densities and DA-mediated adenylate cyclase activity in the olfactory tubercle, which is a component of the striatum, where projections from olfactory bulb neurons and midbrain dopaminergic neurons converge (Gottesfeld et al. 1989; Heimer et al. 1995) in OBX mice. In the present study, to examine DA receptors associated with behavioral changes, we conducted autoradiography to investigate the density distribution of DA receptors in the CPu, NAcC, and NAcSh. As a result of this study, we observed a tendency for an increase in density distribution of DA receptors in the striatum on the 14th day after OBX and especially, a significant increase in D2 receptors in the NAcC. A previous study showed an increase in striatal DA in OBX rats (Masini et al. 2004). However, we evaluated the OBX model in mice not rats. It has been reported that species differences lead to different OBX-induced changes (Hendriksen et al. 2015). Thus, we hypothesized that the present study showing paradoxical alterations of the DA system compared to previously published results may be attributable to species differences. Moreover, we found that OBX mice had reduced TH levels, which is a catecholamine rate-limiting enzyme, in the CPu, NAcC, and NAcSh compared to the sham group. Methamphetamine-induced physiological changes are similar to the reduction in TH levels shown in the present study (Boger et al. 2007; Zhu et al. 2005). The methamphetamine-induced reduction in TH levels caused a decrease in DA levels in the striatum and a decrease in locomotor activity which is a DA-associated behavior (Boger et al. 2007). Interestingly, dopamine-deficient (DD) mice were generated by targeted deletion of the TH gene in the DA neurons (Zhou and Palmiter 1995). Bamford et al. have indicated that DD mice result in morphologically normal striatal terminals with hypersensitive pre-synaptic D2 receptor (Bamford et al. 2004). Previous reports suggest that D2 receptor-deficient mice are hypoactive (Baik et al. 1995; Kelly et al. 1998). The D2 receptor mediates both the hyperactivity and response to amphetamine, as shown by others (Xueliang et al. 2010). In addition, it has been reported that amphetamine depresses striatal γ-aminobutyril acid (GABA)-ergic synaptic transmission through D2 receptors (Centonze et al. 2002). These findings suggest that the reduction of DA in the striatum may induce a supersensitivity of D2 receptors and GABA regulation dysfunction in the area. Actually, the Flinders Sensitive Line of rats, which is a widely accepted and validated model of depression, show decreased DA neurotransmission in the mesolimbic areas (Friedman et al. 2005). Thus, we suggest that OBX may induce a compensatory increase of D2 receptor in the NAcC by reducing TH levels in the striatum.
Anti-depressants are reported to antagonize apomorphine-induced climbing behavior (Balsara et al. 1982). Imipramine, a tricyclic anti-depressant, reduces OBX-induced hyperactivity (Breuer ME et al. 2008; Breuer et al. 2009a, b). These findings suggest that anti-depressants have the potential to reduce apomorphine-induced climbing behavior in OBX. In fact, the present study shows that chronic administration of imipramine significantly decreased apomorphine-induced climbing behavior in OBX mice. Imipramine has been shown to increase striatal DA (Ghosh and Hrdina 1977). Thus, we hypothesize that the effects of imipramine may contribute to normalizing DA receptor densities in the striatum of OBX mice by increasing DA. Therefore, we investigated the density distribution of DA receptors in the CPu, NAcC, and NAcSh following chronic administration of imipramine. We found a decrease in DA receptor density distribution in the striatum of imipramine-treated OBX mice. It is reported that chronic administration of imipramine decreased D1 receptor density in the striatum of rats exposed to chronic mild stress (Papp M et al. 1994). Electrophysiological studies have also provided direct evidence that tricyclic anti-depressant treatment decreases the sensitivity of D2 receptors (Chiodo and Antelman 1980a, b). A previous study indicated that D1 and D2 receptor mRNA levels in the striatum were regulated by chronic administration of imipramine (Dziedzicka-Wasylewska et al. 1997). These results reveal that imipramine may normalize the dopaminergic system in the striatum of OBX mice. However, chronic administration of imipramine in sham mice significantly increased the number of DA receptors in the striatum compared to the sham controls in the present study. Another study reported that repeated imipramine administration increased the affinity for D2 receptors in the striatum in rats (Maj et al. 1996). Moreover, imipramine treated sham mice also significantly increased apomorphine-induced climbing behavior compared to the sham controls in the present study. Imipramine has been shown to decrease TH levels in the striatum of rats (Rosin et al. 1995). Indeed, chronic administration of imipramine significantly decreased TH levels in sham mice. This effect may be involved in the negative feedback that contributes to an increase in catecholamines. These results suggest that chronic administration of imipramine in sham mice may enhance apomorphine-induced climbing behavior due to a compensatory increase in DA receptors in the striatum because of a reduction in striatal TH levels. In contrast, chronic administration of imipramine in OBX mice did not change TH levels compared to saline-treated OBX mice. This result revealed that OBX may induce dysfunction of negative feedback in the striatum.
OBX rodents express anhedonic behavior (Calcagnetti et al. 1996; Sato et al. 2010a). Wistar Albino Glaxo (WAG)/Rij rats also show anhedonic behavior, such as a decrease in sucrose preference, and the anhedonic behavior in WAG/Rij rats is reversed by administration of imipramine (Sarkisova et al. 2008). Sarkisova et al. have proposed that the increased sensitivity of D2 receptors in WAG/Rij rats would appear to be a compensatory reaction to hypofunction of the mesolimbic dopaminergic system of the brain (Sarkisova et al. 2008). Thus, we hypothesized that depressive-like behavior in OBX mice such as anhedonia may be associated with the increase in D2 receptors.
In conclusion, we suggest that OBX in mice causes a supersensitivity of DA receptors in the striatum, especially the D2 receptor. This phenomenon may contribute to a reduction in TH levels in the striatum following OBX. Moreover, abnormal behavior and increased D2 receptor numbers were reversed by chronic administration of imipramine. These studies revealed an anti-depressant effect of imipramine, mediated through an interaction with the D2 receptor.
References
Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377:424–8
Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK (2004) Dopamine modulates release from corticostriatal terminals. J Neurosci 24:9541–52
Balsara JJ, Nandal NV, Mane VR, Chandorkar AG (1982) Experimental evaluation of the antidepressant and neuroleptic activity of maprotiline. Indian J Physiol Pharmacol 26:183–95
Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF (2007) Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci 27:8816–25
Breuer ME, Groenink L, Oosting RS, Buerger E, Korte M, Ferger B, Olivier B (2009a) Antidepressant effects of pramipexole, a dopamine D3/D2 receptor agonist, and 7-OH-DPAT, a dopamine D3 receptor agonist, in olfactory bulbectomized rats. Eur J Pharmacol 616:134–40
Breuer ME, van Gaalen MM, Wernet W, Claessens SE, Oosting RS, Behl B, Korte SM, Schoemaker H, Gross G, Olivier B, Groenink L (2009b) SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn Schmiedebergs Arch Pharmacol 379:101–6
Brennan JA, Graf R, Grauer SM, Navarra RL, Pulicicchio CM, Hughes ZA, Lin Q, Wantuch C, Rosenzweig-Lipson S, Pruthi F, Lai M, Smith D, Goutier W, van de Neut M, Robichaud AJ, Rotella D, Feenstra RW, Kruse C, Broqua P, Beyer CE, McCreary AC, Pausch MH, Marquis KL (2010) WS-50030 [7-{4-[3-(1H-inden-3-yl)propyl]piperazin-1-yl}-1,3-benzoxazol-2(3H)-one]: a novel dopamine D2 receptor partial agonist/serotonin reuptake inhibitor with preclinical antipsychotic-like and antidepressant-like activity. J Pharmacol Exp Ther 332:190–201
Cain DP (1974) The role of the olfactory bulb in limbic mechanisms. Psychol Bull 81:654–71
Calcagnetti DJ, Quatrella LA, Schechter MD (1996) Olfactory bulbectomy disrupts the expression of cocaine-induced conditioned place preference. Physiol Behav 59:597–604
Centonze D, Picconi B, Baunez C, Borrelli E, Pisani A, Bernardi G, Calabresi P (2002) Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology 26:164–75
Chiodo LA, Antelman SM (1980a) Electroconvulsive shock: progressive dopamine autoreceptor subsensitivity independent of repeated treatment. Science 210:799–801
Chiodo LA, Antelman SM (1980b) Repeated tricyclics induce a progressive dopamine autoreceptor subsensitivity independent of daily drug treatment. Nature 287:451–4
Dziedzicka-Wasylewska M, Rogoz R, Klimek V, Maj J (1997) Repeated administration of antidepressant drugs affects the levels of mRNA coding for D1 and D2 dopamine receptors in the rat brain. J Neural Transm (Vienna) 104:515–24
Feltenstein MW, Altar CA, See RE (2007) Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry 61:582–90
Fetsko LA, Xu R, Wang Y (2003) Alterations in D1/D2 synergism may account for enhanced stereotypy and reduced climbing in mice lacking dopamine D2L receptor. Brain Res 967:191–200
Friedman A, Dremencov E, Crown H, Levy D, Mintz M, Overstreet DH, Yadid G (2005) Variability of the mesolimbic neuronal activity in a rat model of depression. Neuroreport 16:513–6
Ghosh PK, Hrdina PD (1977) Effects of tricyclic antidepressants on the content and metabolism of dopamine in the rat striatum. Can J Physiol Pharmacol 55:383–8
Gottesfeld Z, Garcia CJ, Lingham RB, Chronister RB (1989) Prenatal ethanol exposure impairs lesion-induced plasticity in a dopaminergic synapse after maturity. Neuroscience 29:715–23
Greenaway M, Elbe D (2009) Focus on aripiprazole: a review of its use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatr 18:250–60
Heimer L, Zahm DS, Alheid GF (1995) Basal ganglia. In: Paxinos G (ed) The rat nervous system. Academic Press, San Diego, pp 579–628
Hendriksen H, Korte SM, Olivier B, Oosting RS (2015) The olfactory bulbectomy model in mice and rat: one story or two tails? Eur J Pharmacol 753:105–13
Holmes PV, Masini CV, Primeaux SD, Garrett JL, Zellner A, Stogner KS, Duncan AA, Crystal JD (2002) Intravenous self-administration of amphetamine is increased in a rat model of depression. Synapse 46:4–10
Holmes PV (1999) Olfactory bulbectomy increases prepro-enkephalin mRNA levels in the ventral striatum in rats. Neuropeptides 33:206–11
Hozumi S, Nakagawasai O, Tan-No K, Niijima F, Yamadera F, Murata A, Arai Y, Yasuhara H, Tadano T (2003) Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy. Behav Brain Res 138:9–15
Jancsàr SM, Leonard BE (1984) Changes in neurotransmitter metabolism following olfactory bulbectomy in the rat. Prog Neuropsychopharmacol Biol Psychiatry 8:263–9
Jancsár SM, Leonard BE (1983) Behavioural and neurochemical interactions between chronic reserpine and chronic antidepressants. A possible model for the detection of atypical antidepressants. Biochem Pharmacol 32:1569–71
Kelly JP, Wrynn AS, Leonard BE (1997) The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 74:299–316
Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18:3470–9
Larsson K (1971) Impaired mating performances in male rats after anosmia induced peripherally or centrally. Brain Behav Evol 4:463–71
Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B (1992) Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res 587:181–5
Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogóz Z, Skuza G (1996) Antidepressant drugs given repeatedly change the binding of the dopamine D2 receptor agonist, [3H]N-0437, to dopamine D2 receptors in the rat brain. Eur J Pharmacol 304:49–54
Masini CV, Holmes PV, Freeman KG, Maki AC, Edwards GL (2004) Dopamine overflow is increased in olfactory bulbectomized rats: an in vivo microdialysis study. Physiol Behav 81:111–9
Mele A, Avena M, Roullet P, De Leonibus E, Mandillo S, Sargolini F, Coccurello R, Oliverio A (2004) Nucleus accumbens dopamine receptors in the consolidation of spatial memory. Behav Pharmacol 15:423–31
Mucignat-Caretta C, Bondí M, Caretta A (2006) Time course of alterations after olfactory bulbectomy in mice. Physiol Behav 89:637–43
Nakagawasai O, Yamadera F, Iwasaki K, Asao T, Tan-No K, Niijima F, Arai H, Tadano T (2007) Preventive effect of kami-untan-to on performance in the forced swimming test in thiamine-deficient mice: relationship to functions of catecholaminergic neurons. Behav Brain Res 177:315–21
Nakagawasai O, Hozumi S, Tan-No K, Niijima F, Arai Y, Yasuhara H, Tadano T (2003a) Immunohistochemical fluorescence intensity reduction of brain somatostatin in the impairment of learning and memory-related behaviour induced by olfactory bulbectomy. Behav Brain Res 142:63–7
Nakagawasai O, Tadano T, Arai Y, Hozumi S, Oba A, Tan-No K, Yasuhara H, Kisara K, Oreland L (2003b) Enhancement of 5-hydroxytryptamine-induced head-twitch response after olfactory bulbectomy. Neuroscience 117:1017–23
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–9
Papp M, Klimek V, Willner P (1994) Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berl) 115:441–6
Ploeger GE, Spruijt BM, Cools AR (1994) Spatial localization in the Morris water maze in rats: acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci 108:927–34
Protais P, Costentin J, Schwartz JC (1976) Climbing behavior induced by apomorphine in mice: a simple test for the study of dopamine receptors in striatum. Psychopharmacology (Berl) 50:1–6
Randrup A, Mogilnicka E (1976) Spectrum of pharmacological actions on brain dopamine. Indications for development of new psychoactive drugs. Discussion of amantadines as examples of new drugs with special actions on dopamine systems. Pol J Pharmacol Pharm 28:551–6
Rosin DL, Melia K, Knorr AM, Nestler EJ, Roth RH, Duman RS (1995) Chronic imipramine administration alters the activity and phosphorylation state of tyrosine hydroxylase in dopaminergic regions of rat brain. Neuropsychopharmacology 12:113–21
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–85
Sarkisova KY, Kulikov MA, Midzyanovskaya IS, Folomkina AA (2008) Dopamine-dependent nature of depression-like behavior in WAG/Rij rats with genetic absence epilepsy. Neurosci Behav Physiol 38:119–28
Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T (2010a) Effect of non-selective dopaminergic receptor agonist on disrupted maternal behavior in olfactory bulbectomized mice. Behav Brain Res 210:251–6
Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T (2010b) Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behav Brain Res 215:141–5
Segal DS (1976) Differential effects of para-chlorophenylalanine on amphetamine-induced locomotion and stereotypy. Brain Res 116:267–76
Setlow B, McGaugh JL (1998) Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci 112:603–10
Sieck MH (1972) The role of the olfactory system in avoidance learning and activity. Physiol Behav 8:705–10
Song C, Leonard BE (1995) The effect of olfactory bulbectomy in the rat, alone or in combination with antidepressants and endogenous factors, on immune function. Hum Psychopharmacol 10:7–18
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–47
Sutoo D, Akiyama K, Yabe K (2001) Quantitative imaging of tyrosine hydroxylase and calmodulin in the human brain. J Neurosci Res 63:369–76
Todzy I, Coper H, Fernandes M (1978) Interaction between d-amphetamine and ethanol with respect to locomotion, stereotypies, ethanol sleeping time, and the kinetics of drug elimination. Psychopharmacology (Berl) 59:143–9
Willner P (1983) Dopamine and depression: a review of recent evidence. I Empirical studies Brain Res 287:211–24
Xueliang F, Ming X, Ellen J (2010) Hess D2 dopamine receptor subtype-mediated hyperactivity and amphetamine responses in a model of ADHD. Neurobiol Dis 37:228–236
Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83:1197–209
Zhu JP, Xu W, Angulo JA (2005) Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res 1049:171–81
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research (26460102) and Matching Fund Subsidy for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors would like to thank Ms. Reina Hoshi, Mr. Shusuke Kawamura, Ms. Jia-Rong Lin, Ms. Eri Kikuchi, and Ms. Wakana Sakuma of Tohoku Pharmaceutical University for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed with the approval of the Ethics Committee for Animal Experimentation at Tohoku Pharmaceutical University and according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Takahashi, K., Nakagawasai, O., Nemoto, W. et al. Alterations in behavioral responses to dopamine agonists in olfactory bulbectomized mice: relationship to changes in the striatal dopaminergic system. Psychopharmacology 233, 1311–1322 (2016). https://doi.org/10.1007/s00213-016-4224-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4224-y