Abstract
Rationale
L-dopa, the main therapeutic for Parkinson’s disease (PD), has been shown to increase brain dopamine concentrations that are necessary for proper motor control; however, PD patients experience non-motor symptoms that are not improved or could be exacerbated by L-dopa.
Objectives
The purpose of this study is to determine the effects of L-dopa treatment on cognitive and affective behavioral responses of rats, as well as their corresponding monoamine brain concentrations.
Methods
Rats were treated with L-dopa (6 mg/kg; twice daily) for 10 consecutive days. Sodium ascorbate (400 mg/kg) was co-administered with L-dopa to investigate the effects of antioxidant co-treatment on behavior and monoamine concentrations. Rats underwent cognitive and affective behavioral testing. Monoamine concentrations of several brain regions were analyzed.
Results
L-dopa treatment resulted in significant impairment in the performance in the Barnes maze and improvement in conditioned fear stress paradigms. Specifically, L-dopa caused an increase in latency to find the goal box during Barnes maze testing and increased freezing behavior in context-induced conditioned fear testing. Furthermore, the rats in the conditioned fear stress experiments showed corresponding depletions in serotonin (5-HT) and its metabolite, 5-HIAA, in the dorsal raphe nucleus (DRN) and the mPFC. The behavioral impairments as well as monoamine depletions were blocked by ascorbate co-treatment.
Conclusions
Chronic L-dopa may contribute to non-motor symptoms related to spatial memory and fear. These effects may be attributable to a dysregulation of brain 5-HT caused by L-dopa treatment. The results presented here provide further rationale for investigating adjunctive therapeutics to L-dopa for PD, such as antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
L-dopa is the mainstay therapeutic treatment for Parkinson’s disease (PD). As the precursor to dopamine, L-dopa alleviates some of the dopaminergic deficits in the striatum caused by the progressive degeneration of dopamine neurons found in the substantia nigra pars compacta (Hoehn and Yahr 1967; Cotzias 1968). Although L-dopa effectively treats the movement disorders in PD such as bradykinesia and rigidity, chronic L-dopa treatment often results in negative side-effects such as dyskinesias, hallucinations, and impulsive behavior problems (Cotzias et al. 1969; Banerjee et al. 1989; Ceravolo et al. 2009). In addition, the progression of PD is accompanied by many significant and underappreciated non-motor symptoms such as sleep disturbances, depression, fear, anxiety, and cognitive impairments (Mayeux et al. 1981; Post et al. 2011; Bonnet and Czernecki 2013). Importantly, many non-motor symptoms in PD are unresponsive to L-dopa therapy, and in fact, some may be exacerbated by chronic treatment (Choi et al. 2000; Richard et al. 2004; Sethi 2008; Kim et al. 2009), suggestive of the involvement of non-dopaminergic systems in the non-motor sequela of PD.
The neuropathology of PD and the effects of L-dopa are not limited to the dopaminergic system as they also involve the serotonergic and noradrenergic systems (Braak et al. 2003; Huot et al. 2011; Winter et al. 2011). Alterations in these monoamine systems are known to produce behavioral impairments similar to the non-motor symptoms exhibited by PD patients after chronic L-dopa therapy (Eskow Jaunarajs et al. 2011). Pre-clinically, there is mounting evidence for extensive changes in the serotonin (5-HT) systems after L-dopa (Navailles et al. 2011a). In fact, L-dopa has been shown to be transported into 5-HT neurons, decarboxylated to dopamine, and exocytosed in an impulse dependent manner (Ng et al. 1972; Arai et al. 1995; Miller and Abercrombie 1999; Stansley and Yamamoto 2013). 5-HT neuron fibers in the striatum have been shown to contain dopamine after L-dopa treatment (Arai et al. 1995). Further, L-dopa-induced dopamine release from striatal 5-HT terminals can be prevented by 5-HT1A and 5-HT1B agonists (Kannari et al. 2001; Carta et al. 2007) or ablation of the dorsal raphe nucleus (DRN) with the 5-HT neurotoxin 5,7-dihydroxytryptamine (Hollister et al. 1979; Navailles et al. 2010). Thus, there is evidence to support dopamine production and release from 5-HT neurons after systemic L-dopa administration.
While 5-HT neurons may facilitate the elevation of striatal dopamine content toward physiological concentrations after L-dopa in the PD brain, extrastriatal brain regions that are innervated by 5-HT axons have been shown to contain supraphysiologic concentrations of dopamine. For example, extracellular concentrations of dopamine in the prefrontal cortex (PFC) increase approximately 12-fold after acute injection of 12 mg/kg L-dopa (Navailles et al. 2010). High amounts of unsequestered dopamine have been shown to be pro-oxidant as dopamine can be degraded by monoamine oxidase (MAO) to produce 3,4-dihydroxyphenylacetic acid (DOPAC) and the by-product hydrogen peroxide or auto-oxidize into a highly reactive quinone species (Graham 1978). Indeed, L-dopa is toxic to 5-HT neurons in vitro by oxidative mechanisms that are dependent on dopamine production and degradation (Stansley and Yamamoto 2013). In vivo, chronic L-dopa has been shown to reduce the number of 5-HT neurons in the DRN by similar oxidative mechanisms (Stansley and Yamamoto 2014). In addition to decreases in the number of 5-HT cell soma, chronic L-dopa depletes 5-HT content in several brain regions including the DRN, striatum, hippocampus, amygdala, and PFC (Borah and Mohanakumar 2007; Navailles et al. 2011b; Eskow Jaunarajs et al. 2012; Stansley and Yamamoto 2014). Importantly, proper 5-HT function in these regions is crucial for normal behavioral physiology (Lowry et al. 2005; Maier and Watkins 2005; Berger et al. 2009; Monti 2011) such that 5-HT deficits in these areas result in impaired memory consolidation, executive cognitive dysfunction, fear, depression, and anxiety (Lowry et al. 2008; Soh et al. 2011; Euston et al. 2012; Izumi et al. 2012).
Given the similarities between 5-HT deficits produced by L-dopa in animal models and in vitro cell culture, and the 5-HT deficit related symptoms observed in PD, L-dopa may contribute to non-motor symptoms by reducing transmission of 5-HT in critical limbic brain structures in an pro-oxidant manner. Therefore, the present study was designed to specifically test the hypothesis that chronic L-dopa treatment results in the impairment of cognition and affective behaviors associated with 5-HT deficits in limbic and midbrain structures through oxidative mechanisms.
Materials and methods
Animals
Male Sprague–Dawley rats (175–199 g, Harlan Indianapolis, IN) were used in all experiments. Rats were housed two per cage, in clear plastic containers (45 × 24 × 20 cm), and allowed to acclimate for 1 week to the animal colony before any experimentation. The environment in which the rats were housed was under a 12-h light/dark cycle, temperature (~24 °C) and humidity (~40 %) controlled and rats had ad libitum access to food and water. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Toledo Institutional Animal Care and Use Committee.
Pharmacological treatments
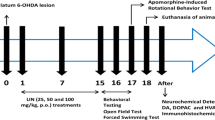
To test the behavioral effects of chronic L-dopa, rats were treated twice daily with either vehicle (0.9 % saline) or L-dopa methyl ester (L-dopa; 6 mg/kg, i.p., Sigma) plus the peripheral decarboxylase inhibitor DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 12 mg/kg, i.p.; Sigma) for 10 days. In experiments testing the effects of an antioxidant, sodium ascorbate (400 mg/kg, ip, Sigma) was administered 15 min before each L-dopa dose. The injection volume for all drugs was 1 ml per kilogram body weight. To ensure that acute effects of L-dopa would not influence behavior, behavioral testing began at least 48 h after last drug injection.
Behavioral experiments
Forced swim test
The modified forced swim test was used to evaluate chronic L-dopa effects on depressive-like behavior. Rats were treated for 10 days with either L-dopa or vehicle saline, and 48 h later underwent a single forced swim test. During the swim test, rats were placed in a cylindrical swimming chamber (60 cm × 20 cm) that contained 30 cm of water (25 °C) for 5 min, and behavior was recorded by video camera. A trained observer, blinded to the treatment conditions, analyzed the video recordings of the 5-min swim test. Behavior was measured continuously as either climbing, swimming, or immobile throughout the entire 5-min test. Immobility was defined as floating with the absence of limb movement. Measures of climbing, swimming, duration of immobility, and latency to immobility were compared between treatment groups, with increased immobility duration and decreased latency suggestive of depressive-like behavior (Tew et al. 2013; Simpson et al. 2014).
Barnes maze
The Barnes maze was used to test acquisition and recall of spatial memory (Roland et al. 2012). Rats were placed on a circular maze (120 cm diameter) that contained 16 holes (7.5 cm diameter) with one hole containing a darkened “goal box” underneath, to which the rat can enter to escape the brightly lit maze. The goal box was always placed in the 2 o’clock position relative to the walls surrounding the maze which contained spatial cues (e.g., lined patterns). The acquisition phase began 48 h after last drug treatment, when rats were placed in the center of the maze inside a dark cylinder start chamber. A bright light (250 W) was turned on over the maze and, 10 s later, the start chamber was lifted and rats were allowed 3 min to find the goal box. During the first acquisition trial, rats that did not find the goal box within 3 min were gently guided to the escape box. The light was turned off immediately upon rat entering the escape box. Rats underwent one trial per day and were individually tested in a randomized order relative to treatment. In addition, after each trial, the maze was cleaned with a disinfectant/deodorizer (1 % cetylcide solution; Cetylcide-II, Cetylite) and rotated one quarter turn to prevent the scent of previous rats from influencing escape latency. After 5 consecutive days of acquisition, all rats were returned to the housing facility for 48 h and then tested on the Barnes maze for recall. The recall trial followed the identical procedure as previous acquisition trials. Each trial was recorded by video camera and subsequently reviewed and analyzed for latency to find the escape box by a researcher blinded to treatment groups.
Elevated plus-maze
Experiments to measure unlearned or innate fear/anxiety-like behavior utilized the elevated plus-maze, a procedure that has been shown to be sensitive to particular anti-anxiety pharmacological agents, when administered at doses free of side-effects (Shimizu et al. 2004). The maze consists of four arms, two open (50 cm × 10 cm) and two enclosed (50 cm × 10 cm × 37.5 cm), with the base elevated 48 cm above the ground. In the present study, 48 h following 10 days of chronic L-dopa, rats were placed in the elevated plus-maze and allowed to explore the maze for 5 min while behavior was recorded by video camera. After each trial, the maze was cleaned with a disinfectant/deodorizer (1 % cetylcide solution; Cetylcide-II, Cetylite). A trained observer, blinded to the treatment conditions, reviewed the video recordings and noted time spent in the open arms, as well as entries into the open arms, which were later analyzed. Typically, time spent in open arm is reflective of anti-anxiety-like behavior. In addition, the frequency of unprotected head dips (while the rat has all four paws in the open arm, any movement of the head over the open arm and toward the floor), which negatively correlates with anxiety (Ducottet and Belzung 2005; Navarro et al. 2006), was recorded and analyzed.
Conditioned fear stress
Conditioned fear response was measured after chronic L-dopa by a conditioned fear stress paradigm. This procedure produces a freezing behavior in response to a context associated with an aversive foot shock stimulus and is a model of conditioned fear or anxiety-like behavior (Peters et al. 2011). Rats were treated chronically with L-dopa or saline vehicle and 48 h after the last dosing underwent conditioning. Rats were individually placed in a chamber (45 × 24 × 20 cm) consisting of four vertical line-patterned walls and an electrifiable grid floor. The fear conditioning consisted of an inescapable electric foot shock in a paradigm lasting for a total of 5 min (2.5 mA scrambled shock, 10 ms every 100 ms; shock duration of 30 s × 4; 30 s intershock interval). Rats were placed in the chamber and acclimated for 2 min before the first footshock interval began. Electric shock was provided by a programmable shock generator (SMSCK). Rats then remained in the chamber for 1 min after the termination of the last footshock interval. Context-induced fear behavior was tested 24 h after conditioning by placing rats into the shock chamber; however, no current was applied to the grid floor during testing. Behavior was recorded by video camera for a total of 30 min (three, 10 min intervals; 0–10, 10–20, 20–30). Each interval was scored independently, by a researcher blinded to treatment groups. Freezing behavior was scored as complete immobility aside from respiration for 10 consecutive seconds.
Determination of monoamine levels HPLC-EC
Brain monoamine content experiments were carried out in rats that underwent conditioned fear stress. Briefly, rats were treated for 10 days with saline vehicle, L-dopa, ascorbate, or the combination of ascorbate plus L-dopa and then underwent the conditioned fear stress paradigm. Rats were killed 48 h after testing, brains were dissected, and concentrations of tissue monoamines were determined by high-performance liquid chromatography with electrochemical detection (HPLC-EC) as described previously (Stansley and Yamamoto 2014). The DRN subregions (−7.3 to −8.3 mm from bregma) consisted of a rectangular dissection with boundaries of the cerebral aqueduct above and the decussation of the superior cerebellar peduncle below, and 1 mm to either side of the midline; the top half was considered dorsal DRN, and the bottom half was ventral DRN. The medial-prefrontal cortex (2.7 to 2.2 mm from bregma) consisted of a rectangular dissection of the prelimbic, infralimbic, and dorsal peduncular cortex, which are bordered on either side by the forceps minor of the corpus callosum. The hippocampus (−3.3 to −3.8 mm from bregma; dorsal hippocampus) and amygdala (−2.3 to −3.3 mm from bregma) were micropunched with a cylindrical flat needle (14.5 gauge; 1.6 mm internal diameter). The tissue was then homogenized in cold 0.25 M perchloric acid and centrifuged at 14,000g for 15 min. Monoamines were measured by HPLC-EC. The supernatant (20 μL) was injected onto a C18 column (100 × 2.0 mm, Phenomenex, Torrance, CA, USA). The mobile phase consisted of 21 g/L citric acid anhydrous, 10.65 g/L disodium phosphate, 500 mg/L octyl sodium sulfate, and 15 % methanol, with a pH 4.2. An LC-4B amperometric detector (BAS Bioanalytical Systems, West Lafayette, IN, USA) was used and data were analyzed with EZChrom_software (Scientific Software Inc., Pleasanton, CA, USA). The protein content of the samples was determined using a Bradford assay (Bio-Rad, Hercules, CA, USA) after re-suspending the pellet in 1 N NaOH. Monoamine content per sample was normalized to protein and expressed as picogram per microgram of protein. Of note, the data from several rats had to be excluded from analyses that pertained to monoamine content because of significant contamination. The same rats were excluded from both the monoamine results (Fig. 5, Table 1) and behavioral experiments (Fig. 4), as well as the correlational analyses, so that valid comparisons can be made between the neurochemical and behavioral data.
Statistical analysis
Statistical comparison of behavioral measures or monoamine content was performed using either a Student’s t test, a two-way analysis of variance (ANOVA), or a two-way ANOVA with repeated measures and post hoc multiple comparison test when appropriate. Analyses were performed using SigmaPlot 11.0 software (SigmaPlot for Windows, Systat Software). All data are presented as mean ± SEM. Significance levels in all studies are p ≤ 0.05.
Results
Effect of chronic L-dopa on depressive-like behaviors in the forced swim test
Initial experiments employed the modified forced swim test to measure behaviors associated with depression or learned helplessness in rodents. Results indicate that chronic L-dopa treatment does not affect depressive-like behaviors. A Student’s t test revealed that there were no significant differences between vehicle or L-dopa-treated rats in measures of immobility (t = 1.00, p = 0.33), latency to immobility (t = −1.03, p = 0.32), climbing (t = −0.72, p = 0.48), or swimming (t = −0.5, p = 0.62) during the forced swim test (Fig. 1). These results indicate that chronic L-dopa neither reduces nor exacerbates depressive-like behaviors in the forced swim.
Effects of chronic L-dopa on depressive-like behaviors in the forced swim test. L-dopa treatment did not alter duration of immobility during the 5-min swim test compared to vehicle-treated controls. Furthermore, latency time to immobility, swimming, and climbing were also not significantly affected by L-dopa treatment (n = 8 per group) (Student’s t test; data represented as mean ± SEM)
Effect of chronic L-dopa on spatial memory
Spatial memory was tested by performance in the Barnes maze after chronic L-dopa. Two additional groups of rats were treated with ascorbate (400 mg/kg, i.p.) or the combination of ascorbate plus L-dopa to investigate antioxidant effects on the Barnes maze task. Following 10 days of treatment, rats underwent a 5-day acquisition phase, consisting of one trial per day, in which latency to find the goal box was measured (Fig. 2a). A two-way ANOVA with repeated measures indicated a main effect of time [F (4112) = 18.5, p < 0.05], suggesting rats became more efficient at finding the goal box throughout the acquisition phase. Indeed, a Tukey’s post hoc analysis revealed that latency time for all treatment groups was significantly decreased on days 3, 4, and 5 when compared to day 1 (Day 3: q = 8.5, p < 0.05; Day 4: q = 8.9, p < 0.05; Day 5: q = 10.4, p < 0.05). These effects were independent of treatment, as latency did not differ between treatment groups at any time-point [F (3112) = 0.83, p = 0.49]. Two days following completion of the acquisition phase, rats were tested for recall on the Barnes maze and latency to find the goal box was measured (Fig. 2b). A two-way ANOVA revealed a significant interaction between L-dopa and ascorbate treatment, indicating that the effect on latency caused by L-dopa varied according to whether ascorbate was co-administered (F (1,28) = 6.73, p < 0.05). Tukey’s post hoc analysis showed a significant effect of L-dopa (q = 4.38, p < 0.05) that was blocked by co-treatment with ascorbate (q = 6.18, p < 0.05). These results suggest that while acquisition of spatial memory is not effected by chronic L-dopa treatment, the recall of spatial memory is impaired by L-dopa through an oxidative mechanism.
Effects of chronic L-dopa on spatial learning and memory in the Barnes maze. a L-dopa treatment did not affect latency to find the goal box during the 5-day acquisition phase. There was an overall effect of day on latency to find the goal box (*p < 0.05 compared to Day 1, two-way ANOVA with repeated measures and Tukey’s post hoc test). b L-dopa treatment significantly increased the latency to find the goal box during the recall trial. Ascorbate co-treatment significantly blocked the increased latency caused by L-dopa (*p < 0.05 compared to vehicle-vehicle, two-way ANOVA with Tukey’s post hoc test; data represented as mean ± SEM) (n = 8 per group)
Effect of chronic L-dopa on anxiety/fear-related behaviors
To investigate the effects of chronic L-dopa on unconditioned anxiety-like behaviors, the elevated plus-maze was used. A Student’s t test revealed that there was no significant difference between treatment groups in time spent in the open arms (t = −0.47, p = 0.65) (Fig. 3a). Additionally, L-dopa treatment did not affect other measures of anxiety-like behavior such as number of unprotected head dips (t = 0.68, p = 0.15) or number of entries into the open arms (t = 0.49, p = 0.62) when compared to vehicle-treated rats (Fig. 3b, c). These results suggest that innate anxiety-like behavior is not altered by chronic L-dopa treatment.
Effects of chronic L-dopa on anxiety-like behaviors in the elevated plus-maze. a L-dopa treatment did not influence total time in open arm compared to vehicle treatment. b Unprotected head dips were also not affected by L-dopa treatment. c The number of entries into the open arms was also not significantly affected by L-dopa (n = 8 per group) (Student’s t test; data represented as mean ± SEM)
To investigate whether chronic L-dopa influences conditioned fear behavior, the conditioned fear stress paradigm was used. This paradigm has been shown to produce significant context-induced freezing, which has been interpreted as anxiety or fear-related behavior (Rodgers 1991). Rats were treated with either saline or L-dopa for 10 days. Two additional groups received either ascorbate alone or the combination of ascorbate plus L-dopa to investigate antioxidant effects on conditioned fear behavior. Rats then underwent fear conditioning 48 h after the last dosing by being placed individually in a chamber and receiving aversive foot shock. Context-induced fear behavior was then tested 24 h after conditioning by returning the rats to the conditioning chamber and recording freezing behavior for three consecutive 10-min intervals. Data were analyzed by two-way ANOVA with repeated measures and a main effects of both treatment [F (3,44) = 5.74, p < 0.05] and time [F (2,44) = 45.1, p < 0.05] were found. A Student-Newman-Keuls post hoc analysis revealed that L-dopa treatment significantly increased amount of freezing compared to saline treated controls from 0 to 10 min (q = 4.81, p < 0.05). Within the 0–10-min interval, ascorbate treatment alone did not affect freezing behavior, but it blocked the increased freezing behavior when administered with L-dopa (q = 3.01, p < 0.05). No significant effects occurred during the 10–20 or 20–30-min interval when treatment groups were compared (Fig. 4).
Effects of chronic L-dopa on conditioned fear behavior. Chronic L-dopa treatment significantly increased freezing behavior during the 0–10-min interval. This increase was blocked when ascorbate was co-administered with L-dopa. No significant effects were observed during the 10–20 or 20–30-min intervals. (*p < 0.05 compared to vehicle-vehicle, two-way ANOVA with repeated measures and Student-Newman-Keuls post hoc test; data represented as mean ± SEM) (n = 6–7 per group)
Effect of chronic L-dopa on brain neurochemistry
To investigate the effects of chronic L-dopa on neurochemistry, brain regions were analyzed for monoamine tissue content. Rats that were administered chronic L-dopa treatment, with or without ascorbate, and underwent conditioned fear were killed 48 h after fear testing and brain regions were dissected and monoamine concentrations analyzed by HPLC-EC. Results indicated that chronic L-dopa significantly decreased 5-HT and 5-HIAA tissue content in the DRN and mPFC (Fig. 5a, b). A two-way ANOVA also revealed a significant interaction between L-dopa and ascorbate [F (1,23) = 4.95, p < 0.05], indicating that changes in 5-HT and 5-HIAA by L-dopa were dependent on ascorbate co-treatment. Specifically, the dorsal but not the ventral DRN was depleted in 5-HT (25 % decrease) and 5-HIAA (25 % decrease) (5-HT: q = 4.04, p < 0.05; 5-HIAA: q = 3.34, p < 0.05) after chronic L-dopa. These effects were completely blocked by ascorbate co-treatment (5-HT: q = 5.19, p < 0.05; 5-HIAA: q = 5.2, p < 0.05). The mPFC was similarly affected as there was a statistically significant interaction between L-dopa and ascorbate with regard to 5-HT and 5-HIAA tissue content [F (1,23) = 4.55, p < 0.05]. L-dopa treatment resulted in statistically significant reductions in 5-HT (22 % decrease) and 5-HIAA (26 % decrease) (5-HT: q = 3.95, p < 0.05; 5-HIAA: q = 5.74, p < 0.05) in the mPFC. While ascorbate alone had no effect on 5-HT or 5-HIAA tissue content (q = 0.57, p = 0.69), ascorbate blocked L-dopa-induced 5-HT and 5-HIAA deficits (5-HT: q = 3.95, p < 0.05; 5-HIAA: q = 5.74, p < 0.05). In addition, 5-HT levels in both the dorsal DRN and mPFC were negatively correlated with freezing behavior (dorsal DRN: r value = −0.44, p < 0.05; mPFC: r value = −0.47, p < 0.05) (Fig. 5c, d). There were no statistically significant treatment effects on 5-HT or 5-HIAA in the amygdala or hippocampus (Fig. 5a, b). Furthermore, norepinephrine as well as dopamine and its metabolites were not significantly different from controls after chronic L-dopa in all brain regions examined (Table 1).
Effects of chronic L-dopa on 5-HT and 5-HIAA tissue content by brain region in rats that underwent fear conditioning. a Chronic L-dopa significantly reduced 5-HT tissue content in the dorsal DRN and mPFC. This effect was blocked by ascorbate co-treatment. b 5-HIAA was similarly reduced in both dorsal DRN and mPFC, which was blocked by ascorbate co-treatment (*p < 0.05 compared to vehicle-vehicle treatment, two-way ANOVA and Tukey’s post hoc test; data represented as mean ± SEM) (n = 6–7 per group). c Graphical scatterplot showing a negative correlation between dorsal DRN 5-HT tissue content and freezing behavior of rats (r value = −0.44). d Graphical scatterplot showing a negative correlation between mPFC 5-HT tissue content and freezing behavior of rats (r value = −0.47). Each point on graph corresponds to a single rat. Rats from all treatment groups that underwent conditioned fear stress are represented (Veh/Veh, Ascorbate/Veh, Veh/L-dopa, Ascorbate/L-dopa). Both analyses had a significance value of p < 0.05, Pearson correlation
Discussion
The effect of chronic L-dopa on affective and cognitive behaviors, as well as corresponding brain monoamine tissue content, was investigated in the present study. It was found that chronic L-dopa impaired spatial memory and caused a potentiation of conditioned fear behavior in rats. There was also a corresponding significant decrease in 5-HT and its metabolite 5-HIAA in both the dorsal DRN and mPFC. The antioxidant ascorbate blocked the behavioral impairments as well as deficits in 5-HT and 5-HIAA caused by chronic L-dopa.
Initial experiments found that chronic L-dopa did not significantly affect behaviors during the forced swim (Fig. 1). This is consistent with previous studies that found no association between L-dopa treatment and impairments in the forced swim (Eskow Jaunarajs et al. 2010, 2012). The forced swim is generally used to investigate anti-depressant agents, and thus a decrease in immobility compared to controls is often measured. Therefore, one possible explanation for lack of L-dopa effects in the forced swim may be that the baseline immobility in control rats is the maximum or “ceiling” that can be measured in the assay. This point is partially supported by the fact that when rats were tested a second time (24 h after the first session) in the forced swim, vehicle-treated rats did not differ significantly in regard to immobility duration from test day 1 to test day 2 (data not shown). However, this is unlikely given that one study found that L-dopa is capable of increasing immobility duration significantly above controls during a two-session forced swim test, indicating enhanced depressive-like behavior in non-lesioned rats after high dose L-dopa for 60 days (Borah and Mohanakumar 2007). Therefore, the discrepancy between those findings with the current study may be the difference in the dose of L-dopa administered (250 mg/kg/day vs. 12 mg/kg/day) or treatment duration (60 days vs. 10 days). Overall, it appears that at lower and more therapeutically relevant doses of L-dopa, L-dopa treatment does not result in detectable alterations in regard to depressive-like behaviors within the forced swim test.
Spatial working memory impairment has been noted in L-dopa medicated PD patients (Owen et al. 1997); however, the effects of PD disease progression and L-dopa treatment on spatial memory have not been differentiated. Experiments conducted in the current study were aimed at delineating the effects of chronic L-dopa on spatial memory in rats. Chronic L-dopa caused an impairment of spatial memory, as latency to find the goal box within the Barnes maze was significantly increased during the recall trial (Fig. 2b). This suggests that chronic L-dopa imparts alterations in a brain area involved in spatial memory as noted by a deficit in recall of a previously learned spatial task. Both the mPFC and hippocampus have been implicated in spatial learning and memory (Cano de la Cuerda et al. 2010; Euston et al. 2012). Studies have demonstrated that remote memory recall requires activation of the mPFC (Tomita et al. 1999; Blum et al. 2006; Margis et al. 2010), an area that has been shown to contain deficits in 5-HT after chronic L-dopa (Navailles et al. 2011a; Eskow Jaunarajs et al. 2012; Stansley and Yamamoto 2014). Further, 5-HT in the mPFC has been shown to play a role in control of memory retrieval, as blockade of 5-HT2A receptors within the mPFC reduces object recognition memory retrieval (Sethi et al. 2010). In the current study, the impairment in spatial memory caused by L-dopa was completely blocked by ascorbate co-treatment, suggesting that oxidative mechanisms underlie this behavioral impairment. Interestingly, L-dopa-induced 5-HT tissue content deficits within the PFC have also been shown to be blocked by ascorbate co-treatment (Stansley and Yamamoto 2014). Therefore, it is likely that 5-HT related deficits in the PFC caused by L-dopa at least partially contribute to subsequent spatial memory impairments.
Symptoms of anxiety and fear are highly prevalent in the PD population; however, the contribution of L-dopa treatment in relation to these non-motor symptoms has not been extensively investigated. Therefore, we attempted to elucidate the effects of L-dopa on both innate anxiety-like/fear behavior using the elevated plus-maze test, and conditioned or learned anxiety-like/fear behavior using the conditioned fear stress paradigm. Rats treated chronically with L-dopa did not display any behavioral differences in the elevated plus-maze compared to controls (Fig. 3). However, L-dopa-treated rats exhibited potentiated freezing behavior during the conditioned fear testing when compared to vehicle-treated rats (Fig. 4). To our knowledge, this is the first pre-clinical report of increased context-induced fear behavior in the conditioned fear stress paradigm caused by chronic L-dopa. Moreover, the fact that ascorbate co-treatment blocked L-dopa-induced improvements of conditioned fear behaviors is significant because it suggests that similar to the spatial memory impairments, L-dopa-induced conditioned fear improvements may be associated with increased oxidative stress produced by the drug. A neurobiological mechanism of these behaviors may be related to the fact that L-dopa-treated rats that underwent fear behavior testing also had significant depletions in 5-HT in the dorsal DRN and mPFC. The 5-HT system is known to greatly influence fear conditioning (Bauer 2015). Moreover, the DRN and mPFC comprise part of a complex brain circuit that contributes to stress reactivity and emotional regulation of fear behaviors (Jasinska et al. 2012). Specifically, the dorsal DRN, which projects and receives dense innervations to and from the mPFC (Van Bockstaele et al. 1993; Amat et al. 2005) has been shown to be activated by a variety of anxiogenic drugs and stressors (Lowry et al. 2008). During conditioned fear stress, 5-HT is released in the amygdala and mPFC (Ducottet and Belzung 2005). However, the temporal differences of release suggest that 5-HT release in the amygdala occurs during acquisition of freezing behavior (Yokoyama et al. 2005), while 5-HT elevation in the mPFC corresponds to the reduction of freezing behavior (Hashimoto et al. 1999). Furthermore, it is known that the PFC exerts inhibitory influence over the amygdala to facilitate extinction of conditioned fear (Quirk et al. 2003; Milad et al. 2004). In the present study, 5-HT tissue content was unaffected in the amygdala, but decreased in the mPFC. Therefore, the potentiation of context-induced freezing behavior (e.g., fear response) could be due to a dysfunction of 5-HT signaling in the mPFC, leading to a lack of inhibition of amygdaloid output during conditioned fear testing. An alternative explanation for the increased context-induced fear behavior could be that increased amygdaloid output during the conditioning phase (day 1) could promote an enhancement of fear learning in L-dopa-treated rats. Therefore, it is somewhat unclear if chronic L-dopa improves fear learning or impairs fear extinction, or both, within this paradigm. Regardless, the notion that decreased mPFC 5-HT concentrations may produce an improvement in the conditioned fear stress is supported by the findings that 5-HT tissue concentrations within the mPFC were negatively correlated with freezing behavior (Fig. 5d), indicating that rats with less mPFC 5-HT exhibited more freezing behavior in the conditioned fear stress test.
It is important to note that a change in nociceptive threshold could affect the conditioned learning of fear. It is known that changes in brain 5-HT concentrations have been positively correlated with change in nociceptive threshold (Messing and Lytle 1977). Therefore, L-dopa treatment and nociceptive threshold may not be able to be separated in the current set of experiments where L-dopa is producing 5-HT deficits. In addition, acute L-dopa alone has been shown to provide analgesic effects and increase nociceptive threshold; however, benserazide co-treatment abolishes these effects (Shimizu et al. 2004). Furthermore, brain L-dopa concentrations should be near baseline levels at the time of behavioral testing. Therefore, in this study, the fact that benserazide was used in combination with L-dopa and behavioral testing began 2 days after the last L-dopa dose makes direct effects of L-dopa on nociception in these rats unlikely.
These conditioned fear stress findings are to some extent in contrast to a previous report (Haaker et al. 2013) that showed a single acute L-dopa dose of 20 mg/kg prevented reinstatement of fear behavior in mice that had underwent fear conditioning. The discrepancy between that report and the present study is the time-point of L-dopa treatment, given that in the current study, L-dopa was given for 10 consecutive days prior to conditioned fear stress, while Haaker et al. administered L-dopa immediately following fear extinction. The acute administration of L-dopa shortly after extinction of fear likely increased dopamine signaling in the mPFC and bolstered extinction memory formation in the mice. This differs from the current study because L-dopa was likely absent during all behavioral testing to permit the detection of any persistent effects of chronic L-dopa.
While the differential effects of L-dopa on behavior in the elevated plus-maze and the conditioned fear stress paradigm appear contradictory given that they both measure anxiety-like or fear behaviors, these differences may be explained by the fact that innate fear and conditioned fear behaviors are controlled by overlapping but essentially different brain circuitry. For example, activity of the prelimbic cortex has been shown to mediate conditioned fear but has no role in the expression of innate fear (Corcoran and Quirk 2007). Similarly, lesioning of amygdaloid nuclei has been shown to reduce conditioned fear behavior but does not affect fear behavior in response to predator odor (Wallace and Rosen 2001), supporting the notion that innate fear and conditioned fear behavior may occur through different neurological mechanisms. Furthermore, 5-HT neuron activation in the DRN has been shown to have opposing effects on conditioned or innate fear/anxiety behaviors (Maier et al. 1994; Graeff et al. 1996). Therefore, alterations in 5-HT caused by L-dopa may alter conditioned and unconditioned fear/anxiety differently, as suggested by the present study.
It is important to note that the dopaminergic system has been shown to be involved in cognitive and emotional behaviors (Morrow et al. 1999; Fadok et al. 2009). However, dopamine and norepinephrine were not altered by chronic L-dopa in this treatment paradigm (Table 1). Therefore, we conclude that 5-HT deficits likely contribute to the behavioral impairments. This notion is supported by the fact that ascorbate co-treatment rescues both the 5-HT deficits and behavioral impairments observed after chronic L-dopa.
The effects of ascorbate at preventing L-dopa-induced behavioral changes suggest that the mechanism by which L-dopa exerts these effects is pro-oxidative. Indeed, previous studies in our lab have shown that chronic L-dopa treatment results in 5-HT neuron loss within the dorsal DRN, as well as 5-HT neurotransmitter concentrations within the dorsal DRN and PFC brain tissue, effects which were blocked by ascorbate co-treatment (Stansley and Yamamoto 2014). Therefore, the behavioral changes observed after chronic L-dopa treatment in the current study may be attributable to the loss of 5-HT neurons and 5-HT tissue content, as blocking these deficits with ascorbate also blocks the behavioral alterations in spatial memory and conditioned fear response.
PD patients often exhibit a large number of non-motor symptoms that may be attributable to disease progression and/or therapeutic treatment (Choi et al. 2000; Garcia-Ruiz et al. 2014). The results presented here argue that L-dopa alone is capable of impairing cognitive and fear or anxiety-related behaviors, possibly through alterations in brain 5-HT. Indeed, cognitive impairment as well as fear of falling are prevalent in PD patients (Cumming et al. 2000; Owen 2004). These non-motor symptoms significantly impact quality of life and adjunctive therapies to prevent or mitigate impairments of cognition and fear by strengthening 5-HTergic tone would be beneficial.
References
Amat J, Baratta MV et al (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8(3):365–371
Arai R, Karasawa N et al (1995) L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett 195(3):195–198
Banerjee AK, Falkai PG et al (1989) Visual hallucinations in the elderly associated with the use of levodopa. Postgrad Med J 65(764):358–361
Bauer EP (2015) Serotonin in fear conditioning processes. Behav Brain Res 277:68–77
Berger M, Gray JA et al (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366
Blum S, Hebert AE et al (2006) A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport 17(3):341–344
Bonnet AM, Czernecki V (2013) Non-motor symptoms in Parkinson’s disease: cognition and behavior. Geriatr Psychol Neuropsychiatr Vieil 11(3):295–304
Borah A, Mohanakumar KP (2007) Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol 27(8):985–996
Braak H, Del Tredici K et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Cano de la Cuerda R, Vela L et al (2010) Quantitative measurement of axial rigidity, functional status and health-related quality of life in patients with Parkinson’s disease. Rev Neurol 51(4):193–200
Carta M, Carlsson T et al (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130(Pt 7):1819–1833
Ceravolo R, Frosini D et al (2009) Impulse control disorders in Parkinson’s disease: definition, epidemiology, risk factors, neurobiology and management. Parkinsonism Relat Disord 15(Suppl 4):S111–S115
Choi C, Sohn YH et al (2000) The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson’s disease. J Neurol Sci 172(1):12–16
Corcoran KA, Quirk GJ (2007) Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27(4):840–844
Cotzias GC (1968) L-Dopa for Parkinsonism. N Engl J Med 278(11):630
Cotzias GC, Papavasiliou PS et al (1969) Modification of Parkinsonism—chronic treatment with L-dopa. N Engl J Med 280(7):337–345
Cumming RG, Salkeld G et al (2000) Prospective study of the impact of fear of falling on activities of daily living, SF-36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci 55(5):M299–M305
Ducottet C, Belzung C (2005) Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res 156(1):153–162
Eskow Jaunarajs KL, Dupre KB et al (2010) Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav Pharmacol 21(7):627–637
Eskow Jaunarajs KL, Angoa-Perez M et al (2011) Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of l-DOPA treatment. Neurosci Biobehav Rev 35(3):556–564
Eskow Jaunarajs KL, George JA et al (2012) L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson’s disease. Neuroscience 218:243–256
Euston DR, Gruber AJ et al (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76(6):1057–1070
Fadok JP, Dickerson TM et al (2009) Dopamine is necessary for cue-dependent fear conditioning. J Neurosci 29(36):11089–11097
Garcia-Ruiz PJ, Chaudhuri KR et al (2014) Non-motor symptoms of Parkinson’s disease. A review from the past. J Neurol Sci 338(1–2):30–33
Graeff FG, Viana MB et al (1996) Opposed regulation by dorsal raphe nucleus 5-HT pathways of two types of fear in the elevated T-maze. Pharmacol Biochem Behav 53(1):171–177
Graham DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14(4):633–643
Haaker J, Gaburro S et al (2013) Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proc Natl Acad Sci U S A 110(26):E2428–E2436
Hashimoto S, Inoue T et al (1999) Effects of conditioned fear stress on serotonin neurotransmission and freezing behavior in rats. Eur J Pharmacol 378(1):23–30
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Hollister AS, Breese GR et al (1979) Role of monoamine neural systems in L-dihydroxyphenylalanine-stimulated activity. J Pharmacol Exp Ther 208(1):37–43
Huot P, Fox SH et al (2011) The serotonergic system in Parkinson’s disease. Prog Neurobiol 95(2):163–212
Izumi T, Ohmura Y et al (2012) Effects of serotonergic terminal lesion in the amygdala on conditioned fear and innate fear in rats. Eur J Pharmacol 696(1-3):89–95
Jasinska AJ, Lowry CA et al (2012) Serotonin transporter gene, stress and raphe-raphe interactions: a molecular mechanism of depression. Trends Neurosci 35(7):395–402
Kannari K, Yamato H et al (2001) Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem 76(5):1346–1353
Kim HJ, Park SY et al (2009) Nonmotor symptoms in de novo Parkinson disease before and after dopaminergic treatment. J Neurol Sci 287(1-2):200–204
Lowry CA, Johnson PL et al (2005) Modulation of anxiety circuits by serotonergic systems. Stress 8(4):233–246
Lowry CA, Hale MW et al (2008) Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci 1148:86–94
Maier SF, Watkins LR (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29(4–5):829–841
Maier SF, Kalman BA et al (1994) Chlordiazepoxide microinjected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behav Neurosci 108(1):121–130
Margis R, Donis KC et al (2010) WHOQOL-OLD assessment of quality of life in elderly patients with Parkinson’s disease: influence of sleep and depressive symptoms. Rev Bras Psiquiatr 32(2):125–131
Mayeux R, Stern Y et al (1981) Depression, intellectual impairment, and Parkinson disease. Neurology 31(6):645–650
Messing RB, Lytle LD (1977) Serotonin-containing neurons: their possible role in pain and analgesia. Pain 4(1):1–21
Milad MR, Vidal-Gonzalez I et al (2004) Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci 118(2):389–394
Miller DW, Abercrombie ED (1999) Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem 72(4):1516–1522
Monti JM (2011) Serotonin control of sleep-wake behavior. Sleep Med Rev 15(4):269–281
Morrow BA, Elsworth JD et al (1999) The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience 92(2):553–564
Navailles S, Bioulac B et al (2010) Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis 38(1):136–143
Navailles S, Bioulac B et al (2011a) Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis 41(2):585–590
Navailles S, Carta M et al (2011b) L-DOPA and serotonergic neurons: functional implication and therapeutic perspectives in Parkinson’s disease. Cent Nerv Syst Agents Med Chem 11(4):305–320
Navarro JF, Buron E et al (2006) Anxiolytic-like activity of SB-205384 in the elevated plus-maze test in mice. Psicothema 18(1):100–104
Ng LK, Chase TN et al (1972) L-dopa in Parkinsonism. A possible mechanism of action. Neurology 22(7):688–696
Owen AM (2004) Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 10(6):525–537
Owen AM, Iddon JL et al (1997) Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia 35(4):519–532
Peters M, Fitzpatrick R et al (2011) Does self-reported well-being of patients with Parkinson’s disease influence caregiver strain and quality of life? Parkinsonism Relat Disord 17(5):348–352
Post B, Muslimovic D et al (2011) Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson’s disease. Mov Disord 26(3):449–456
Quirk GJ, Likhtik E et al (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23(25):8800–8807
Richard IH, Frank S et al (2004) The ups and downs of Parkinson disease: a prospective study of mood and anxiety fluctuations. Cogn Behav Neurol 17(4):201–207
Rodgers RJ (1991) A step in the right direction: comment on ‘5-HT and mechanisms of defence’. J Psychopharmacol 5(4):316–319
Roland KP, Jakobi JM et al (2012) Quality of life as a determinant of frailty phenotype in community-dwelling persons with Parkinson’s disease. J Am Geriatr Soc 60(3):590–592
Sethi K (2008) Levodopa unresponsive symptoms in Parkinson disease. Mov Disord 23(Suppl 3):S521–S533
Sethi K, Factor S et al (2010) Quality of life in Parkinson’s disease patients following adjunctive tolcapone therapy: results of an open-label, multicenter, community-based trial. CNS Spectr 15(1):27–32
Shimizu T, Iwata S et al (2004) Antinociceptive mechanism of L-DOPA. Pain 110(1–2):246–249
Simpson J, Lekwuwa G et al (2014) Predictors of quality of life in people with Parkinson’s disease: evidence for both domain specific and general relationships. Disabil Rehabil 36(23):1964–1970
Soh SE, McGinley J et al (2011) Measuring quality of life in Parkinson’s disease: selection of-an-appropriate health-related quality of life instrument. Physiotherapy 97(1):83–89
Stansley BJ, Yamamoto BK (2013) L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 67:243–251
Stansley BJ, Yamamoto BK (2014) Chronic L-dopa decreases serotonin neurons in a subregion of the dorsal raphe nucleus. J Pharmacol Exp Ther 351(2):440–447
Tew EH, Naismith SL et al (2013) Quality of life in Parkinson’s disease caregivers: the contribution of personality traits. Biomed Res Int 2013:151872
Tomita H, Ohbayashi M et al (1999) Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401(6754):699–703
Van Bockstaele EJ, Biswas A et al (1993) Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 624(1–2):188–198
Wallace KJ, Rosen JB (2001) Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci 21(10):3619–3627
Winter Y, von Campenhausen S et al (2011) Health-related quality of life and its determinants in Parkinson’s disease: results of an Italian cohort study. Parkinsonism Relat Disord 17(4):265–269
Yokoyama M, Suzuki E et al (2005) Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett 379(1):37–41
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research supported by NIDA: DA007606
Rights and permissions
About this article
Cite this article
Stansley, B.J., Yamamoto, B.K. Behavioral impairments and serotonin reductions in rats after chronic L-dopa. Psychopharmacology 232, 3203–3213 (2015). https://doi.org/10.1007/s00213-015-3980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3980-4