Abstract
Rationale
Mesolimbic dopamine (DA) regulates behavioral activation and effort-related decision-making in motivated behaviors. Mesolimbic DA D2 receptors are co-localized with adenosine A2A receptors, and they interact in an antagonistic manner.
Objectives
A T-maze task was developed to assess dopaminergic involvement in preference between a reinforcer that involves vigorous voluntary activity (running wheel) and a reinforcer that requires minimal behavioral activation (sucrose pellets). Haloperidol (D2 antagonist) was administered to adenosine A2A receptor knockout (A2AKO) and wild-type (WT) littermate controls to assess the involvement of these two receptors in the selection of running wheel activity versus sucrose consumption.
Results
Under control conditions, mice spent more time running and less time eating. In WT mice, haloperidol reduced time running but actually increased time-consuming sucrose. However, A2AKO mice did not show the haloperidol-induced shift from running wheel activity to sucrose intake. Prefeeding reduced sucrose consumption in the T-maze in both strains, indicating that this paradigm is sensitive to motivational devaluation. Haloperidol increased c-Fos immunoreactivity in anterior cingulate cortex (ACg) and nucleus accumbens (Acb) core of WT but not KO mice.
Conclusions
These results indicate that after DA antagonism, the preference for vigorous physical activity is reduced, while palatable food selection increases. Adenosine A2A receptor deletion provides resistance to these effects of D2 receptor antagonism. These two receptors in Acb core and ACg seem to be involved in the regulation of the intrinsic reinforcing characteristics of voluntary exercise but not in the regulation of the primary reinforcing characteristics of palatable sedentary reinforcers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleus accumbens (Acb) dopamine (DA) is an important component of the neural circuitry that regulates behavioral activation, energy expenditure, and the ability of organisms to overcome work-related response costs in motivated behaviors (Salamone and Correa 2002, 2009, 2012; Robbins and Everitt 2007; Cagniard et al. 2006; Floresco et al. 2008a; Beeler et al. 2012; Mai et al. 2012; Floresco 2015). The effects of Acb DA depletions or DA receptor antagonism on food-reinforced behavior interact powerfully with the response requirements of instrumental tasks. Research with concurrent choice tasks involving distinct food reinforcers that can be obtained by instrumental behaviors with different work requirements has shown that rodents with accumbens DA depletions or DA receptor antagonism reallocate their instrumental behavior away from food-reinforced tasks that have high response requirements (e.g., ratio requirements or vigorous activities such as climbing) and instead select a less-effortful type of food-seeking behavior (Salamone et al. 1991, 1994; Floresco et al. 2008b; Randall et al. 2012; Mai et al. 2012; Pardo et al. 2012, 2015).
Considerable evidence indicates also that brain adenosine interacts with DA systems in the regulation of effort-related choice behavior (Salamone and Correa 2009, 2012). Acb and neostriatum have a high concentration of adenosine A2A receptors (Ferré et al. 2004). DA D2 and adenosine A2A receptors are co-localized on enkephalin-containing medium spiny neurons, and these receptors interact by convergence onto the same signal transduction pathways, where they have opposite effects (Ferré et al. 2004). Evidence indicates that DA D2 and adenosine A2A receptors interact to regulate behavioral activation and effort-related functions (Salamone and Correa 2009; Nunes et al. 2013; Randall et al. 2012; Pardo et al. 2012, 2013; Pereira et al. 2011). Moreover, genetic deletion of the adenosine A2A receptor in mice has been shown to alter the locomotor response to DA antagonists (El Yacoubi et al. 2001; Pardo et al. 2013) and to attenuate the impact of D2 antagonism in mice tested on a T-maze barrier task that assesses effort-related decision-making (Pardo et al. 2012).
In addition to being an instrumental requirement for obtaining access to motivational stimuli, considerable research indicates that physical activities can have intrinsic motivational or reinforcing properties (Belke et al. 2005; Belke and Pierce 2014). In research with rodents, one of the most commonly studied voluntary physical activities is wheel running. Running appears to be motivationally regulated like other appetitive behaviors (Mueller et al. 1997; Sherwin 1998; Belke and Pierce 2014). Thus, wheel running can be used as the motivational stimulus for the establishment of a conditioned place preference (Lett et al. 2000) and as an explicit reinforcer in operant-conditioning procedures (Premack and Premack 1963; Belke and Pierce 2014).
Of course, the choice to engage in voluntary physical activity is always undertaken in relation to the possible selection of other alternatives, such as sedentary behaviors, drugs, or food consumption. If a running wheel (RW) is present in a complex environment that offers other alternatives such as drugs of abuse, rats will spend a considerable amount of time engaged in running activity (McMillan et al. 1995; Cosgrove et al. 2002). Some studies have demonstrated that when given a choice between food and RW, rats often choose running over food (Premack and Premack 1963; Routtenberg 1968), and food consumption decreases on days that rats have access to RW (Premack and Premack 1963; Mueller et al. 1997). However, little is known about the neural mechanisms involved in the decision-making processes that establish those preferences.
In the present study, we developed a rodent task for investigating the decision-making processes that allow for the selection of voluntary physical activity relative to other more sedentary activities such as sucrose pellet consumption. Thus, we employed a maze task that allowed mice to choose between an arm that offers the opportunity to engage in wheel running as the reward versus selecting another arm that leads to palatable pellets containing 50 % sucrose. With this paradigm, we assessed the impact of the DA D2 antagonist haloperidol in wild-type (WT) and A2A receptor knockout (A2AKO) mice and compared it with conditions that reduce motivation such as free access to RW and sucrose pellets prior to the test session. To provide a neural marker of the effects of haloperidol, c-Fos immunoreactivity in WT and A2AKO mice was evaluated in DA and A2A receptor-rich brain areas that are involved in activational aspects of motivation.
Materials and methods
Animals
Male mice lacking A2A adenosine receptors and their WT littermates (N = 18 and 17, respectively) initially weighed 25–30 g. Mice were generated by C. Ledent at Université Libre de Bruxelles (Belgium), as previously reported (Ledent et al. 1997). Subjects were maintained at 22 ± 2 °C with 12-h light/dark cycles. Mice were housed in groups with standard laboratory rodent chow and tap water available ad libitum (see specific conditions for each experiment). Sweet food for the testing procedures consisted of 45 mg precision pellets for rodents with a content of 50 % sucrose (TestDiet, Richmond, IN, USA). All animals were under a protocol approved by the Institutional Animal Care and Use Committee of Universitat Jaume I, and all experimental procedures complied with European Community Council directive (86/609/ECC).
Drugs
Haloperidol (Sigma Quimica C.O) was dissolved in a 0.3 % tartaric acid solution (pH = 4.0), which also was used as the vehicle control. Drugs were administered intraperitoneally (IP), 50 min before testing started.
Apparatus and testing procedures
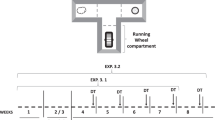
The T-maze apparatus consisted of a central corridor with two opposed arms (for details, see Fig. 1). Half of the mice had the RW consistently located on the left arm, while half the mice had the RW on the right arm. Training as well as test sessions lasted 15 min. Training phase 1: To avoid neophobia, the RW arm was blocked for 5 days to force animals to explore the other arm and to try the sucrose pellets. Training phase 2: During five more days, animals were exposed, once a day to the T-maze with the two reinforcers. Test session: Animals received drug injections (exp. 1) or pre-exposure to the reinforcers (exp. 2), and after that, they were introduced in the T-maze. Sessions were videotaped to later evaluate all the dependent variables: first choice, latency to reach the reinforcers from the onset of the first trial, accumulated time spent in the wheel or consuming the sucrose pellets, and exploration time on the areas of the T-maze with no reinforcer. A “bout” was recorded every time the animal initiated running or eating. The number of bouts was used to calculate the average bout length (i.e., time spent/number of bouts). Testing sessions started 2 h after light period onset. The behavioral test room was illuminated with a soft light, and external noise was attenuated.
Top: top view of the mouse T-maze apparatus used in the present study. All the surfaces and the doorway were constructed out of Plexiglas, and the top of all arms were open. The sucrose arm contained 15 food pellets during the training phase and 25 during the testing phase, and the other arm contained a metallic RW (12.5 cm in diameter). Bottom: schematic depicting training and testing phases
c-Fos immunohistochemistry procedure
Mice were anesthetized with CO2 and perfused with a solution that contained 0.9 % saline and 0.06 % heparin for 5 min, followed by paraformaldehyde for an additional 5 min. Perfusion was initiated 90 min after receiving haloperidol or vehicle. Brains were processed for c-Fos immunoreactivity as previously described (Pardo et al. 2012). Processed sections were then mounted and photographed using a Nikon Eclipse E600 microscope equipped with an Insight Spot digital camera (Melville, NY, USA). Placements for the photographs were counterbalanced between right and left hemispheres for all animals and structures. Images of the regions of interest were magnified at 20× and captured digitally using Stereo Investigator software. Cells that were positively labeled for c-Fos were quantified with ImageJ software (v. 1.42, National Institutes of Health sponsored image analysis program) in three sections per animal, and the average value per mm2 was used for statistical analysis.
Experiments
Experiment 1: effect of haloperidol on preference for RW versus sucrose pellets in the T-maze
Mice (WT N = 9 and A2AKO N = 8) were trained as described above. During the drug-testing phase, there was one drug treatment day per week and four baseline days before the next drug day. A within-group design was used. On test days, mice received the following haloperidol doses in a randomly varied order: 0.0, 0.05, 0.1, and 0.2 mg/kg.
Experiment 2: effect of pre-exposure to both RW and sucrose pellets on preference in the T-maze
Mice (WT N = 8 and A2AKO N = 10) were trained as described above, and a within-group design was used. The test day mice received either vehicle or the highest dose of haloperidol, or for the pre-exposure condition, the day prior to testing, mice had 24 h access to the sucrose pellets and RW in their home cage.
Experiment 3: effect of haloperidol on c-Fos immunoreactivity
Once experiments1 and 2 were completed, mice were trained two additional weeks, and on the test day, they received either vehicle or the highest dose of haloperidol 90 min before being anesthesized and perfused. Brain sections were stained for c-Fos immunoreactivity as described above. A between-group design was used.
Statistical analyses
The main dependent variable that was used as a marker of choice was time spent in each activity. Time was selected because it allowed both choices to be measured by the same units and also because of the behavioral literature demonstrating that time allocation is a useful measure of relative reinforcement value and response choice (Baum and Rachlin 1969). Data for this key dependent variable (time with the reinforcer, RW, or sweet food) in experiments 1 and 2 were analyzed first by a MANOVA comparing strain (WT vs A2AKO) × dose of haloperidol (0.0, 0.05, 0.1, and 0.2 mg/kg) × reinforcer (RW vs sweet food). Because the three-way interaction was significant in both cases (experiment 1 F(3, 28) = 4.35, p < 0.01 and experiment 2 F(2, 29) = 6.67, p < 0.01), univariate ANOVAs were conducted for reinforcer and strain factors in all the dependent variables. One-way repeated measures ANOVA for the dose of haloperidol (0.0, 0.05, 0.1, and 0.2 mg/kg) was performed followed by non-orthogonal planned comparisons using the overall error term, which compared vehicle to all the other doses or conditions (Keppel 1991). In experiment 2, the one-way repeated measures ANOVA analyzed condition (vehicle, haloperidol, and pre-exposure). Non-parametric chi-squared test for goodness of fit was used to analyze proportion data. Data for experiment 3 were analyzed using a two-way factorial ANOVA (strain × treatment) for every brain structure. All data were expressed as mean ± SEM, and significance was set at p < 0.05. STATISTICA 7 software was used.
Results
Experiment 1: effect of haloperidol on preference for RW versus sucrose pellets in the T-maze
Independent ANOVAs were performed to analyze dependent measures related to interaction with RW and with sucrose pellets in the WT and in the A2AKO mice. Thus, repeated measures ANOVA for haloperidol dose indicated a significant effect (F(3, 24) = 3.51, p < 0.05) on time spent in the RW for the WT mice. Planned comparisons revealed significant differences between vehicle and haloperidol 0.2 mg/kg (p < 0.05). The ANOVA for the time spent consuming sucrose yielded also a significant effect of haloperidol (F(3, 24) = 12.02, p < 0.01) in the WT. Significant differences were found after planned comparisons between vehicle and the two highest doses of haloperidol (0.1 and 0.2 mg/kg) (p < 0.01). Thus, haloperidol shifted choice behavior in WT mice, decreasing wheel running but increasing sucrose consumption. However, repeated measures ANOVA showed no significant effect of treatment for A2AKO mice, either on time spent in the RW, or time-consuming sucrose pellets (Fig. 2a–b).
a–f Effect of different doses of haloperidol (0.0, 0.05, 0.1, and 0.2 mg/kg) on time spent in the RW or consuming sucrose (a, b), average bout length (c and d), and latency to reach each reinforcer (e, f) for WT and A2AKO mice. Mean (±SEM) seconds in 15 min. ■ sucrose,  RW. *p < 0.05, **p < 0.01 significantly different from 0.0 mg/kg haloperidol for the same reinforcer
RW. *p < 0.05, **p < 0.01 significantly different from 0.0 mg/kg haloperidol for the same reinforcer
Figure 2c, d shows the average duration of a bout for RW and sucrose in WT and A2AKO mice. The ANOVA for the mean duration of a RW bout in WT mice yielded no significant effect of haloperidol. However, for the mean duration of a sucrose bout, the repeated measures ANOVA showed a significant effect (F(3, 24) = 11.70, p < 0.01), and planned comparisons revealed significant differences between vehicle and 0.2 mg/kg of haloperidol, indicating that mice had longer sucrose bouts when receiving the highest dose of haloperidol. In the A2AKO animals, ANOVAs for mean bout duration for RW or sucrose consumption did not yield significant effects, indicating again that these animals were resistant to the effects of haloperidol.
Data for latency to start interacting with the RW or the sucrose for the WT and the A2AKO mice are shown in Fig. 2e, f, respectively. WT mice significantly increased latency to reach the RW after haloperidol treatment (F (3, 21) = 10.56, p < 0.01), and planned comparisons yielded significant differences between vehicle and the highest dose of haloperidol (0.2 mg/kg). The ANOVA for the latency to interact with the sucrose pellets in the WT mice was not significant. These results indicate that WT mice delay their interaction with the RW when receiving haloperidol, but this treatment does not delay the interaction with sucrose. The ANOVAs on the impact of haloperidol on latency to initiate interaction with the RW or with sucrose for A2AKO mice were not significant.
Experiment 2: effect of pre-exposure to both RW and sucrose pellets on the preference in the T-maze
Figure 3a, b shows time spent running and time-consuming sucrose in WT and A2AKO mice after receiving vehicle or haloperidol 0.2 mg/kg (the most effective dose in experiment 1) or after pre-exposure to both reinforcers. Repeated measures ANOVA for the WT mice data showed that there was a significant effect of condition on time spent on the RW (F(2, 16) = 5.65, p < 0.05). Planned comparisons indicated that haloperidol condition differed from both the control (p < 0.05) and pre-exposed conditions (p < 0.01). There was also a significant treatment effect on time spent consuming sucrose in WT mice (F(2, 16) = 31.63, p < 0.01). Planned comparisons demonstrated that both haloperidol and pre-exposed conditions differed from vehicle (p < 0.01). The univariate repeated measures ANOVA with A2AKO mice showed that only time spent consuming sucrose yielded a significant effect (F(2, 14) = 6.76, p < 0.01). Planned comparisons showed that the pre-exposure condition differed from vehicle (p < 0.01). ANOVA for time on the RW was not significant. Thus, reinforcer devaluation had an impact in A2AKO mice, as it did in WT mice, by reducing time-consuming sucrose.
a–f Effect on WT and A2AKO mice of different experimental conditions: vehicle control (Veh), haloperidol 0.2 mg/kg (HP), and pre-exposure (Pre-exp) on time spent in the RW or consuming sucrose (a, b), average bout length (c, d), and number of bouts for each reinforcer (e, f). Mean (±SEM) seconds or counts in 15 min. ■ sucrose,  RW. *p < 0.05 **p < 0.01 significantly different from Veh condition for the same type of reinforcer; ##p < 0.01 significantly different from HP condition for the same type of reinforcer
RW. *p < 0.05 **p < 0.01 significantly different from Veh condition for the same type of reinforcer; ##p < 0.01 significantly different from HP condition for the same type of reinforcer
The repeated measures ANOVA for average bout length in the WT mice (Fig. 3c) was statistically significant for sucrose (F(2, 14) = 30.91, p < 0.01), and planned comparisons indicated that both the haloperidol (p < 0.01) and pre-exposed (p < 0.05) conditions significantly differed from vehicle. While haloperidol increased the average sucrose bout length, pre-exposure to sucrose reduced this parameter. The pre-exposed condition was also different from haloperidol (p < 0.01). However, the repeated measures ANOVA for average bout length in the RW for the WT mice was not significant. Figure 3d shows the average bout length for the two reinforcers in the A2AKO mice. There was a significant effect on the sucrose bout length (F(2, 14) = 3.71, p < 0.05), and planned comparisons revealed that pre-exposure to sucrose significantly shortened bout length compared to vehicle and also to haloperidol (p < 0.05). As with the WT mice, the ANOVA for the average bout length in the RW for the A2AKO mice was not significant.
The total number of RW and sucrose bouts is shown in Fig. 3e–f. For the WT mice, the repeated measures ANOVA of the RW bouts under the three conditions was not significant. However, the number of bouts for sucrose was statistically significant (F(2, 16) = 10.02, p < 0.01). Under the pre-exposed condition, WT mice had fewer bouts than under vehicle or haloperidol conditions (p < 0.01). Similarly, in the A2AKO mice, although the repeated measures ANOVA for the number of RW bouts was not significant, the ANOVA for the sucrose bouts was (F(2, 14) = 7.89, p < 0.01). Pre-exposed A2AKO mice differed from both vehicle (p < 0.01) and haloperidol treatment (p < 0.05).
To analyze differences between the three conditions in relation to the proportion of animals making a first choice of RW versus sucrose, we used a non-parametric chi-squared test for goodness of fit comparing every condition (haloperidol or pre-exposed) with vehicle. Among the WT animals, haloperidol (0.2 mg/kg) did not change their first choice, but pre-exposing animals to both reinforcers produced a significant effect (χ 2 = 15.65, df = 1, p < 0.01); pre-exposed mice switched from sucrose to RW as their first choice (Fig. 4a). A similar pattern was observed among A2AKO mice; haloperidol did not significantly affect first choice, but pre-exposure to reinforcers did (χ 2 = 10.00, df = 1, p < 0.01) increasing RW selection as well (Fig. 4b).
a–f Effect on WT and A2AKO mice of Veh, HP, and Pre-exp conditions on first choice (% of animals choosing every reinforcer) (a, b), latency to first choice (c, d), and time exploring the T-maze (e, f). Mean (±SEM) seconds in 15 min. ■ sucrose,  RW for a and b. *p < 0.05 **p < 0.01 significantly different from Veh; ##p < 0.01 significantly different from HP
RW for a and b. *p < 0.05 **p < 0.01 significantly different from Veh; ##p < 0.01 significantly different from HP
Latency to reach their first selected reinforcer is shown in Fig. 4c, d. The repeated measures ANOVA for the WT mice yield a significant effect of treatment condition (F(2, 18) = 8.20, p < 0.01). Planned comparisons indicated that pre-exposed WT mice increased latency to select their first choice compared to when they received vehicle or haloperidol (p < 0.01). However, for the A2AKO mice, there was no significant effect.
Finally, repeated measures ANOVA for time of general exploration (not directed to any of the reinforces) showed no significant effect, neither for WT nor for A2AKO mice. Data are shown in Fig. 4e, f.
Experiment 3: effect of haloperidol on c-Fos immunoreactivity
c-Fos immunoreactivity was determined in different brain areas (Fig. 5) after vehicle or the highest dose of haloperidol in WT and A2AKO mice. A separate two-way factorial ANOVA was done for each brain region: infralimbic (IL), prelimbic (PrL), anterior cingulate cortex (ACg), Acb core, Acb shell, dorsomedial striatum (DMS), and dorsolateral striatum (DLS). The two-way factorial ANOVA yielded significant interactions only for the ACg cortex and the Acb core. In the ACg, there was an effect of haloperidol (F(1, 34) = 18.18, p < 0.01), although no effect of strain was shown. However, the haloperidol dose × strain interaction was significant (F(1, 34) = 6.60; p < 0.05). Planned comparisons showed significant differences between haloperidol and vehicle only in WT animals (p < 0.01). The two-way factorial ANOVA for the Acb core data yielded a similar pattern of results. Although the mouse strain factor was not significant, there was an effect of haloperidol (F(1, 33) = 13.0, p < 0.01) and an interaction (F(1, 33) = 3.93, p < 0.05). As seen in the ACg, planned comparisons showed significant differences between haloperidol and vehicle in WT animals (p < 0.01) but not in A2AKO mice. Separate factorial ANOVAs for Acb shell, DMS, and DLS did not yield significant interactions or effects of strain. However, the factor haloperidol dose showed significant effects in all the structures independently of the strain: IL (F(1, 17) = 9.73, p < 0.01), PrL (F(1, 17) = 19.47, p < 0.01), shell (F(1, 33) = 9.85, p < 0.05), DMS (F(1, 33) = 9.07, p < 0.05), and DLS (F(1, 34) = 11.5, p < 0.05). Thus, in all areas, there was a clear effect of haloperidol increasing c-Fos immunoreactivity, but in ACg and Acb core, this effect was reduced in A2A KO mice.
Top: effect of Veh or HP on c-Fos expression in different prefrontal cortex, Acb, and striatum areas of WT and A2AKO mice. Mean (±SEM) number of c-Fos-positive cells per mm2. **p < 0.01 significantly different from WT/Veh in the same brain area; ##p < 0.01, significantly different from WT/HP in the same brain area. Middle: diagram of mice brain coronal sections with bregma coordinates from Franklin and Paxinos 2007, showing location areas for c-Fos counting. Bottom: photomicrographs of c-Fos staining in ACg and Acb core from representative WT and A2AKO animals in each treatment group. Images were taken at 20×, and scale bar is 250 μm
Discussion
The present studies evaluated the role of DA and adenosine transmission in regulating the preference for two different types of natural reinforcers: a highly palatable food reinforcer versus an activity-based reinforcer (wheel running). For this purpose, a novel T-maze preference task was developed, in which animals chose to distribute their time between wheel running, sucrose intake, or maze exploration. Mice have a high preference for engaging in physical activities (Routtenberg 1968), and wheel running has been used as a reinforcer in rodents; for example, rats will press a lever to gain access to a RW (Pierce et al. 1986). Sucrose is a highly preferred substance that induces strong hedonic reactivity in rodents (Berridge 2000; Levine et al. 2003; Peciña et al. 2003) and can be used to reinforce instrumental behaviors (Pardo et al. 2015). When mice were given the choice between these options, we observed that under control conditions, they spent about 55 % of the time on the RW and only about 15 % of the time eating sucrose pellets. While some of the reinforcing effects of wheel running in the present experiment were probably due to the immediate effects of running, the aftereffects of running may also have contributed (Belke and Wagner 2005). In WT mice, the DA antagonist haloperidol dose dependently produced a shift on several measures of preference, significantly reducing time spent on the RW and increasing latency to go to the wheel for the first time, while concomitantly increasing total time spent eating sucrose pellets, as well as the duration of each bout of sucrose consumption. Despite these effects, DA D2 antagonism did not change the first choice (sucrose), did not increase initial latency to reach sucrose, and did not reduce time spent exploring the T-maze. All these parameters indicate that the main effect produced by low doses of haloperidol was to shift choice behavior from RW activity to sucrose.
This shift in relative preference produced by a D2 antagonist, from a reinforcer that involves vigorous physical activity to a gustatory reinforcer that requires little energy expenditure, supports the role of Acb DA in behavioral activation but not in the consumption of food reinforcement (Salamone 1988; Ikemoto and Panksepp 1996; Peciña et al. 2003; Salamone and Correa 2002, 2009, 2012; Robbins and Everitt 2007; Floresco 2015). It has previously been demonstrated that local blockade of D1 and D2 receptors in Acb in rats suppressed spontaneous motor activity and shifted the structure of feeding toward longer bout durations but did not reduce the total amount of food consumed (Baldo et al. 2002). Acb DA antagonism reduced speed to approach a sucrose solution at the end of a runway but did not affect sucrose intake (Ikemoto and Panksepp 1996). Furthermore, increasing DA levels in DAT knockdown mice reduced latency to reach sweet rewards but did not affect “liking” (Peciña et al. 2003). Although high doses of DA antagonists such as haloperidol do affect food intake, evidence indicates that these effects differ from those of prefeeding to reduce food motivation and are mainly due to effects of motor parameters such as rate of feeding and food handling (Salamone et al. 1990). Moreover, these effects appear to depend upon disruption of DA transmission in the lateral neostriatum, rather than the nucleus accumbens (Salamone et al. 1990, 1993; Bakshi and Kelley 1991; Salamone and Correa 2002).
The intrinsic reinforcing value of voluntary physical activities such as lever pressing, barrier climbing, or wheel running is of critical importance for understanding several aspects of motivation and decision-making (Salamone and Correa 2002, 2012; Hosking et al. 2015). In previous research conducted under conditions in which sucrose was not concurrently available, haloperidol was shown to reduce spontaneous wheel running (Pardo et al. 2013). The effects observed in the present work were obtained using a choice procedure, in which vigorous behavioral activity was not required for the animals to obtain the sucrose reward, and running in the wheel competed with sucrose consumption. The primary dependent variable that was used to mark choice was time spent in each activity. Time was selected because it allowed both choices to be measured by the same units and also because of the classic behavioral literature demonstrating that time allocation is a critical measure of relative reinforcement value and response choice (Baum and Rachlin 1969). Thus, the present results suggest that DA antagonism reduces the relative intrinsic reinforcing characteristics of wheel running in an empirical sense, though the processes that underlie this effect (e.g., reduced behavioral activation, aversion to running, increased perceived response costs or fatigue) are not certain. Inspection of the videotapes did not reveal any gross effects of haloperidol on gait, such as ataxia or incoordination, and no animals ever showed problems with paw placement on the floor of the running wheel, but subtle effects on parameters such as response slowing cannot be completely ruled out. Nevertheless, in contrast to the effects on wheel running, haloperidol in the dose range tested did not impair the primary reinforcing characteristics of sucrose, actually increasing the relative preference for sucrose.
Using our T-maze procedure, sucrose reinforcement can be devalued by pre-exposure. In experiment 2, pre-exposing the animals to the reinforcers reduced the percentage of animals that chose sucrose as their first choice from 80 to 20 %. It also reduced time spent eating, number of bouts, and average bout length. All these changes indicate a relative reduction of sucrose reinforcement in the devalued condition, a pattern of effects very different from that produced by haloperidol. Previous choice studies using different types of food have demonstrated that DA antagonists or Acb DA depletions do not produce effects that closely resemble those produced by prefeeding-induced devaluation or appetite suppressant drugs (Salamone et al. 1990, 1991; Aberman and Salamone 1999; Randall et al. 2012; Nunes et al. 2013). In studies using a T-maze task with different effort-related choices (arm with barrier with high density of food versus arm with no barrier for low density of food), prefeeding increased the number of omission trials (no visit to any arm) and produced indifference for the arm selected in mice, effects that differed from those produced by haloperidol (Pardo et al. 2012). In the T-maze barrier test, rats with Acb core DA depletion showed a decrease in preference for effortful/large-reward options under both food restricted and sated conditions (Mai et al. 2012). Together with the present results, these findings demonstrate that interference with DA transmission changes motivation by reducing behavioral activation and exertion of effort rather than simply reducing appetite (Salamone and Correa 2009, 2012; Mai et al. 2012). However, in the present experiments, RW pre-exposure did not change most parameters in relation to the control condition. In fact, mice chose RW as their first option (Fig. 4a) because they were probably sucrose satiated, and also, since sucrose was normally their first option, it took them longer to choose initially where to go (Fig. 4c). This lack of effect of pre-exposure to RW on any parameter related to RW activity could be due to the amount of RW exposure time used in the pre-exposed condition, to the fact that RW “satiation” follows a different dynamic than sucrose satiation (Belke and Pierce 2014), or could also be due to the development of running in the wheel as habitual and therefore relatively insensitive to devaluation.
Adenosine A2A receptors are co-localized with DA D2 receptors on medium spiny neurons throughout the striatal complex; these receptors can form heteromers and converge onto the same signal transduction pathways, having opposite effects on the adenylyl cyclase-related signal transduction cascade (Ferré et al. 2004; Santerre et al. 2012; Nunes et al. 2013). Previous studies reported that adenosine A2A antagonists reverse the effort-related effects of DA D2 antagonists (Pardo et al. 2012; Santerre et al. 2012). In the present work, A2AKO mice were resistant to the effects of haloperidol on performance of the T-maze procedure. While haloperidol redirected behavior in WT animals away from RW activity and toward the less demanding reinforcer (sucrose), no effect was seen in A2AKO mice. Recent data in our laboratory showed that A2AKO mice were also resistant to the effects of haloperidol on spontaneous locomotion in the OF and RW (Pardo et al. 2013) and on effort-based decision-making in the T-maze barrier choice task (Pardo et al. 2012).
In order to provide a marker of haloperidol-induced changes in neural activity, c-Fos immunoreactivity was measured in different subregions of Acb, striatum, and prefrontal cortex. Haloperidol increased c-Fos-positive cells in all regions compared to vehicle. However, there was a significant difference between WT and A2AKO mice in ACg and Acb core; the lack of A2A receptors appeared to attenuate the effects of haloperidol on c-Fos expression, which is possibly a neural marker of the resistance to the behavioral effects of haloperidol shown by A2AKO animals. The present data on c-Fos in Acb agree with previous findings on the effects of haloperidol in A2AKO mice (Pardo et al. 2012), as well as studies employing A2A antagonists (Santerre et al. 2012; Farrar et al. 2010; Pinna et al. 1999). Moreover, although Acb DA is a vital component of the brain circuitry regulating work output and effort-based choice behavior, and D2/A2A interactions in Acb are known to be important for effort-related processes (Farrar et al. 2010; Santerre et al. 2012; Pardo et al. 2012), ACg also plays an important role. Lesions and DA depletions in the ACg cause rats to shift their choice behavior from high effort/high reward options to low effort/low reward alternatives (Schweimer et al. 2005; Hauber and Sommer 2009), which is similar to the effects of Acb DA depletions (Salamone et al. 1994). Cell body lesions or inactivations of Acb core, as well as combined contralateral lesions of ACg and Acb core, also were shown to affect effort-related choice (Hauber and Sommer 2009; Ghods-Sharifi and Floresco 2010), indicating that effort-related processes are regulated by an interconnected neural system that requires serial information transfer between those two brain areas (Hauber and Sommer 2009; Floresco et al. 2008a). In humans, prefrontal cortex plays an important role in the sensation of fatigue and the perception of effort during exercise (Berchicci et al. 2013), and willingness to exert effort correlated with increases in striatal and medial prefrontal cortex DA transmission (Treadway et al. 2012).
A lack of physical activity can contribute to the development of depression (Lambert 2006), and exercise in humans has been suggested as an intervention for the prevention of disease and the treatment of various metabolic, neurological, or psychiatric symptoms including obesity and drug abuse (Dishman et al. 2006; Friedman 2009; Smith et al. 2008). Moreover, there is a striking similarity between brain mechanisms involved in behavioral activation and effort-related processes in rats and those involved in effort-related motivational symptoms such as anergia, fatigue, and psychomotor slowing seen in depressed humans (Salamone et al. 2012). For these reasons, it is important to identify the neural factors that influence the choice to engage in physical activity relative to other more sedentary rewards.
References
Aberman JE, Salamone JD (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92:545–552
Bakshi VP, Kelley AE (1991) Dopaminergic regulation of feeding behavior: I. differential effects of haloperidol microinjection in three striatal subregions. Psychobiology 19:223–232
Baldo BA, Sadeghian K, Basso AM, Kelley AE (2002) Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137:165–177
Baum WM, Rachlin HC (1969) Choice as time allocation. J Exp Anal Behav 12:861–874
Beeler JA, Frazier CR, Zhuang X (2012) Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci 6:49
Belke TW, Pierce WD (2014) Effect of sucrose availability and pre-running on the intrinsic value of wheel running as an operant and a reinforcing consequence. Behav Process 103:35–42
Belke TW, Wagner JP (2005) The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Process 68(2):165–172
Belke TW, Oldford AC, Forgie MY, Beye JA (2005) Responding for sucrose and wheel-running reinforcement: effect of D-amphetamine. Behav Pharmacol 16:219–225
Berchicci M, Menotti F, Macaluso A, Di Russo F (2013) The neurophysiology of central and peripheral fatigue during sub-maximal lower limb isometric contractions. Front Hum Neurosci 7:135
Berridge KC (2000) Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24:173–198
Cagniard B, Balsam PD, Brunner D, Zhuang X (2006) Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31:1362–1370
Cosgrove KP, Hunter RG, Carroll ME (2002) Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav 73:663–671
Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton R, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ (2006) Neurobiology of exercise. Obesity 14:345–356
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2001) Adenosine A2A receptor knockout mice are partially protected against drug-induced catalepsy. Neuroreport 12:983–986
Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD (2010) Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience 166:1056–1067
Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casadó V, Hillion J, Torvinen M, Fanelli F, Benedetti PD, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A (2004) Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord 10:265–271
Floresco SB (2015) The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66:25–252
Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA (2008a) Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision-making. Cogn Affect Behav Neurosci 8:375–389
Floresco SB, Tse MT, Ghods-Sharifi S (2008b) Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33:1966–1979
Friedman JH (2009) Fatigue in Parkinson’s disease patients. Curr Treat Options Neurol 11:186–190
Ghods-Sharifi S, Floresco SB (2010) Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behav Neurosci 124:179–191
Hauber W, Sommer S (2009) Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex 19:2240–2247
Hosking JG, Floresco SB, Winstanley CA (2015) Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 40:1005–1015
Ikemoto S, Panksepp J (1996) Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci 110:331–345
Keppel G (1991) Design and analysis: a researcher’s handbook. Prentice-Hall, Englewood Cliffs
Lambert KG (2006) Rising rates of depression in today’s society: consideration of the roles of effort based rewards and enhanced resilience in day-to-day functioning. Neurosci Biobehav Rev 30:497–510
Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388:674–678
Lett BT, Grant VL, Byrne MJ, Koh MT (2000) Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite 34:87–94
Levine AS, Kotz CM, Gosnell BA (2003) Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr 78:834S–842S
Mai B, Sommer S, Hauber W (2012) Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci 12:74–84
McMillan DE, McClure GY, Hardwick WC (1995) Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend 40:1–7
Mueller DT, Loft A, Eikelboom R (1997) Alternate-day wheel access: effects on feeding, body weight, and running. Physiol Behav 62:905–908
Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn S, Baqi Y, Müller CE, Lopez-Cruz L, Correa M, Salamone JD (2013) Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression. J Neurosci 33:19120–19130
Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, Salamone JD, Correa M (2012) Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-based decision making in mice. Neuropharmacology 62:2068–2077
Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, Salamone JD, Correa M (2013) Effect of subtype-selective adenosine receptor antagonists on basal or haloperidol-regulated striatal function: studies of exploratory locomotion and c-Fos immunoreactivity in outbred and A2AR KO mice. Behav Brain Res 247:217–226
Pardo M, Lopez-Cruz L, San Miguel N, Salamone JD, Correa M (2015) Selection of sucrose concentration depends on the effort required to obtain it: studies using tetrabenazine, D1, D2 and D3 receptor antagonists. Psychopharmacology 232:2377–2391
Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X (2003) Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23:9395–9402
Pereira M, Farrar AM, Hockemeyer J, Müller CE, Salamone JD, Morrell JI (2011) Effect of the adenosine A2A receptor antagonist MSX-3 on motivational disruptions of maternal behavior induced by dopamine antagonism in the early postpartum rat. Psychopharmacology 213:69–79
Pierce WD, Epling WF, Boer DP (1986) Deprivation and satiation: the interrelations between food and wheel running. J Exp Anal Behav 46:199–210
Pinna A, Wardas J, Cozzolino A, Morelli M (1999) Involvement of adenosine A2A receptors in the induction of c-fos expression by clozapine and haloperidol. Neuropsychopharmacology 20:44–51
Premack D, Premack AJ (1963) Increased eating in rats deprived of running. J Exp Anal Behav 6:209–212
Randall PA, Pardo M, Nunes EJ, López-Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD (2012) Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7:e47934
Robbins TW, Everitt BJ (2007) A role for mesencephalic dopamine in activation: commentary on Berridge (2006). Psychopharmacology 191:433–437
Routtenberg A (1968) “Self-starvation” of rats living in activity wheels: adaptation effects. J Comp Physiol Psychol 66:234–238
Salamone JD (1988) Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule induced activity, feeding and foraging in rats. Psychobiology 16:196–206
Salamone JD, Correa M (2002) Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137:3–25
Salamone JD, Correa M (2009) Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite 53:422–425
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485
Salamone JD, Zigmond MJ, Stricker EM (1990) Characterization of the impaired feeding behavior in rats given haloperidol or dopamine depleting brain lesions. Neuroscience 39(17):24
Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K (1991) Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 104:515–521
Salamone JD, Mahan K, Rogers S (1993) Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav 44:605–610
Salamone JD, Cousins MS, Bucher S (1994) Anhedonia or anergia? effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65:221–229
Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M (2012) The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. J Exp Anal Behav 97:125–146
Santerre JL, Nunes EJ, Kovner R, Leser CE, Randall PA, Collins-Praino LE, Lopez-Cruz L, Correa M, Baqi Y, Müller CE, Salamone JD (2012) The novel adenosine A(2A) antagonist prodrug MSX-4 is effective in animal models related to motivational and motor functions. Pharmacol Biochem Behav 102:477–487
Schweimer J, Saft S, Hauber W (2005) Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci 119:1687–1692
Sherwin CM (1998) Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27
Smith MA, Schmidt KT, Iordanou JC, Mustroph ML (2008) Aerobic exercise decreases the positive reinforcing effects of cocaine. Drug Alc Depend 988:129–135
Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH (2012) Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32:6170–6176
Acknowledgments
This work was supported by a grant to Mercè Correa from Pla Promoció Investigació UJI (P1.1 A 2013-01), to John D. Salamone from the National Institute of Mental Health (MH078023), and to Olga Valverde from Ministery of Economy, Spain (SAF-2013-41761-R). Personal grants were awarded to Marta Pardo (Predoc-UJI/ 2007/43), Noemí San Miguel (Predoc-UJI/2012/28), and Laura Lopez-Cruz (FPU AP2010-3793, Ministerio de Educación, Spain).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Correa, M., Pardo, M., Bayarri, P. et al. Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: effects of haloperidol in wild type and adenosine A2AKO mice. Psychopharmacology 233, 393–404 (2016). https://doi.org/10.1007/s00213-015-4127-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4127-3