Abstract
Rationale
Phosphodiesterase 3 (PDE3) inhibitor cilostazol ameliorates negative effects of cerebral hypoperfusion against cerebral ischemic injury through the phosphodiesterase 3-cyclic adenosine monophosphate (cAMP) signaling cascade.
Objectives
We investigated the question of whether cilostazol would have an anti-depressant effect on chronic mild stress (CMS)-treated mice after ischemic stroke.
Methods
An animal model of post-stroke depression was developed by additional CMS procedures in middle cerebral artery occlusion (MCAO). We performed behavioral, histological, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), immunohistochemical, Western blot and enzyme linked immunosorbent assays (ELISA).
Results
In the open field, sucrose preference, forced swim and Morris water maze test, treatment with cilostazol resulted in reduction of all depressive behaviors examined, particularly in the Morris water maze test. Treatment with cilostazol reduced prominent atrophic changes in the ipsilateral striatum and hippocampus of CMS-treated ischemic mice through inhibition of neuronal cell death and microglial activation. In addition, treatment of the CMS-treated ischemic mice with cilostazol resulted in significantly increased phosphorylation of cAMP response element-binding protein (CREB) and expression of mature brain-derived neurotrophic factor (BDNF) with its receptor tropomyosin receptor kinase B (TrkB) in the ipsilateral striatum and hippocampus. Phosphorylation of CREB was also demonstrated in the dopaminergic neurons of the midbrain. Treatment with cilostazol also resulted in an increased number of newly formed cells and enhanced differentiation into neurons in the ipsilateral striatum and hippocampus.
Conclusions
Our results suggest that phosphodiesterase 3 inhibitor cilostazol may have anti-depressant effects on post-stroke depression through inhibition of neurodegeneration in the primary lesion and secondary extrafocal sites and promotion of neurogenesis. These beneficial effects on post-stroke depression may be involved in activation of CREB/BDNF signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-stroke depression is considered within the broad category of vascular depression, in which cerebrovascular diseases are closely connected with some depressive syndromes (Taylor et al. 2013). More than one-third of stroke survivors suffer from depression, and these mood symptoms negatively impact stroke recovery with increased mortality and poorer functional outcomes (Kronenberg et al. 2012; Loubinoux et al. 2012). Post-stroke depression involves neuronal loss and impaired neurogenesis in the primary lesion sites as well as in remote brain areas that contribute to mood symptoms (Kronenberg et al. 2012; Sun and Alkon 2013). However, these symptoms of post-stroke depression share common underlying mechanisms with nonvascular depression (Sun and Alkon 2013).
Cilostazol, a selective phosphodiesterase 3 (PDE3) inhibitor, acts as an antiplatelet agent and has been widely approved for treatment of intermittent claudication with peripheral arterial disease and for secondary prevention of ischemic stroke (Barnett et al. 2004; Oyama et al. 2011; Shinohara et al. 2010). Cilostazol produces various powerful pleiotropic effects via restoration of intracellular second messenger cyclic adenosine monophosphate (cAMP) (Kwon et al. 2015). Treatment with cilostazol against cerebral ischemic injury ameliorates negative effects of cerebral hypoperfusion through the PDE3–cAMP signaling cascade with subsequent activation of inducible transcription factor cAMP response element-binding protein (CREB), suggesting the importance of the CREB signaling pathway (Lee et al. 2004; Miyamoto et al. 2010).
Activation of CREB promotes the gene expression of neuroprotective molecules that activate subsequent anti-apoptotic pathways with the gene expression of brain-derived neurotrophic factor (BDNF) (Miyamoto et al. 2010; Nishimura et al. 2007; Watanabe et al. 2006). BDNF also regulates neurogenesis, proliferation, and survival of neural stem or progenitor cells, as well as neuronal survival (Kwon et al. 2015; Nishimura et al. 2007). In addition, CREB and BDNF signaling play an important role in the pathophysiology of major depressive disorder (Gundersen et al. 2013; Zhou et al. 2013).
Psychopharmacotherapy with antidepressants or mood stabilizers provides an effective treatment for depression after stroke, but alternative therapy by activation of CREB and BDNF signaling may exert beneficial effects on various aspects of negative mood (Hackett et al. 2008; Nishimura et al. 2007). Drugs that activate CREB and BDNF signaling may provide a potential therapeutic approach for treatment of post-stroke depression via neural cell survival and proliferation of neural progenitor cells. Beneficial roles of cilostazol in reduction of infarct volume with reduced apoptosis and enhanced neurogenesis by a CREB-mediated signaling pathway have been demonstrated (Miyamoto et al. 2013; Tanaka et al. 2010; Watanabe et al. 2006).
Chronic mild stress (CMS) after ischemic stroke leads to severe depressive behaviors via neurodegeneration in the lesion and extrafocal sites, and degradation of neurogenesis (Kim et al. 2015). Prevention of primary and secondary neuronal injury after ischemic stroke may provide a novel target for post-stroke depression with regard to functional recovery. Thus, we hypothesize that cilostazol may have anti-depressant effects on post-stroke depression via neuroprotection and neurogenesis against summative neuronal injury by ischemia and additional chronic mild stressors. Therefore, we aimed to determine whether cilostazol treatment could prevent the depressive behavior phenotypes in the CMS-treated mice after ischemic stroke and its underlying mechanism.
Materials and methods
Animals
Male C57BL/6 mice aged 10 weeks were obtained from Dooyeol Biotech (Seoul, Korea). The mice were housed at 22 °C under alternating 12-h cycles of dark and light and were fed a commercial diet and allowed tap water ad libitum throughout the study. All experiments were approved by the Pusan National University Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines (approval number, PNU-2015-0850). All treatments were administered under 2 % isoflurane (Choongwae, Seoul, Korea) anesthesia to minimize mouse suffering, using a model VIP 3000 calibrated vaporizer (Midmark, Orchard Park, OH, USA).
Focal cerebral ischemia
Focal cerebral ischemia was induced by occluding the middle cerebral artery (MCA) using the intraluminal filament technique. A fiber-optic probe was affixed to the skull over the middle cerebral artery for measurement of regional cerebral blood flow using a PeriFlux Laser Doppler System 5000 (Perimed, Stockholm, Sweden). Left middle cerebral artery occlusion (MCAO) model was induced by a silicon-coated 4–0 monofilament in the internal carotid artery, and the monofilament was advanced to occlude the MCA. The filament was withdrawn 30 min after occlusion, and reperfusion was confirmed using laser doppler. All groups were kept in adjacent cages in the same area, and the MCAO mice did not receive any stress and also had free access to food and water. Following induction of MCAO, the condition of post-operative mice was assessed and monitored daily for signs of decreased activity, decline of feed intake; however, no sign of these conditions was observed.
Chronic mild stress
The CMS procedure was applied from the study by Willner et al. with slight modification (Willner et al. 1987). Vehicle and cilostazol groups underwent the CMS procedure from 2 days after MCAO. The CMS regimen included a total of seven different stressors, which were arranged day and night in order for 16 consecutive days as follows: food and water deprivation (20 h), water deprivation (18 h), 45° cage tilt (17 h), overnight illumination (36 h), soiled cage (21 h), swimming in 4 °C water (5 min), and paired caging (2 h).
Drug administration
Cilostazol [Cls, OPC-13013, 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl) butoxy]-3,4-dihydro-2-(1H)-quinolinone] was donated by Otsuka Pharmaceutical (Tokushima, Japan). Cls was administered orally into the mice from 2 days after MCAO during the CMS process (successive 16 days) using a sonde. Cilostazol was used at concentration of 3, 10 and 30-mg/kg body weight [10 % dimethyl sulfoxide (DMSO) in distilled water].
BrdU labeling
5-Bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich Corporation, St. Louis, MO, USA), a synthetic thymidine analog, becomes incorporated into a cell’s DNA when the cell is dividing during the S-phase of the cell cycle. For labeling of proliferating cells, all animals were injected with BrdU (50 mg/kg intraperitoneal) once daily for successive 5 days after MCAO.
Behavioral experiments
Behavioral experiments including open field, sucrose preference and forced swim test were performed from the fourth week after MCAO to the sixth week once a week. Morris water maze test was performed during successive 4 days at the sixth week after MCAO.
Open field test
The open field apparatus consisted of a white box (30 × 30 × 40 cm). After 10 min of adaptation in a white box, measurement was performed for 30 min as the total distance traveled by placing the mice. Results of the experiment were recorded using SMART 2.5.18 (Panlab S.L.U., Barcelona, Spain).
Sucrose preference test
Mice were deprived of food and water for 20 h after habituation of 1 % sucrose solution (AMRESCO Inc., Solon, OH, USA) for 24 h. Subsequently, their preference for 1 % sucrose solution and water was measured for 1 h by weighing the whole bottle with solution or water.
Forced swim test
One day before the first test, mice were exposed in a glass cylinder (15 cm in height × 10-cm diameter) of 25 °C water for 15 min. On the test day, behavior was recorded using a digital camera (E8400, Nicon Corporation, Japan) for 5 min and scored until immobile time in a cylinder.
Morris water maze test
Mice were trained on the Morris water maze for 4 days (five trials per day) before MCAO. The tank had a diameter of 100 cm, an altitude of 50 cm. The platform was placed 0.5 cm beneath the surface of the water. Each trial was performed for 90 s or until the mouse arrived on the platform. Results of the experiment were recorded using SMART 2.5.18 (Panlab, S.L.U.).
Histological assessment
Mice anesthetized with 8 % chloral hydrate received intraperitoneal perfusion with saline followed by 4 % paraformaldehyde in phosphate buffered saline (PBS). Brains were removed, post-fixed in the same fixative for 24 h at 4 °C, and immersed in 30 % sucrose solution for 72 h at 4 °C for cryoprotection. For evaluation of brain damage or atrophy, frozen 20-μm-thick sections were stained in 0.1 % cresyl violet (Sigma-Aldrich Corporation). The contralateral and ipsilateral subarea sizes of each section (striatum, corpus callosum, cortex, hippocampus and midbrain) were measured using i-solution (IMT i-Solution Inc. Burnaby, BC, Canada).
TUNEL assay
Apoptotic neuronal death was characterized in frozen 20-μm-thick sections by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). TUNEL assay was performed using a DeadEnd™ Fluorometric TUNEL System kit (Promega Corporation, Madison, WI, USA), and TUNEL-positive cells were counted. Quantitative analysis was performed blindly by counting the number of apoptotic cells using a fluorescence microscope (Carl Zeiss, Inc., Gottingen, Germany). The data are presented as total number of apoptotic cells.
Immunohistochemistry
The 20-μm-thick sections were incubated with a blocking buffer (1× PBS/5 % normal goat serum/0.3 % triton X-100) for 1 h. The sections were incubated with the primary antibodies overnight in PBS with 1:500 dilution of each following antibodies at 4 °C: BDNF (Cat. ab101747, Abcam, Cambridge, UK), BrdU (Cat. OBT0030G, AbD Serotec, Oxford, UK), cluster of differentiation 68 (CD68, Cat. MCA1957, AbD Serotec), glial fibrillary acidic protein (GFAP, Cat. MAB360, Millipore Corporation, Billerica, MA, USA), ionized calcium-binding adaptor molecule 1 (Iba1, Cat. 019–19741, Wako Pure Chemical Industries, Ltd., Osaka, Japan), neuronal nuclei (NeuN, Cat. MAB377; ABN78, Millipore Corporation), phospho-CREB (Cat. sc-7978-R, Santa Cruz Biotechnology, Santa Cruz, CA, USA; Cat. #9196, Cell Signaling Technology, Danvers, MA, USA), tyrosine hydroxylase (TH, Cat. AB152, Millipore Corporation), and tropomyosin receptor kinase B (TrkB, phospho Y515, Cat. ab131483, Abcam). After washes with PBS, the sections were incubated with the secondary biotinylated antibody for 2 h and then washed with PBS. The sections were incubated with ABC reagent (Vector Laboratories, Burlingame, CA, USA) for 1 h followed by staining with diaminobenzidine (Vector Laboratories) for 2–10 min. For immunofluorescent staining, without blocking endogenous peroxidase, the sections were incubated with fluorescent secondary antibody (Vector Laboratories) and 4',6-diamidino-2-phenylindole (DAPI, Invitrogen Corporation, Carlsbad, CA, USA) for 2 h and 30 min in the dark, respectively. Slides were mounted in the mounting medium (Vector Laboratories) and captured using an optical microscope (Carl Zeiss, Inc.).
Measurement of BDNF concentration
Blood samples were drawn into micro tubes at room temperature for 30 min, which were spun at 10,000 g for 15 min at 4 °C. Brain tissue samples tested in this study were homogenized in RIPA buffer (25 mM Tris-Cl, 250 mM NaCl, 5 mM EDTA, 1 % NP40) containing protease inhibitors (GenDEPOT, Inc., Baker, TX, USA). Brain tissue samples were homogenized and then centrifuged at 13,000 g for 25 min at 4 °C. Serum and tissue supernatant samples were isolated and stored in liquid nitrogen. The mBDNF levels were measured using a Quantikine BDNF ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) (Lim et al. 2015).
Western blot
Each brain tissue punch was washed in cold HEPES buffer and homogenized in lysis buffer [200 mM Tris (pH 8.0), 150 mM NaCl, 2 mM EDTA, 1 mM NaF, 1 % NP40, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM Na3VO4 and protease inhibitor cocktail]. Equal amounts of proteins were then separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer of the resolved proteins to a nitrocellulose membrane (Whatman, Dassel, Germany). The membranes were incubated with the primary antibody used in immunohistochemistry overnight at 4 °C with following antibody: BDNF (1:1000 dilution, Cat. ab101747, Abcam). Subsequently, membranes were incubated with rabbit secondary antibody (1:5000 dilution, Cat. ADI-SAB-301-J, Enzo Life Sciences, Inc., Farmingdale, NY, USA). Actin was used as a loading control for all experiments. Immunoreactivity corresponding to the bands was quantified by densitometric analysis using an Image Quant LAS 4000 (Fujifilm, Tokyo, Japan).
Data analyses
All data are expressed as mean ± SEM and were analyzed using the Sigmastat statistical program Version 11.2 (Systat Software, San Jose, CA, USA). Statistical analysis of data was performed using one-way analysis of variance (ANOVA) via Tukey’s post hoc comparison when comparing more than two groups. A p < 0.05 was considered statistically significant.
Results
Effect of cilostazol on depressive behaviors
To determine whether cilostazol can suppress depressive behaviors in the CMS-treated mice after ischemic stroke, we assessed multiple dimensions of depressive behavioral phenotypes from 4 to 6 weeks after MCAO. In the open field, number of entries into the central zone (anxiety-like behavior) was lower in the vehicle-treated mice than control mice at 6 weeks after MCAO. However, mice treated with Cls 30 mg/kg showed significantly higher number of entries compared to the vehicle-treated mice (F (4,17) = 12.395, p < 0.001) (Fig. 1a). Sucrose consumption in the sucrose preference test (anhedonia) and latency to float by forced swim test (despair-like behavior) were significantly decreased in the vehicle treated-mice compared to those in the control from fourth weeks after MCAO. Mice treated with Cls 30 mg/kg showed significantly higher sucrose intake and time floating at the fifth and sixth weeks compared to the vehicle-treated mice (sucrose preference test: 5 weeks, F (4,30) = 9.965, p < 0.001; 6 weeks, F (4,30) = 43.326, p < 0.001, forced swim test: 5 weeks, F (4,23) = 31.971, p < 0.001; 6 weeks, and F (4,23) = 104.888, p < 0.001) (Fig. 1b, c). In the Morris water maze test (spatial memory), the vehicle-treated mice took a longer time on average to find the platform than the control mice at the sixth week after MCAO. However, mice treated with Cls 30 mg/kg attained a significantly lower time compared to the vehicle-treated mice (1 day, F (4,22) = 129.334, p < 0.001; 2 day, F (4,26) = 316.308, p < 0.001; 3 day, F (4,26) = 209.899, p < 0.001; and 4 day, F (4,26) = 107.310, p < 0.001) (Fig. 1d). These results suggest that treatment with cilostazol caused a reversal of depressive behavioral phenotypes such as anxiety-like behavior, anhedonia and despair-like behavior with enhancement of spatial memory.
Effect of cilostazol on depressive behaviors. a Open field (n = 5), b sucrose preference (n = 7), c forced swim (n = 5), and d Morris water maze test (n = 6) were performed in the control and CMS-treated ischemic mice. Treatment with high dose of cilostazol showed significant recovery from depressive behaviors with spatial memory. Mean ± SEM. # P < 0.05, ## P < 0.01 and ### P < 0.001 versus the control mice; * P < 0.05, ** P < 0.01 and *** P < 0.001 versus the vehicle-treated mice; && P < 0.01 versus the Cls 3 mg/kg-treated mice; $$ P < 0.01 versus the Cls 10 mg/kg-treated mice
Effect of cilostazol on neuronal cell death and microglial activation
To determine whether cilostazol can suppress regional atrophy of the brain, we first measured atrophic volumes that were expressed as a percentage of the contralateral hemisphere. Severe atrophic changes of the CMS-treated ischemic mice were seen in the striatum and hippocampus compared to the control, but these changes were recovered by treatment with Cls 30 mg/kg (striatum: F (2,12) = 11.583, p < 0.001; hippocampus: and F (2,12) = 12.440, p < 0.001) (Fig. 2). Next, we performed assays for neuronal death and inflammation in the ipsilateral regions of the brain. TUNEL-positive cells of the striatum, hippocampus and midbrain showed significantly increased apoptotic features in the CMS-treated ischemic mice compared to those in the control. However, significantly lower numbers of TUNEL (striatum: F (2,12) = 82.394, p < 0.001) and TUNEL/PI-double positive cells [hippocampus (DG): F (2,12) = 82.757, p < 0.001] were detected in the striatum and hippocampus of the Cls 30 mg/kg-treated mice compared with those of vehicle-treated mice (Fig. 3a, b). Microglia activation related to inflammatory response was determined by detection of Iba1/CD68-double positive cells. Iba1/CD68-double positive cells were significantly increased in both the striatum and cornu ammonis (CA) 3 of the hippocampus, but not in the dentate gyrus (DG) of the hippocampus and midbrain. Treatment with Cls 30 mg/kg reduced these microglial activations in the CMS-treated ischemic mice [striatum: F (2,12) = 27.683, p < 0.001; and hippocampus (CA3): F (2,12) = 162.174, p < 0.001] (Fig. 3c, d). These results suggest that treatment with cilostazol caused amelioration of atrophic changes in the striatum and hippocampus, the primary lesion site by ischemic assaults, via inhibition of neuronal cell death and microglial activation.
Comparison of volume atrophy in each region of the brain. a Schematic diagram and photomicrograph (cresyl violet stain) shows the regions of the cortex, striatum, corpus callosum, hippocampus and midbrain of the brain and b its histogram for histological analysis (n = 5). Treatment with cilostazol significantly increased the volume of the ipsilateral striatum (arrow) and hippocampus (arrow head) compared to those of the vehicle-treated one. CC corpus callosum, Hippo hippocampus. # P < 0.05 versus the control mice; * P < 0.05 versus the vehicle-treated mice; scale bars = 5 mm
Effect of cilostazol on neuronal cell death and microglial activation. a Photomicrograph and b its histogram for apoptosis marker TUNEL/PI (n = 5) and c, d activated microglia marker Iba1/CD68 (n = 5). TUNEL/PI-double positive cells were significantly decreased by treatment with cilostazol in the striatum and DG of the hippocampus. Iba1/CD68-double positive cells were also significantly decreased by treatment with cilostazol. Cls cilostazol, DG dentate gyrus, Hippo hippocampus, CA cornu ammonis. # P < 0.05, ## P < 0.01 and ### P < 0.001 versus the control mice; * P < 0.05, ** P < 0.01 and *** P < 0.001 versus the vehicle-treated mice. Scale bars = 100 (A) and 50 μm (B)
Effect of cilostazol on CREB/BDNF signaling
Because cilostazol is associated with cAMP signaling, we determined the effects of this compound on the activation of CREB/BDNF signaling in the ipsilateral regions of the brain. pCREB/NeuN-double positive cells with pCREB-positive were significantly decreased in the striatum and hippocampus compared to those in the control, but these changes were reversed by treatment of Cls 30 mg/kg [pCREB: striatum, F (2,12) = 69.958, p < 0.001; hippocampus (DG), F (2,12) = 91.883, p < 0.001]. Although there was no significance difference in pCREB-positive cells of the midbrain, deceased pCREB/TH-double positive cells of the CMS-treated ischemic mice was recovered by treatment of Cls 30 mg/kg (F (2,12) = 111.479, p < 0.001) (Fig. 4). mBDNF-positive cells were markedly decreased in the striatum and hippocampus of the CMS-treated ischemic mice compared to those of the control. However, significantly recovered mBDNF-positive cells were detected in the hippocampus of Cls 30 mg/kg-treated mice [hippocampus (DG): F (2,12) = 225.071, p < 0.001]. In mBDNF staining with NeuN, there were no significant changes among all mice examined (Fig. 5a, b). Phosphorylation of BDNF receptor TrkB also showed a significant decrease in the striatum and hippocampus of the CMS-treated ischemic mice compared to that of the control, but these changes were recovered by treatment with Cls 30 mg/kg [striatum: F (2,10) = 42.550, p < 0.001; hippocampus (DG): F (2,11) = 12.009, p = 0.002] (Fig. 5c, d). These results suggest that treatment with cilostazol may induce neuroprotective effects in CMS-treated ischemic mice via activation of CREB/BDNF signaling.
Effect of cilostazol on the phosphorylation of CREB. a Photomicrograph and b its histogram for pCREB and pCREB/NeuN or TH-double positive cells (n = 5). The number of pCREB-positive cells was significantly increased in the striatum and hippocampus by treatment with cilostazol. pCREB/TH-double positive cells also showed similar patterns. Cls cilostazol, DG dentate gyrus, Hippo hippocampus. # P < 0.05, ## P < 0.01 and ### P < 0.001 versus the control mice; ** P < 0.01 versus the vehicle-treated mice. Scale bars = 100 μm
Effect of cilostazol on the expression of mBDNF and its receptor TrkB. a Photomicrograph and b its histogram for mBDNF and mBDNF/NeuN-double positive cells (n = 5) and c, d for pTrkB and pTrkB/NeuN (n = 4). mBDNF-positive cells in the hippocampus were significantly increased by treatment with cilostazol. pTrkB-positive cells and pTrkB/NeuN-double positive cells were also markedly increased in the striatum, hippocampus and midbrain, respectively. Cls cilostazol, DG dentate gyrus, Hippo hippocampus. # P < 0.05, ## P < 0.01 and ### P < 0.001 versus control mice; * P < 0.05 and ** P < 0.01 versus the vehicle-treated mice. Scale bars = 100 μm
Effect of cilostazol on proliferation of neuronal progenitor cells
To determine whether cilostazol can induce neuronal proliferation and differentiation, we observed positive cells for BrdU and BrdU/NeuN in the stratum and hippocampus. Significantly increased BrdU-positive cells were detected in the ipsilateral striatum and hippocampus compared to those in the control. BrdU-positive cells were significantly enhanced in the striatum by treatment with Cls 30 mg/kg (F (2,12) = 75.360, p < 0.001). BrdU/NeuN-double positive cells for identification of differentiated cells were significantly increased in both the striatum (F (2,11) = 29.601, p < 0.001) and hippocampus (F (2,15) = 14.384, p < 0.001) by treatment with Cls 30 mg/kg (Fig. 6). These results suggest that treatment with cilostazol may enhance the number of proliferative cells and differentiation into neurons in the ipsilateral striatum and hippocampus.
Effect of cilostazol on the proliferation and differentiation of neuronal progenitor cells. Photomicrograph and its histogram for BrdU and BrdU/NeuN-double positive cells in the a, b striatum (n = 5) and c, d hippocampus (n = 6). Treatment with cilostazol resulted in a significant increase of BrdU-positive and BrdU/NeuN-double positive cells in the striatum and BrdU/NeuN-double positive cells in the hippocampus. Cls cilostazol. # P < 0.05, ## P < 0.01 and ### P < 0.001 versus the control mice; * P < 0.05 and ** P < 0.01 versus the vehicle-treated mice. Scale bars = 200 μm
Effect of cilostazol on the mBDNF concentration in the blood and striatum
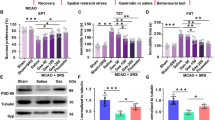
To clarify whether cilostazol plays a crucial role in CMS-treated ischemic mice via mBDNF expression, levels of mBDNF in the blood and striatum were estimated and compared between the vehicle- and cilostazol-treated mice. In the ELISA results, levels of mBDNF in the blood (F (1,7) = 10.050, p = 0.016) and ipsilateral striatum (F (3,14) = 14.914, p < 0.001) of the Cls 30 mg/kg-treated mice were significantly increased compared to those of the vehicle-treated mice. When these changes were confirmed by Western blot analysis, significant increase of mBDNF expression was observed in the blood of the Cls 30 mg/kg-treated mice (F (1,4) = 60.182, p = 0.001) (Fig. 7). These results suggest that treatment with cilostazol may increase the level of mBDNF in the blood of systemic circulation and ipsilateral striatum of the brain and enhance neuroprotective effects and neurogenesis.
Effect of cilostazol on the concentration of mBDNF in the blood and striatum. a ELISA assay for mBDNF (n = 5), b Western blot analysis (n = 3) and c its densitometry. Concentration levels of mBDNF were markedly increased in the blood and ipsilateral striatum by treatment with cilostazol, and these changes were confirmed by Western blot analysis. Cls cilostazol. * P < 0.05 and *** P < 0.001 versus the vehicle-treated mice
Discussion
We evaluated the in vivo beneficial effects of cilostazol, a PDE3 inhibitor, on depressive behaviors with underlying mechanism in a mice model of post-stroke depression. Treatment with cilostazol induced recovery from depressive behaviors with reduction of ischemia-induced apoptotic cell death and microglial activation, resulting in reduced atrophic changes such as in the stratum and hippocampus, and enhanced proliferation and differentiation of neuronal progenitor cells. These anti-depressant effects of cilostazol are involved in activation of CREB/BDNF signaling, leading to protection of neuronal cells in primary and secondary exofocal lesion sites and to enhanced neurogenesis after focal ischemia.
As social deprivation following stroke shows an overall negative effect on post-stroke depression, chronic mild stressors in the experimental stroke strongly enhance behavioral symptoms of depression (Kim et al. 2015; Venna et al. 2012; Wang et al. 2012). Thus, we first assessed two distinguished kinds of behavioral tests reflecting mood changes and memory impairment to investigate beneficial effects of cilostazol for depressive behaviors. Continued treatment with cilostazol improved the depressive behaviors including anxiety-like behavior and anhedonia and despair-like behavior as well as spatial memory impairment at late phase of experiment, showing more severe depressive symptoms as synthetic effect of MCAO and CMS (Kim et al. 2015). Our data showed that cilostazol exerted clear anti-depressant effects in CMS-treated mice after ischemic stroke.
Neuronal loss and neuroinflammation by CMS after ischemic insults may represent further important pathophysiological consequence of post-stroke depression. Cilostazol is used for the secondary prevention of ischemic stroke and activates anti-apoptotic signaling pathways in the brain of neurodegenerative disease models (Oyama et al. 2011; Tanaka et al. 2010; Watanabe et al. 2006). Both neuronal cell death and microglial activation were most prominent in the striatum and hippocampus of the CMS-treated ischemic mice, which suggested the primary lesion site by ischemic assaults. Cilostazol prevented atrophic changes in the striatum and hippocampus of the CMS-treated ischemic mice via inhibition of apoptotic cell death and microglial activation, suggesting that cilostazol can ameliorate neurodegenerative symptoms following stroke and probably enhance recovery of neuronal function.
Phosphorylation levels of CREB are generally important for neuroprotection of cilostazol, which could be induced by PDE3–cAMP intracellular signaling (Lee et al. 2004; Miyamoto et al. 2013; Watanabe et al. 2006). cAMP/CREB signaling activates a subsequent anti-apoptotic cascade and promotes the gene expression of BDNF in both in vitro and vivo system, which regulates neuronal survival (Daniele et al. 2015; Miyamoto et al. 2013; Nishimura et al. 2007; Watanabe et al. 2006). Neuroprotection is also temporally associated with increased expression of mBDNF, phosphorylation of its receptor TrkB (Lazarovici et al. 2012). BDNF and its receptor TrkB agonist confer anti-depressant effects in animal models of depression, suggesting involvement of the BDNF–TrkB pathway in depression (Blugeot et al. 2011; Hashimoto 2010). Therefore, we attempted to determine whether CREB/BDNF responses are involved in our models. Phosphorylation of CREB by cilostazol treatment was significantly increased in the striatum and hippocampus, primary lesion sites by ischemic assaults of the CMS-treated ischemic mice. Cilostazol also prevented the reduced mBDNF expression with phosphorylation of its receptor TrkB in these regions. Neuroprotective effects of cilostazol for neurodegenerative sites can be mediated through CREB/BDNF signaling, and this signaling may be involved in alleviation of depressive symptoms after stroke.
Cerebral ischemia entails secondary extrafocal neurodegeneration of the midbrain which contributes to mood symptoms (Dihne et al. 2002; Kronenberg et al. 2012). Cilostazol improves expression of TH through the CREB phosphorylation signaling pathway, ultimately maintains the levels of dopamine (Zhang et al. 2009). Dopaminergic neuronal loss is detected in the midbrain of ischemic mice, in the ipsilateral substantia nigra and ventral tegmental area, as delayed exofocal neurodegeneration (Kim et al. 2015; Kronenberg et al. 2012). Even if there were no significant anthropic changes in the midbrain, losses of dopaminergic neuronal cells were recovered in the substantia nigra and ventral tegmental area by cilostazol treatment, which showed an additional important pathophysiological consequence of post-stroke depression.
BDNF coupled to CREB phosphorylation is thought to be a key modulator of neurogenesis, which contributes to the proliferation and survival of neural stem and progenitor cells (Dworkin et al. 2009; Kwon et al. 2015). Cilostazol enhances generation of neuroblasts and differentiation and survival of progenitor cells through the CREB-mediated signaling pathway after focal ischemia (Miyamoto et al. 2009; Tanaka et al. 2010). Treatment with cilostazol resulted in a substantial increase in the numbers of newly generated cells and differentiated cells into neurons, suggesting that cilostazol can cause proliferation and differentiation of neuronal progenitor cells. This enhanced neurogenesis by cilostazol may be involved in cAMP-dependent signaling pathways after focal ischemia (Tanaka et al. 2010; Yoneyama et al. 2015).
Peripheral changes of BDNF concentration can be used to monitor brain tissue neurotrophin alterations, and reduced expression of growth factor expression fails the CREB activation (Miyamoto et al. 2013; Sartorius et al. 2009). Neuroprotective effects against post-stroke infection are exerted through its systemic BDNF regulatory actions (Yousuf et al. 2013). The levels of mBDNF and TrkB in the blood have negative correlations with the severity of major depression, thus levels of blood BDNF indicate a biomarker for major depressive disorder (Hashimoto 2010, 2013; Zhou et al. 2013). When we measured concentration of systemic blood mBDNF with the striatum of the brain, levels of mBDNF in the blood and ipsilateral striatum, particularly in the blood, were significantly increased by cilostazol treatment in CMS-treated ischemic mice. Thus, neuroprotective effects and neurogenesis of cilostazol may be exerted through systemic regulation of mBDNF.
Current biological theories for major depressive disorder center on hypothalamus-pituitary-adrenal axis, neurotrophin signaling, neurogenesis, neuroinflammation, and others (Kronenberg et al. 2014). However, the correlation between neuroprotection and enhanced neurogenesis and behavioral recovery from depressive phenotypes by cilostazol suggests the full significance of cilostazol treatment for post-stroke depression via attenuation of neuronal injury and activating proliferation of progenitor cells. Collectively, our results suggest that cilostazol reduced depressive behaviors in the CMS-treated mice after ischemic stroke through inhibition of neuronal cell death, and these beneficial effects for post-stroke depression may be involved in activation of CREB/BDNF signaling. Therefore, we proposed a schematic diagram of the CREB/BDNF signaling pathways involved in cilostazol-induced neuronal cell survival with neurogenesis (Fig. 8). We demonstrated new curative effects of cilostazol for post-stroke depression as an auditory therapy and indicated a new therapeutic target in post-stroke depression. Further basic studies are needed for greater understanding of the therapeutic potency and for elaborating the detailed mechanism; however, drugs activating cAMP/CREB/BDNF signaling cascade may provide another therapeutic approach for post-stroke depression.
References
Barnett AH, Bradbury AW, Brittenden J, Crichton B, Donnelly R, Homer-Vanniasinkam S, Mikhailidis DP, Stansby G (2004) The role of cilostazol in the treatment of intermittent claudication. Curr Med Res Opin 20:1661–1670
Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel JJ, Becker C (2011) Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci 31:12889–12899
Daniele S, Da Pozzo E, Zappelli E, Martini C (2015) Trazodone treatment protects neuronal-like cells from inflammatory insult by inhibiting NF-kappaB, p38 and JNK. Cell Signal 27:1609–1629
Dihne M, Grommes C, Lutzenburg M, Witte OW, Block F (2002) Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 33:3006–3011
Dworkin S, Malaterre J, Hollande F, Darcy PK, Ramsay RG, Mantamadiotis T (2009) cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 27:1347–1357
Gundersen BB, Briand LA, Onksen JL, Lelay J, Kaestner KH, Blendy JA (2013) Increased hippocampal neurogenesis and accelerated response to antidepressants in mice with specific deletion of CREB in the hippocampus: role of cAMP response-element modulator tau. J Neurosci 33:13673–13685
Hackett ML, Anderson CS, House A, Xia J (2008) Interventions for treating depression after stroke. Cochrane Database Syst Rev CD003437
Hashimoto K (2010) Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci 64:341–357
Hashimoto K (2013) Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol 100:15–29
Kim YR, Kim HN, Pak ME, Ahn SM, Hong KH, Shin HK, Choi BT (2015) Studies on the animal model of post-stroke depression and application of antipsychotic aripiprazole. Behav Brain Res 287:294–303
Kronenberg G, Balkaya M, Prinz V, Gertz K, Ji S, Kirste I, Heuser I, Kampmann B, Hellmann-Regen J, Gass P, Sohr R, Hellweg R, Waeber C, Juckel G, Hortnagl H, Stumm R, Endres M (2012) Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry 72:273–281
Kronenberg G, Gertz K, Heinz A, Endres M (2014) Of mice and men: modelling post-stroke depression experimentally. Br J Pharmacol 171:4673–4689
Kwon KJ, Lee EJ, Kim MK, Kim SY, Kim JN, Kim JO, Kim HJ, Kim HY, Han JS, Shin CY, Han SH (2015) Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol Dis 73:12–23
Lazarovici P, Cohen G, Arien-Zakay H, Chen J, Zhang C, Chopp M, Jiang H (2012) Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models. J Mol Neurosci 48:526–540
Lee JH, Kim KY, Lee YK, Park SY, Kim CD, Lee WS, Rhim BY, Hong KW (2004) Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther 308:896–903
Lim Y, Zhong JH, Zhou XF (2015) Development of mature BDNF-specific sandwich ELISA. J Neurochem 134:75–85
Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A (2012) Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med 16:1961–1969
Miyamoto N, Tanaka R, Zhang N, Shimura H, Onodera M, Mochizuki H, Hattori N, Urabe T (2009) Crucial role for Ser133-phosphorylated form of cyclic AMP-responsive element binding protein signaling in the differentiation and survival of neural progenitors under chronic cerebral hypoperfusion. Neuroscience 162:525–536
Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, Mochizuki H, Hattori N, Urabe T (2010) Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab 30:299–310
Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K (2013) Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44:2573–2578
Nishimura K, Ishigooka J, Imamura Y, Ihara S (2007) Cilostazol, a cAMP phosphodiesterase 3 inhibitor, in the treatment of poststroke depression. J Neuropsychiatry Clin Neurosci 19:471–472
Oyama N, Yagita Y, Kawamura M, Sugiyama Y, Terasaki Y, Omura-Matsuoka E, Sasaki T, Kitagawa K (2011) Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke 42:2571–2577
Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P (2009) Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 42:270–276
Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C, Group C (2010) Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 9:959–968
Sun MK, Alkon DL (2013) Cerebral ischemia-induced difference in sensitivity to depression and potential therapeutics in rats. Behav Pharmacol 24:222–228
Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T (2010) Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience 171:1367–1376
Taylor WD, Aizenstein HJ, Alexopoulos GS (2013) The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 18:963–974
Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, Conti LH, McCullough LD (2012) NF-kappaB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol 124:425–438
Wang S, Yuan Y, Xia W, Li F, Huang Y, Zhou Y, Guo Y (2012) Neuronal apoptosis and synaptic density in the dentate gyrus of ischemic rats’ response to chronic mild stress and the effects of Notch signaling. PLoS One 7:e42828
Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T (2006) Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke 37:1539–1545
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berlin) 93:358–364
Yoneyama M, Tanaka M, Hasebe S, Yamaguchi T, Shiba T, Ogita K (2015) Beneficial effect of cilostazol-mediated neuronal repair following trimethyltin-induced neuronal loss in the dentate gyrus. J Neurosci Res 93:56–66
Yousuf S, Atif F, Sayeed I, Wang J, Stein DG (2013) Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: immunomodulation and neuroprotection by progesterone. Neuroscience 239:92–102
Zhang N, Miyamoto N, Tanaka R, Mochizuki H, Hattori N, Urabe T (2009) Activation of tyrosine hydroxylase prevents pneumonia in a rat chronic cerebral hypoperfusion model. Neuroscience 158:665–672
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF (2013) Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord 150:776–784
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2014R1A5A2009936).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were approved by the Pusan National University Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines (approval number, PNU-2015-0850).
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kim, Y.R., Kim, H.N., Hong, K.W. et al. Anti-depressant effects of phosphodiesterase 3 inhibitor cilostazol in chronic mild stress-treated mice after ischemic stroke. Psychopharmacology 233, 1055–1066 (2016). https://doi.org/10.1007/s00213-015-4185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4185-6