Abstract
Rationale
Pharmacological intervention for autism spectrum disorder (ASD) is an important addition to treatment, yet currently available agents target co-morbid psychiatric concerns, such as aggression and irritability. Propranolol, a beta-adrenergic antagonist with anxiolytic effects, has been shown to improve verbal fluency and working memory in adults and adolescents with ASD in single-dose challenges.
Objectives
The present pilot study explores the acute effects of propranolol on a measure of conversational reciprocity in this population. We also examined whether autonomic activity and anxiety moderate or mediate response to the drug, given relationships between these variables and ASD, as well as the drug’s effects.
Methods
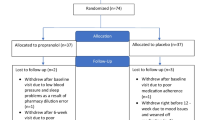
In a within-subject crossover design, 20 individuals with ASD received a single dose of propranolol or placebo during two sessions in a double-blinded, counterbalanced manner. After drug administration, participants performed a conversational reciprocity task by engaging in a short conversation with the researcher. Measurements of autonomic activity and anxiety were obtained before and after drug administration.
Results
Propranolol significantly improved performance on the conversational reciprocity task total [d = 0.40] and nonverbal communication domain scores when compared to the placebo condition. However, neither autonomic activity nor anxiety was significantly associated with drug response.
Conclusions
Acute propranolol administration improved conversational reciprocity in ASD. Further exploration of these preliminary findings, as well as other potential treatment response predictors, with serial doses is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social communication impairments and restricted, repetitive behaviors (American Psychiatric Association 2013). Pharmacological intervention can be an important component of treatment for ASD symptoms. However, most currently available agents target psychiatric symptoms associated with ASD, such as irritability and agitation (Myers and Johnson 2007). No pharmacological treatments have been reliably shown to improve core symptoms of ASD (Volkmar 2001), especially when it comes to impairments in social functioning (Farmer et al. 2013). A few pilot investigations involving the neurohormone oxytocin (Hollander et al. 2007) and glutamatergic (Erickson et al. 2007) and GABAergic agents (Erickson et al. 2014) have shown some promise in the social domain, but larger, randomized trials have not yet demonstrated significant benefits (Dadds et al. 2014; Young and Findling 2015). Exploring additional agents for these symptoms is crucial for the advancement of pharmacological intervention for ASD, especially in the adult population, for which behavioral therapy is less developed and studied (Gaus 2011; Myers and Johnson 2007).
The noradrenergic system may be a potential target for effective pharmacological intervention for ASD. Previous studies have suggested increased noradrenergic activity in autism (Lake et al. 1977; Launay et al. 1987), which may relate negatively to vocabulary ability (Cook et al. 1990). However, some studies have not supported these noradrenergic alterations (Minderaa et al. 1994). Other studies have shown that stress responses (Tordjman et al. 1997) and anxiety levels (Gillott et al. 2001), both of which involve increases in noradrenergic activity (Charney and Redmond 1983; Ward et al. 1983), are heightened in ASD. Previous studies have also indicated that these symptoms relate to social impairments in ASD (Bellini 2004), further suggesting the potential benefit of a pharmacological agent aimed at the noradrenergic system.
Propranolol, a centrally and peripherally active nonselective beta-adrenergic antagonist, reduces noradrenergic system activity. This known anxiolytic has been used off-label for test anxiety (Faigel 1991) and performance anxiety (Lader 1988) for several decades. Propranolol was first explored within the context of ASD in an uncontrolled case series, which reported improvements in language and sociability (Ratey et al. 1987). Our laboratory has since investigated the effects of this agent on a wide range of behaviors known to be affected in ASD, including verbal abilities (Beversdorf et al. 2008; Beversdorf et al. 2011), working memory (Bodner et al. 2012), facial scanning (Zamzow et al. 2014), as well as functional connectivity (Narayanan et al. 2010) in single-dose psychopharmacological challenge studies. To date, no double-blinded, placebo-controlled study has examined the effects of propranolol on social-related impairments in ASD. In the present pilot study, we explored whether acute propranolol administration improves social functioning, in the form of conversational reciprocity, in ASD during a single-dose psychopharmacological challenge. We predicted that propranolol would improve conversational reciprocity.
It is also important to explore potential moderators and mediators of this agent’s effects, as ASD is characterized by profound clinical heterogeneity (Geschwind 2009). Although there are many potential pathways through which propranolol could influence behavior, both at the central and peripheral level (Damasio 1996; Hasselmo et al. 1997), we explored two hypotheses informed by the expected effects of propranolol and known characteristics of ASD. Given the role of noradrenergic activity in autonomic nervous system functioning, changes in autonomic activity associated with propranolol may mediate this agent’s other effects. It is also possible that baseline levels of autonomic activity could determine which individuals experience benefits from this agent. Furthermore, previous studies have suggested autonomic dysregulation in the ASD population (Bal et al. 2010; Ming et al. 2005), which may be related to social and emotional functioning, language, and cognition (Guy et al. 2014; Neuhaus et al. 2014; Patriquin et al. 2013). Additionally, the known anxiolytic actions of propranolol, evidence of increased anxiety in ASD (Gillott et al. 2001), and a potential association between autonomic dysregulation and anxiety in this population (Guy et al. 2014) suggest that anxiety may influence propranolol’s effects. Accordingly, in the present study, we examined how autonomic activity and anxiety levels might moderate or mediate the effects of propranolol, suggesting potential treatment response markers for future explorations of this agent.
Patients and methods
Patients
A sample of 20 individuals with high-functioning ASD [mean age 21.39 ± 4.55 (SD), 19 males] was recruited from the University of Missouri Thompson Center for Autism and Neurodevelopmental Disorders (Table 1). In line with our previous studies, ASD diagnoses were confirmed by the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994). Eighteen participants met full criteria for autism. Both remaining participants met criteria for clinical diagnoses of Asperger syndrome according to DSM-IV criteria (American Psychiatric Association 2000), and one met criteria for ASD on three ADI-R subscales but was one point below criteria on the restricted, repetitive behavior subscale. All participants had an estimated full-scale IQ of at least 85, as confirmed by the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999), were native English speakers, and had no other secondary language or learning disorders. None of the participants were taking any noradrenergic agents or stimulants at the time of study participation, but noncontraindicated medications were allowed to continue. As such, five participants were concurrently taking selective serotonin reuptake inhibitors, and two were taking atypical antipsychotics. Participants with risk factors for exposure to propranolol, such as current diagnoses of asthma, bradycardia, and depression, were excluded. All participants provided informed consent in accordance with the Health Sciences Institutional Review Board of the University of Missouri.
Procedure and drug administration
Each participant completed two testing sessions in a within-subject, crossover design. At the start of each session, baseline psychophysiological measurements of autonomic activity were recorded. Drug was then administered in a double-blinded, counterbalanced manner. For the first study session, participants were randomized to receive 40 mg propranolol, the dose used in our previous studies, or placebo via oral capsule. At the second study session, participants received the drug not given at the first study session. Following drug administration and a 60-min waiting period to allow for peak drug effects, participants were then administered a series of behavioral tasks, including our primary social outcome measure of conversational reciprocity, and questionnaires, during which psychophysiological measurements were continuously recorded. Study sessions for each participant were conducted at least 24 h apart to allow for complete clearance of the drug.
Conversational reciprocity task
Conversational reciprocity was assessed using the Conversational Reciprocity task of the General Social Outcome Measure (GSOM CR), a psychometrically valid measure of social competence that has been shown to be sensitive to changes in behavior over time (Stichter et al. 2012). During this task, several social skills were assessed during a brief conversation between the participant and a researcher. At the start of the task, participants chose between two topics for the conversation (e.g., “plans for the weekend” or “hobbies”), and then, the researcher provided an open-ended comment or question to initiate a conversation. Following standard administration procedures, a single, consistent researcher, blind to drug conditions, rated the participant’s performance across six domains of social skills related to maintaining a reciprocal conversation: (1) staying on topic—maintaining the chosen topic or appropriately shifting to a related topic, (2) sharing information—the frequency of sharing new information, (3) reciprocity—providing or eliciting information (i.e., asking a question) beyond simple or vague comments, (4) transitions/interruptions—ability to transition appropriately from listener to speaker role, (5) nonverbal communication—indication of physical engagement via nodding and body posture, and (6) eye contact—degree of appropriateness of provided eye contact. Each domain was scored on a scale of 0 to 2. An operational definition was provided for each score across each domain, but generally, a score of 0 was given when the social skill was not demonstrated or was presented in an inappropriate manner. Demonstrations of skills to a fair but less than typical degree were given a score of 1, and a score of 2 was reserved for demonstrations of skills that were representative of typical performance. During the conversation, the researcher could provide a single prompt, such as a question or “tell me more about . . .” statement, encouraging the participant to provide additional information and continue the conversation. Additional prompts required to accurately assess the conversation resulted in a 0.5-point deduction from the sharing information domain score. A total score for the task was also computed by summing the domain scores.
Self-report anxiety questionnaires
Self-report anxiety was assessed via the Spence Children’s Anxiety Scale (SCAS) (Spence 1998) and the Beck Anxiety Inventory (BAI) (Beck et al. 1988). The SCAS was administered prior to drug administration to assess baseline anxiety levels. This well-validated, 45-item self-report questionnaire is scored across six subscales: separation anxiety, social phobia, obsessive compulsive, panic/agoraphobia, physical injury fears, and generalized anxiety. The SCAS has been used previously to assess anxiety in individuals with autism, including adults, given a paucity of assessment measures for this population (Gillott and Standen 2007). The BAI was administered after drug administration to assess between-drug differences in anxiety levels. This 21-item self-report measure assesses the degree to which a participant is experiencing anxiety-related symptoms.
Psychophysiological measurements
Autonomic activity was assessed via psychophysiological measurements of electrocardiography (ECG) and skin conductance. During each study session, ECG and skin conductance data were recorded at baseline for a period of 5 min after 3 min of acclimation, as well as continuously during task administration, using a BIOPAC MP150 acquisition unit with GSR100c and ECG100c amplifier modules (BIOPAC Systems, Inc., Goleta, CA). For ECG recordings, two disposable electrodes were placed on the chest: one just below the right collarbone and the other below the left breast. For skin conductance recordings, transducers prepared with isotonic gel were placed on the middle and index fingers of the nondominant hand and secured with Velcro straps.
Psychophysiological data processing
Baseline and during-task ECG and skin conductance data were collected using AcqKnowledge 4.1 (BIOPAC Systems, Inc.). R-R intervals were extracted from ECG data in AcqKnowledge 4.1 and then edited as previously described (Lewis et al. 2012) to remove artifacts with CardioEdit software (Brain-Body Center, University of Illinois at Chicago, 2007). The intervals were linearly interpolated and filtered (0.12–0.40 Hz) to remove the effects of spontaneous breathing (Porges 1995). CardioBatch software (Brain-Body Center, University of Illinois at Chicago, 2007) (Porges 1985; Porges and Bohrer 1990) was then used to analyze the data and measure respiratory sinus arrhythmia (RSA), naturally occurring heart rate variability associated with respiration. RSA is commonly used as an index of parasympathetic activity (Berntson et al. 1993) and has been proposed as an indicator of behavioral and physiological functioning in ASD (Porges 2005). Data for RSA was quantified for sequential 15-s epochs and averaged across each baseline or during-task data collection period. Prior to skin conductance analyses, data were visually examined and segments containing motion artifact, as confirmed by experimenter notes indicating participant movement, were manually removed using AcqKnowledge 4.1. Skin conductance data was averaged to determine mean skin conductance level (SCL), an index of sympathetic activity (Dawson et al. 2007), both at baseline and during task administration.
Statistical analyses
All statistical analyses were conducted by a blinded researcher using the Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics 22, Chicago, IL). To explore our a priori hypothesis of an effect of propranolol on conversational reciprocity, we used paired samples t tests and Wilcoxon signed-rank tests, when the data were non-normal according to the Kolmogorov-Smirnov test, to compare the GSOM CR task total and domain scores between drug conditions. Bonferroni corrections for multiple comparisons were employed for the task domain tests. We examined the data for potential order effects on task performance via paired samples t tests between study sessions 1 and 2. We used Wilcoxon signed-rank tests to explore drug effects on BAI scores, as this data was not normally distributed.
A series of regression models were used to evaluate relationships between baseline measurements of autonomic activity and response to propranolol for the GSOM CR task. To define response to propranolol, the placebo condition task scores were subtracted from the propranolol condition scores. Regressions were only run for GSOM CR task domains for which there was a significant between-drug difference in task performance. In these regressions, we tested the average of baseline data for each autonomic variable (RSA and SCL) across both study visits as potential moderators. We also explored the individual study visits separately, as missing or excessively noisy data resulted in only one session of data for some participants. We used simple linear regressions when examining the response to propranolol on the GSOM CR total score and binary logistic regressions for the nonverbal communication score, as the data was distributed in a binomial fashion. Simple linear regressions and binary logistic regressions were also used to assess relationships between baseline anxiety (SCAS scores) and response to propranolol for the GSOM CR task total score and nonverbal communication score, respectively. Bonferroni corrections were used to adjust for the number of hypotheses tested.
We also explored drug effects on autonomic variables via two-way (drug by time point) repeated measures ANOVAs, in which baseline and during-task autonomic data for each drug condition were included as repeated measures. These tests were followed by mediation analyses to explore whether drug effects on conversational reciprocity were mediated by indirect effects on autonomic activity or anxiety. These analyses were performed as described in Judd et al. 2001 with the MEMORE macro for SPSS, which makes inferences about indirect effects based on 5000 bootstrap samples and conducts Sobel tests of significance (Montoya and Hayes 2015). In three separate tests, autonomic activity (RSA and SCL) and anxiety data collected during each drug condition were included as mediators and GSOM CR task total scores from each condition were included as dependent variables. The binomial distribution of the nonverbal communication domain scores precluded mediation analyses for this outcome.
Results
Behavioral tasks and questionnaires
Propranolol improved performance on the GSOM CR task total score when compared to the placebo condition [t(19) = 2.36, p = 0.03, d = 0.40] (Fig. 1a). As expected with the scales used, data for each domain of the task was not distributed normally [D(20) > 0.20, p < 0.001 in all instances]. Performance on the nonverbal communication domain scores improved with propranolol, as compared to placebo, according to Wilcoxon signed-rank tests [Z = 2.00, p < 0.05, r = 0.45]. However, this finding did not withstand Bonferroni corrections for multiple comparisons for the domain scores. There was also a trend suggesting that propranolol improved sharing information scores [Z = 1.64, p = 0.10] (Fig. 1b). Task performance did not differ between propranolol and placebo for any other domain [all p > 0.10]. As the length of the conversations in the GSOM CR task varied between study sessions and participants, we compared conversation length between drug conditions but found no significant difference [p > 0.10]. Additionally, we did not observe any order effects on task performance, as the total and subscale scores did not differ significantly between the first and second study visits [p > 0.50 in each instance].
Effects of propranolol on the General Social Outcome Measure Conversational Reciprocity task (GSOM CR) total and domain scores. a The total task score was significantly greater in the propranolol condition as compared to the placebo condition. b Within individual conversation domains, there was a significant increase in the nonverbal communication score and a trend for an increase in the sharing information score in the propranolol condition. There was a ceiling effect for the transitions/interruptions domain. Bars represent SEM. *p < 0.05, #p = 0.10 (uncorrected)
The BAI scores were also non-normally distributed [D(20) > 0.20, p < 0.01 in both instances]. Propranolol did not influence scores on this self-report measure of anxiety, according to a Wilcoxon signed-rank test [Z = 0.18, p = 0.86].
Evaluation of potential treatment response predictors
Prior to RSA analyses, missing data or excessive motion artifact resulted in the exclusion of two participants’ baseline data from one study session and eight participants’ during-task data from one or both sessions. Using the remaining baseline data (N = 20 with at least one session), we then examined relationships between RSA and response to propranolol on the GSOM CR task (both total score and nonverbal communication score). Baseline RSA did not significantly influence the drug effect on this task in the form of linear or logistic relationships, when we explored RSA averaged across both study sessions or each study session separately [all p > 0.10]. However, we did observe a positive (U-shaped) quadratic relationship between baseline RSA and response to propranolol on the GSOM CR total score but only for the study session during which placebo was later administered, and this finding did not withstand correction for multiple comparisons [F(2,15) = 6.39, p = 0.01 (uncorrected), R 2 = 0.46].
We then explored whether propranolol affected RSA via two-way ANOVAs and found a significant main effect of time point, in which during-task RSA was significantly greater than baseline RSA [F(1,10) = 18.74, p < 0.01, η p 2 = 0.65] but no main effect of drug [F(1,10) = 1.39, p = 0.27] or drug by time point interaction [F(1,10) = 2.79, p = 0.13]. Additionally, mediation analyses did not reveal a significant indirect effect of propranolol on GSOM CR total scores through RSA [ab = −0.30, 95 % CI [−0.98, 0.67], Sobel z = −0.85, p = 0.39].
Examination of skin conductance data revealed that 15 participants had some degree of motion in their baseline data, which was corrected, and no participants were subsequently excluded. Data collected during the GSOM CR task was missing for two of the participants. Nine of the remaining participants’ data (N = 18) had motion artifact, which was corrected, and no additional participants were excluded. There were no significant differences between drug conditions in the amount of data removed due to artifact from baseline or during-task data [all p > 0.10]. Following calculations of mean SCL, natural logarithmic transformations were performed, as some of the data was positively skewed [p < 0.001]. Baseline SCL did not significantly influence drug effect on the GSOM CR task [all p > 0.05]. Two-way ANOVAs revealed a significant main effect of time point, in which during-task SCL was significantly greater than baseline SCL [F(1,17) = 39.86, p < 0.001, η p 2 = 0.70] but no main effect of drug [F(1,17) = 0.09, p = 0.77] or drug by time point interaction [F(1,17) = 0.25, p = 0.62]. Moreover, mediation analyses did not show a significant indirect effect of propranolol on GSOM CR task performance through mean SCL [ab = 0.001, 95 % CI [−0.24, 0.38], Sobel z = 0.01, p = 0.99].
We also explored relationships between baseline anxiety, measured with SCAS scores, and response to propranolol on the GSOM CR task. Data from the separation anxiety, panic/agoraphobia, and physical injury fears SCAS subscales were transformed via square root, as they were positively skewed [all p < 0.05]. Additionally, one participant was missing data for the social phobia subscale. SCAS scores were not significantly associated with drug response on the GSOM CR task total score [all p > 0.10], though these analyses may have been hindered by a considerable floor effect in the data. The SCAS obsessive compulsive subscale score was associated with drug effect on the GSOM nonverbal communication domain score [χ 2(1) = 3.94, p = 0.047 (uncorrected), Nagelkerke R 2 = 0.28], though this finding did not withstand Bonferroni correction. Prior to explorations of anxiety as a potential mediator of the effect of propranolol on GSOM CR performance, BAI scores for both drug conditions were transformed via natural logarithm. Subsequent mediation analyses did not reveal a significant indirect effect [ab = −0.02, 95 % CI [−0.32, 0.22], Sobel z = −0.17, p = 0.87].
Discussion
The present pilot study is the first single-dose, placebo-controlled investigation of the acute effects of propranolol on conversational reciprocity in individuals with ASD. As we predicted, propranolol significantly improved performance on a conversational reciprocity task. This benefit may be partly driven by an improvement in nonverbal communication abilities in response to propranolol. These findings extend the previously demonstrated effects of propranolol in this population (Beversdorf et al. 2008; Beversdorf et al. 2011; Bodner et al. 2012; Narayanan et al. 2010; Ratey et al. 1987; Zamzow et al. 2014) and suggest the social domain as an additional target for future investigations of this agent. Further exploration of these findings with a trial of serial doses is warranted.
Contrary to our predictions, autonomic activity at baseline did not significantly influence drug effect. Moreover, it does not appear that the effect of propranolol on conversational reciprocity was specifically mediated by changes in autonomic activity. Although parasympathetic activity may regulate social behavior (Porges 2007) and beta-adrenergic antagonists have been previously shown to increase RSA (Pitzalis et al. 1998), we did not observe these relationships in the present study. Increases in RSA between baseline and during-task time points were not specific to propranolol and thus did not seem to influence drug response. These findings may reflect generalized increases in RSA in response to a social task, as has been demonstrated previously in typically developing children (Heilman et al. 2008). Sympathetic activity was also not related to drug response, though this finding could have been influenced by the sensitivity of skin conductance measurements to motion (Hirstein et al. 2001). Lastly, the presence and manifestation of autonomic dysregulation is heterogeneous across individuals with autism (Schaaf et al. 2013). Accordingly, a more in-depth investigation of the relationship between autonomic activity and propranolol response with a larger sample of individuals with ASD is necessary.
Additionally, propranolol did not reduce anxiety in the present study, and anxiety levels did not definitively moderate or mediate response to propranolol on the conversational reciprocity task. It has been argued that self-report measures, which were used to assess anxiety in the present study, may not accurately reflect symptom severity in individuals with ASD due to difficulties with introspection (Gillott et al. 2001). This is supported by considerable floor effects observed in both anxiety measures used in the present study. Future studies should utilize parent or clinician report measures and newer assessments designed specifically for measuring anxiety in individuals with ASD (Kerns et al. 2015). Further investigation is needed to explore these and other potential factors that may influence the effects of propranolol in individuals with ASD, as the identification of treatment response markers is critical for this heterogeneous population.
Despite the promising nature of these preliminary findings, a few other limitations should be noted. The present study used only the CR task of the GSOM, limiting the generalizability of our results to additional domains of social functioning, such as interpreting the emotional expressions of others. Future investigations would benefit from using the now-developed full set of GSOM tasks (Stichter et al. 2012), which assesses additional social behaviors. Although it is not the standard for this task, recording testing sessions in future studies would allow for multiple raters and calculations of inter-rater reliability. Although it is notable that conversational reciprocity improvements were observed with a single dose of propranolol, the relatively small effect sizes for these findings may limit their clinical salience. It is currently unknown whether serial doses will result in a greater effect, though an uncontrolled case series suggests a potential benefit (Ratey et al. 1987). Lastly, the present study’s small sample size and relatively limited age and IQ range also limit the generalizability of our findings.
Overall, the present pilot study demonstrates a potential benefit of propranolol for conversational reciprocity in ASD, suggesting an effect on social interaction in this population. Given the paucity of agents affecting ASD symptomatology, this finding warrants further exploration of propranolol as a potential therapeutic agent. Future studies exploring propranolol’s effects in larger randomized, controlled clinical trials are imperative for further characterizing its benefits for this population, as well as predicting which individuals are most likely to respond.
References
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Press, Washington
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Press, Washington
Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW (2010) Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J Autism Dev Disord 40:358–370
Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56:893–897
Bellini S (2004) Social skill deficits and anxiety in high-functioning adolescents with autism spectrum disorders. Focus Autism Other Dev Stud 19:78–86
Berntson GG, Cacioppo JT, Quigley KS (1993) Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 30:183–196
Beversdorf DQ, Carpenter AL, Miller RF, Cios JS, Hillier A (2008) Effect of propranolol on verbal problem solving in autism spectrum disorder. Neurocase 14:378–383
Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE (2011) Effect of propranolol on word fluency in autism. Cogn Behav Neurol 24:11–17
Bodner KE, Beversdorf DQ, Saklayen SS, Christ SE (2012) Noradrenergic moderation of working memory impairments in adults with autism spectrum disorder. J Int Neuropsychol Soc 18:556–564
Charney DS, Redmond D (1983) Neurobiological mechanisms in human anxiety evidence supporting central noradrenergic hyperactivity. Neuropharmacology 22:1531–1536
Cook EH, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX (1990) Autistic children and their first-degree relatives: relationships between serotonin and norepinephrine levels and intelligence. J Neuropsychiatry Clin Neurosci 2:268–274
Dadds M, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J (2014) Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord 44:521–531
Damasio AR (1996) The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351:1413–1420
Dawson ME, Schell AM, Filion DL (2007) The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG (eds) The handbook of psychophysiology, 3rd edn. Cambridge University Press, New York, pp 159–181
Erickson C, Posey D, Stigler K, Mullett J, Katschke A, McDougle C (2007) A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology (Berl) 191:141–147
Erickson C, Veenstra-Vanderweele J, Melmed R, McCracken J, Ginsberg L, Sikich L et al (2014) STX209 (Arbaclofen) for autism spectrum disorders: an 8-week open-label study. J Autism Dev Disord 44:958–964
Faigel HC (1991) The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr 30:441–445
Farmer C, Thurm A, Grant P (2013) Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs 73:303–314
Gaus V (2011) Adult Asperger syndrome and the utility of cognitive-behavioral therapy. J Contemp Psychother 41:47–56
Geschwind DH (2009) Advances in autism. Annu Rev Med 60:367–380
Gillott A, Furniss F, Walter A (2001) Anxiety in high-functioning children with autism. Autism 5:277–286
Gillott A, Standen PJ (2007) Levels of anxiety and sources of stress in adults with autism. J Intellect Disabil 11:359–370
Guy L, Souders M, Bradstreet L, DeLussey C, Herrington J (2014) Brief report: emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. J Autism Dev Disord 44:2614–2620
Hasselmo ME, Linster C, Patil M, Ma D, Cekic M (1997) Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol 77:3326–3339
Hirstein W, Iversen P, Ramachandran VS (2001) Autonomic responses of autistic children to people and objects. Proc R Soc Lond B Biol 268:1883–1888
Heilman KJ, Bal E, Bazhenova OV, Sorokin Y, Perlman SB, Hanley MC et al (2008) Physiological responses to social and physical challenges in children: quantifying mechanisms supporting social engagement and mobilization behaviors. Dev Psychobiol 50:171–182
Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L et al (2007) Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61:498–503
Judd CM, Kenny DA, McClelland GH (2001) Estimating and testing mediation and moderation in within-subjects designs. Psychol Methods 6:115–134
Kerns CM, Maddox BB, Kendall PC, Rump K, Berry L, Schultz RT et al (2015) Brief measures of anxiety in non-treatment-seeking youth with autism spectrum disorder. Autism 19(8):969–979. doi:10.1177/1362361314558465
Lader M (1988) β-adrenoceptor antagonists in neuropsychiatry: an update. J Clin Psychiatry 49:213–223
Lake C, Ziegler MG, Murphy DL (1977) Increased norepinephrine levels and decreased dopamine-β-hydroxylase activity in primary autism. Arch Gen Psychiatry 34:553–556
Launay J, Bursztejn C, Ferrari P, Dreux C, Braconnier A, Zarifian E et al (1987) Catecholamines metabolism in infantile autism: a controlled study of 22 autistic children. J Autism Dev Disord 17:333–347
Lewis GF, Furman SA, McCool MF, Porges SW (2012) Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent? Biol Psychol 89:349–364
Lord C, Rutter M, Couteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685
Minderaa RB, Anderson GM, Volkmar FR, Akkerhuis GW, Cohen DJ (1994) Noradrenergic and adrenergic functioning in autism. Biol Psychiatry 36:237–241
Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML (2005) Reduced cardiac parasympathetic activity in children with autism. Brain Dev 27:509–516
Montoya AK, Hayes AF (2015) Two condition within-participant statistical mediation analysis: a path-analytic framework., Under review
Myers SM, Johnson CP (2007) Management of children with autism spectrum disorders. Pediatrics 120:1162–1182
Narayanan A, White C, Saklayen S, Scaduto M, Carpenter A, Abduljalil A et al (2010) Effect of propranolol on functional connectivity in autism spectrum disorder: a pilot study. Brain Imaging Behav 4:189–197
Neuhaus E, Bernier R, Beauchaine T (2014) Brief report: social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. J Autism Dev Disord 44:730–737
Patriquin MA, Scarpa A, Friedman BH, Porges SW (2013) Respiratory sinus arrhythmia: a marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Dev Psychobiol 55:101–112
Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Totaro P, Forleo C et al (1998) β-blocker effects on respiratory sinus arrhythmia and baroreflex gain in normal subjects. Chest 114:185–191
Porges SW (1985) Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. Patent Number: 4,510,944. U.S. Patent Office, Washington
Porges SW (1995) Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 19:225–233
Porges SW (2005) The vagus: a mediator of behavioral and physiologic features. In: Bauman ML, Kemper TL (eds) The neurobiology of autism, 2nd edn. The Johns Hopkins University Press, Baltimore, pp 65–78
Porges SW (2007) The polyvagal perspective. Biol Psychol 74:116–143
Porges SW, Bohrer RE (1990) Analyses of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG (eds) Principles of psychophysiology: physical, social, and inferential elements. Cambridge University Press, New York, pp 708–753
Ratey JJ, Bemporad J, Sorgi P, Bick P, Polakoff S, O’Driscoll G et al (1987) Brief report: open trial effects of beta-blockers on speech and social behaviors in 8 autistic adults. J Autism Dev Disord 17:439–446
Schaaf RC, Benevides TW, Leiby BE, Sendecki JA (2013) Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. J Autism Dev Disord 45:461–472
Spence SH (1998) A measure of anxiety symptoms among children. Behav Res Ther 36:545–566
Stichter JP, Herzog MJ, O’Connor KV, Schmidt C (2012) A preliminary examination of a general social outcome measure. Assess Eff Interv 38:40–52
Tordjman S, McBride PA, Hertzig ME, Snow ME, Anderson GM, Hall LM et al (1997) Plasma β-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry 38:705–715
Volkmar FR (2001) Pharmacological interventions in autism: theoretical and practical issues. J Clin Child Adolesc 30:80–87
Ward MM, Mefford IN, Parker SD, Chesney MA, Taylor CB, Keegan DL et al (1983) Epinephrine and norepinephrine responses in continuously collected human plasma to a series of stressors. Psychosom Med 45:471–486
Wechsler D (1999) Wechsler abbreviated scale of intelligence. The Psychological Coorporation, San Antonio
Young NJ, Findling RL (2015) An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr Opin Psychiatry 28:91–101
Zamzow RM, Christ SE, Saklayen SS, Moffitt AJ, Bodner KE, Higgins KF et al (2014) Effect of propranolol on facial scanning in autism spectrum disorder: a preliminary investigation. J Clin Exp Neuropsychol 36:431–445
Acknowledgments
The study was supported by a grant from the Health Resources and Services Administration (1R40MC19926). We thank our many research assistants for their help with data collection and Nicole Takahashi and Jill Akers at the University of Missouri Thompson Center for Autism and Neurodevelopmental Disorders for their assistance with recruitment. We are grateful to all participants and their families who participated in this study. The experiments presented in this manuscript comply with the current US laws. This study was supported in part by the Center for Cognitive Aging and Memory at the University of Florida, the McKnight Brain Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest related to the present study.
Rights and permissions
About this article
Cite this article
Zamzow, R.M., Ferguson, B.J., Stichter, J.P. et al. Effects of propranolol on conversational reciprocity in autism spectrum disorder: a pilot, double-blind, single-dose psychopharmacological challenge study. Psychopharmacology 233, 1171–1178 (2016). https://doi.org/10.1007/s00213-015-4199-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4199-0