Abstract
Rationale
Mitragynine (MG) is the primary active alkaloid extracted from the leaves of Mitragyna speciosa or kratom and exhibits pharmacological activities mediated by opioid receptors. The plant has been traditionally used for its opium and psychostimulant-like effects to increase work efficiency or as a substitute in the self-treatment of opiate addiction.
Objectives
The present study was performed to investigate the discriminative stimulus effects of MG in rats. The pharmacological mechanism of MG action and its derivative, 7-hydroxymitragynine (7-HMG) with a specific focus on opioid receptor involvement was examined in rats trained to discriminate morphine from vehicle. In order to study the dual actions of MG, the effect of cocaine substitution to the MG discriminative stimulus was also performed in MG-trained rats.
Methods
Male Sprague Dawley rats were trained to discriminate MG from vehicle in a two-lever drug discrimination procedure under a tandem variable-interval (VI 60’) fixed-ratio (FR 10) schedule of food reinforcement.
Results
Rats acquired the MG discrimination (15.0 mg/kg, i.p.) which was similar to the acquisition of morphine discrimination (5.0 mg/kg, i.p.) in another group of rats. MG substituted fully to the morphine discriminative stimulus in a dose-dependent manner, suggesting pharmacological similarities between the two drugs. The administration of 7-HMG derivative in 3.0 mg/kg (i.p.) dose engendered full generalisation to the morphine discriminative stimulus. In addition, the MG stimulus also partially generalised to cocaine (10.0 mg/kg, i.p.) stimulus.
Conclusion
The present study demonstrates that the discriminative stimulus effect of MG possesses both opioid- and psychostimulant-like subjective effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitragyna speciosa belongs to the family of Rubiaceae and is found in tropical and sub-tropical regions of Asia (Hassan et al. 2013). It is commonly known as ‘biak-biak’ or ‘ketum’ in Malaysia and ‘kratom’ in Thailand. M. speciosa leaves have been traditionally used by locals for their opium-like effect and psychostimulant-like ability to combat fatigue and pain and to boost tolerance to work under intense sunlight (Reanmongkol et al. 2007). In addition, it has also been used as an alternative treatment for fever, malaria, hypertension and cough and as a substitution therapy of opiate addiction (Assanangkornchai et al. 2007; Chan et al. 2005).
Mitragynine (MG) is the main active alkaloid which accounts for 66 % of the total alkaloid contents extracted from the leaves of M. speciosa Korth (Shellard 1974; Ponglux et al. 1994). There are many other alkaloids which are structurally related to MG that includes 7-hydroxymitragynine (7-HMG), speciogynine, speciociliatine and paynantheine (Takayama 2004). For this reason, MG is assumed to be the major chemical responsible for the effects of the plant. Thus, there has been an increased interest in characterising the chemical, toxicological and pharmacological properties of MG in recent years (Matsumoto et al. 1996a; Matsumoto et al. 2005b; Reanmongkol et al. 2007; Takayama 2004; Takayama et al. 2002; Jansen and Prast 1988; Azizi et al. 2010; Janchawee et al. 2007; Macko et al. 1972; Shellard 1974). Many scientific reports have indicated that MG produces a variety of pharmacologic effects, both in vivo and in vitro. The findings show that MG can elicit analgesic effects by acting on opioid systems (Matsumoto et al. 1996a; Stolt et al. 2014). Other physiological effects include the inhibition of ileum motility (Watanabe et al. 1997) and vas deferens contractions of smooth muscle (Matsumoto et al. 2005b), as well as the inhibition of gastric acid secretion (Tsuchiya et al. 2002), which is consistent with the actions of morphine. In addition, studies with opioid receptor antagonists indicate that the effects are primarily mediated by the actions on opioid receptors (Matsumoto et al. 2005b; Watanabe et al. 1997).
However, the anti-nociceptive effect of MG was reported to be less potent than that of the crude extract of the plant (Watanabe et al. 1997; Yamamoto et al. 1999; Matsumoto et al. 2006). Thus, the opium-like effect of the plant extract cannot fully explain the effects of MG alone. This suggests that other constituents of M. speciosa may contribute to the opium-like effect, which may involve 7-HMG, a minor constituent of the leaves and an oxidised derivative of MG (Ponglux et al., 1994). This claim is supported by evidence that 7-HMG is relatively more potent than MG and morphine (Matsumoto et al. 2004; Takayama 2004). In addition, a study from Matsumoto et al. (2006) reported that 7-HMG demonstrated full agonist properties on μ-opioid receptors in vitro (Matsumoto et al. 2006). Taken together, MG and its 7-HMG derivative were reported to act on the central nervous system (CNS) and to exhibit a high affinity towards μ-opioid receptor subtype (Yamamoto et al. 1999; Matsumoto et al. 2006).
However, the psychoactive properties of M. speciosa appear to be contradictory as there are reports claiming it possesses both narcotic and stimulant properties (Assanangkornchai et al. 2007; Macko et al. 1972; Ward et al. 2011; Grewal 1932a, b; Suwanlert 1975, Reanmongkol et al. 2007). The pharmacology of MG was first studied by Grewal (1932a, b) in which a series of experiments were performed in cats and in a group of five male volunteers. These studies revealed that MG possesses a central nervous stimulant effect that resembled actions similar to cocaine (Grewal 1932a, b). The stimulatory effects were also observed with large doses of MG administered to cats which were qualitatively different from opiates (Macko et al. 1972).
Interestingly, clinical trials have reported that the effects of kratom are dose-dependent with lower doses resulting in stimulant effects while higher doses produced opioid-like effects (Suwanlert 1975; Grewal 1932b; Jansen and Prast 1988). This suggests that kratom produces an unusual combination of opioid- and psychostimulant-like effects. Although the profile of MG is unique and not well characterised compared to psychostimulants or opioids, it nevertheless shares some common properties with these drugs. However, the pharmacological basis underlying the dual effects of MG is still unclear.
The stimulant and euphoric effects exerted by kratom caused the plant to be extensively misused as a herbal drug. The earliest use of kratom in Malaysia was reported in 1836 when it was used as a substitute for opium (Burkill 1935). The use of the substance for the treatment of opium dependence emerged in Malaysia and Thailand during the nineteenth century (Burkill 1935).
Recent surveys have shown that besides improving physical effort and endurance, kratom was used for substitution to compensate for more expensive opiates (Ahmad and Aziz 2012; Vicknasingam et al. 2010; Singh et al. 2014). Therefore, kratom is widely used as a recreational drug and can lead to abuse. Kratom abuse has gained significant attention in Malaysia and Thailand (Chan et al. 2005). Furthermore, the wide availability of kratom to purchase through the internet reflects an increasing demand of misusing this herbal product globally (Boyer et al., 2008).
The likelihood that a drug will be abused is generally based on the subjective effects produced by the drug (Schuster et al. 1981). Drug discrimination in laboratory animals is used as a model to study the subjective effects of psychoactive drugs since the action of a drug’s discriminative stimulus in animals is closely related to its subjective effects in human (Overton 1988). Given that the action of MG resembles morphine-like effects, we hypothesised that MG may share discriminative stimulus properties with morphine. Thus, the present study aimed to investigate the pharmacology of the discriminative stimulus properties of MG in rats. To gain a better understanding on the pharmacological similarities shared between MG and morphine, a similar series of generalisation tests were conducted in rats trained to discriminate morphine from vehicle.
Materials and methods
Animals
Male Sprague Dawley rats (n = 20) weighing 200–250 g at the start of the experiment were used. Rats were housed in groups of 6 rats per cage and acclimatised to the housing conditions. The rats were handled for 3–4 days prior to the start of the experiment to minimise handling stress. Rats were maintained on a 12-h light/dark cycle and had free access to water, but were gradually food restricted to approximately 85 % of their free-feeding weight (15 g/day for each rat) before training began. All experimental procedures complied with local ethical requirements and were carried out according to the guidelines for the use of experimental animals and approved by Animal Ethics Committee, Universiti Sains Malaysia (AECUSM).
Apparatus
The experiment utilised standard two-lever operant conditioning chambers (Med-Associates, VT, US). The operant conditioning chambers were enclosed in sound attenuating cubicles. Each cubicle was equipped with an overhead house light to provide general illumination and a fan to provide ventilation and to mask extraneous sounds. The operant panel of each chamber consisted of two levers (4.5 cm wide, extending 2.5 cm from the wall and located 7.0 cm above the floor) located equidistant from a food receptacle where a pellet dispenser delivered food pellets (45-mg pellets). The house light was on during food availability, and it was turned off during food presentation and time-out periods. The operant chambers were controlled by a computer using the MED-PC software package (MED-Associates, Vermont, US).
Drugs
Mitragynine (MG) was extracted, isolated and verified from fresh leaves of M. speciosa at the Malaysian Institute of Pharmaceuticals and Neutraceuticals, Universiti Sains Malaysia as described previously (Utar et al. 2011). 7-Hydroxymitragynine (7-HMG) was hydrolysed from MG in our laboratory. The hydrolysis was adapted from that of Ishikawa et al. (2002), resulting in purity of >99 % after confirmation by GCMS and NMR (500 MHz) analysis, which is similar to the purity of 7-HMG previously reported (Ishikawa et al. 2002). These compounds were kept at 4 °C until the time of experiment. Morphine hydrochloride and cocaine hydrochloride were purchased from Pharmaniaga Logistics SDN BHD (Malaysia). Naloxone hydrochloride was obtained from Sigma Chemical Co. (USA). All drugs were dissolved in physiological saline (0.9 % NaCI) with 20 % (v/v) Tween-80 (poly-oxyethylenesorbitanmonooleate, Sigma-Aldrich Co.) and diluted to the desired concentration prior to the experiment. The drugs were injected in a volume of 1.0 ml/kg per body weight, and were administered intraperitoneally (i.p.).
Drug discrimination procedure
Acquisition of mitragynine and morphine baseline discrimination
The drug discrimination training procedure was adapted from those described previously for rats (Stolerman et al. 1989; Wing and Shoaib 2012). Initially, rats were trained in 15-min daily sessions to press levers for food delivered on a fixed-ratio (FR) schedule. Responding was established on both levers but only one lever was present at a time by producing a single 45-mg food pellet. Under a FR schedule, the rat must complete a fixed number of responses in order to obtain reinforcement. The FR value was gradually increased from FR-1 to FR-10. Once animals exhibited stable levels of responding under FR-10, a variable-interval (VI) component was introduced. Under this tandem schedule of reinforcement, the tenth lever press was reinforced after a randomly determined variable interval of time. The VI schedule was gradually increased from VI-5 s until the terminal of VI-30-s schedule. After this period of shaping over a few sessions, the drug discrimination training began.
The rats were randomly allocated to two groups (n = 10) for each training drug. One group was injected with MG (15.0 mg/kg, i.p.) or vehicle (20 % Tween 80 diluted in 0.9 % saline) 30 min prior to the start of sessions. The dose of MG was selected from previous studies assessing the effect of 15.0 mg/kg i.p. that resulted in the greatest pharmacological effects (Apryani et al. 2010; Farah Idayu et al. 2011; Fakurazi et al. 2013). In the other group, rats were injected with morphine (5.0 mg/kg, i.p.) or vehicle (20 % Tween 80 diluted in 0.9 % saline) 30 min prior to the training sessions. The morphine dose (5.0 mg/kg i.p.) was selected from previous reports (Bartoletti et al. 2010). In half of the animals, operant responses on the right lever were reinforced by food presentation following MG injection, and operant responses on the left lever were reinforced following vehicle injection. The allocation of the levers was reversed in the other half of the animals. MG and vehicle training sessions took place in a random order. After several weeks of training, the duration of the VI component of the schedule was increased up to VI-60. That is, the rats received food pellet reinforcement after pressing the correct lever under the FR-10 and VI-60 schedules, with no reinforcement for the wrong lever presses.
After the rats had acquired the MG discrimination as shown by performance of 80 % accuracy on lever selection in a block of 10 consecutive sessions, test sessions took place twice weekly. Two training sessions took place between each test session to prevent extinction of the discrimination learning. In contrast to the training sessions, test sessions were 5 min in length, rats had access to both levers and responses did not result in food presentation. For each animal, a test session was run only if, during the two most recent training sessions, at least 80 % of total responses were made on the correct lever. For all rats, tests with drug treatments were conducted in a randomised order while substitution tests were completed prior to antagonism tests.
Dose-response evaluations of mitragynine- and morphine-associated discrimination
To evaluate dose-response effects for MG- and morphine-induced discrimination, a dose range for MG (0, 0.3, 1.0, 3.0, 10.0 and 15.0 mg/kg) and morphine (0, 0.16, 0.5, 1.6 and 5.0 mg/kg) were tested in animals corresponding to each group. MG and morphine dose-response curves were also determined prior to testing each compound at the end of the series of experiment to ensure the stability of both MG and morphine discrimination.
Effect of substitution of morphine- on mitragynine-associated discrimination
To evaluate substitution of morphine in MG-trained rats, three doses of morphine (0.5, 1.6 and 5.0 mg/kg) were tested after MG-induced discrimination (15.0 mg/kg) was achieved. This test allowed the determination of whether morphine could substitute for the MG discriminative stimulus.
Full substitution was defined when the percentage of drug-lever responding was greater or equal to 80 %, while partial substitution was defined when the percentage of drug-lever responses was greater than or equal to 20 % but less than 80 %. Percentages of drug-lever responding that were less than or equal to 20 % were considered as vehicle-appropriate responding.
Effect of substitution of mitragynine- on morphine-associated discrimination
Substitution of MG to the morphine-induced discrimination was determined next. Following the acquisition of morphine discrimination (5.0 mg/kg), the animals were pretreated with various doses of MG (1.0, 3.0, 15.0 and 30.0 mg/kg). This was to establish whether MG could substitute for morphine to maintain the discriminative effects previously associated with morphine.
Effect of substitution of 7-Hydroxymitragynine on morphine-associated discrimination
The substitution effect of a derivative of MG, 7-HMG, on the discriminative stimulus effects of morphine (5.0 mg/kg) was also investigated. Three doses of 7-HMG ranging from 0.3 to 3.0 mg/kg were injected 30 min prior to the test.
Effect of substitution of cocaine (psychostimulant) on mitragynine-associated discrimination
To assess the effect of cocaine substitution to the MG-induced discriminative stimulus, rats that were initially trained on MG (15.0 mg/kg) were injected with one of two doses of cocaine (5.0 and 10.0 mg/kg) administered 15 min prior to the test.
Effect of substitution of cocaine (psychostimulant) on morphine-associated discrimination
The effect of cocaine substitution to the morphine-induced discriminative stimulus was also determined. Rats that were initially trained on morphine (5.0 mg/kg) were also injected with one of two doses of cocaine (5.0 and 10.0 mg/kg).
Effect of naloxone pretreatment on the discriminative stimulus effects of mitragynine and morphine
To investigate the ability of a pharmacological blocking agent to block the internal cue generated by the training drug, antagonism tests were carried out. In groups reliably discriminating MG or morphine, pretreatment tests with naloxone (0.1, 0.3 and 1.0 mg/kg) administered 15 min prior to the training doses were conducted.
Complete antagonism of the discriminative stimulus was occurred when the percentage of drug-lever selection decreased to vehicle-appropriate response levels (≤20 % drug-lever responding) while partial antagonism occurred when the percentage of drug-lever selection was between 20 and 80 %. No antagonism occurred when the response level was greater or equal to 80 % of drug-lever responding.
Effect of naloxone pretreatment on 7-Hydroxymitragynine substitution on morphine-associated discrimination
Animals that achieved full substitution (≥80 % of morphine-paired lever responding) when pretreated with 7-HMG were tested for opioid receptor involvement by pretreatment with naloxone (0.3 mg/kg) prior to discrimination testing.
Data analysis
For each rat, the number of responses made on each lever during extinction tests was recorded for the whole 5-min test session. The percentage of total responses made on the drug-appropriate lever served as a measure for stimulus generalisation/substitution, while the total number of responses served as a measure for the response rate. A lever was defined as ‘selected’ when it was the first lever that the rats pressed ten times. Subjects not demonstrating good stimulus control, as defined by an at least 80 % difference between drug- and vehicle-appropriate responding before the dose-response test, were excluded from analyses for that treatment regimen.
Analyses were conducted in SPSS Version 20.0 (SPSS Inc., Chicago, IL, US). One-way ANOVAs for repeated measures were conducted followed by Bonferroni post hoc tests to establish if a particular drug treatment had a significant effect on the percentage of drug-appropriate lever and total responses. ED50 and 95 % confidence intervals (95 % CI) of the dose-response curves of MG, morphine and 7-HMG were determined using GraphPad Prism 5.0 software. Antagonist functions prior to vehicle and naloxone pretreatment were compared by two-tailed Student’s t test for comparison of two groups. GraphPad Prism 5.0 software was used for graphical presentation of processed data. The data are expressed as means ± SEM. A p value of less than 0.05 was the critical criterion for statistical significance.
Results
Mitragynine and morphine produced discriminative stimulating effects
Figure 1 shows the acquisition of the two groups in which the correct lever selection following MG (Fig. 1a) or morphine (Fig. 1b) and vehicle. Tests under extinction with the training dose and vehicle provided an index of stimulus control with MG (Fig. 1c) and morphine (Fig. 1d). A total of 16 out of 20 rats trained (n = 7 for the MG group and n = 9 for the morphine group) reached the 80 % criterion for accuracy and were used for subsequent tests. Approximately 100 trials of training (50 MG and 50 vehicle sessions) were required before the rats could discriminate MG from vehicle. The group of rats trained to discriminate morphine from vehicle required fewer training trials, i.e. approximately 60 training sessions in total (30 morphine and 30 vehicle). Figure 1c illustrates the percentage of drug-paired lever selections after MG (15.0 mg/kg, i.p.) and vehicle administration, indicating that MG produced discriminative stimulus effects as assessed by over 80 % correct lever responding after being paired with MG injection. Subsequent experiments were carried out with the rats that met this criterion. As shown in Fig. 1d, the percentage of responses on the drug lever was comparable to the level of discrimination with morphine (5.0 mg/kg, i.p.) and vehicle. Robust discriminative stimulus effects were demonstrated for the MG-trained [F(6,7) = 3.4, p < 0.0001] and morphine-trained [F (8,8) = 1.1, p < 0.001] groups.
a Training data for acquisition of MG (15.0 mg/kg i.p.) as a discriminative stimulus. Each block represents the mean ± SEM accuracy of drug-lever selection of 10 trials (sessions) of MG (closed bars) or vehicle (open bars). b Training data for acquisition of morphine (5.0 mg/kg i.p.) as a discriminative stimulus. Each block represents the mean ± SEM accuracy of drug-lever selection of 10 trials (sessions) of morphine (closed bars) or vehicle (open bars). The training data for MG and morphine acquisition were obtained in 15-min training sessions. c Percentage of drug-lever selection during tests for stimulus control exerted by MG under extinction conditions. Each block represents the mean ± SEM of MG (closed bar) or vehicle (open bar) extinction tests. d Percentage of drug-lever selection during test for stimulus control exerted by morphine under extinction conditions. Each block represents the mean ± SEM of morphine (closed bar) or vehicle (open bar) extinction tests. The data for stimulus test of MG and morphine were obtained in 5-min extinction tests
Generalisation tests with graded doses of mitragynine and morphine
The results of the stimulus generalisation tests with a range of MG doses are shown in Fig. 2a. There was a dose-dependent generalisation with graded doses of MG on responses on the MG-paired lever ranging from 20 % (0 mg/kg MG) to a maximum of 90 % at 15.0 mg/kg training dose [F(5,30) = 12.3, p < 0.001]. Likewise, as shown in Fig. 2b, graded doses of morphine produced dose-related increases in the percentage of responses on the morphine-appropriate lever [F(4,32) = 31.0, p < 0.0001]. For both compounds, these significant levels of generalisation were observed without any appreciable reductions on total lever-press responses [Fig. 2; MG F(5,30) = 1.1, n.s; morphine F(4,32) = 0.45, n.s.].
a Dose-response curve in rats trained to discriminate 15.0 mg/kg MG from vehicle. Top panel shows the percentage of responses on the MG-paired lever (mean ± SEM, n = 7). Bottom panel shows the total number of responses (mean ± SEM, n = 7). b Dose-response curve in rats trained to discriminate 5.0 mg/kg morphine from vehicle. Top panel shows the percentage of responses on the morphine-paired lever (mean ± SEM, n = 9). Bottom panel shows the total number of responses (mean ± SEM, n = 9)
Morphine substitution tests in mitragynine-trained rats
The results from morphine substitution tests in the MG-trained rats are illustrated in Fig. 3a. Graded doses of morphine (0.5, 1.6 and 5.0 mg/kg) substituted for the MG discriminative stimulus [F(3,15) = 6.2, p < 0.001]. The highest dose of morphine (5.0 mg/kg) engendered a maximum of 85 % MG-lever responding (p < 0.001) without significantly modifying the total number of lever-press responses [F(3,15) = 0.36, n.s.]. The ED50 (95 % CI) of morphine substitution to mitragynine was 0.94 (0.45–1.97) mg/kg.
a Morphine substitution (black down-pointing triangle) maintained discriminative behaviour in rats previously treated with 15.0 mg/kg MG. Top panel shows the percentage of responses on the MG-paired lever (mean ± SEM, n = 6). Bottom panel shows the total number of responses (mean ± SEM, n = 6). ( ) represents baseline response of MG training dose (15.0 mg/kg) and (
) represents baseline response of MG training dose (15.0 mg/kg) and ( ) represents baseline response of vehicle. b MG substitution (black up-pointing triangle) for morphine maintained drug discriminative behaviour in rats previously trained with 5.0 mg/kg morphine. Top panel shows the percentage of responses on the morphine-paired lever (mean ± SEM, n = 7). Bottom panel shows the total number of responses (mean ± SEM, n = 7). Black diamond suit represents baseline response of morphine training dose (5.0 mg/kg), and (
) represents baseline response of vehicle. b MG substitution (black up-pointing triangle) for morphine maintained drug discriminative behaviour in rats previously trained with 5.0 mg/kg morphine. Top panel shows the percentage of responses on the morphine-paired lever (mean ± SEM, n = 7). Bottom panel shows the total number of responses (mean ± SEM, n = 7). Black diamond suit represents baseline response of morphine training dose (5.0 mg/kg), and ( ) represents baseline response of vehicle. *p < 0.05; ***p < 0.001 compared with the baseline response of vehicle
) represents baseline response of vehicle. *p < 0.05; ***p < 0.001 compared with the baseline response of vehicle
Mitragynine substitution tests in morphine-trained rats
The results of MG substitution tests in morphine-treated rats are shown in Fig. 3b. Tests with several doses of MG (1.0, 3.0, 15.0 and 30.0 mg/kg) produced dose-dependent substitution for the morphine training dose [F(4,28) = 18.8, p < 0.0001]. The data shows that 15.0 mg/kg MG substitution produced full substitution for morphine and demonstrated the highest responding (82 %) to the morphine-appropriate lever compared to vehicle (p < 0.001). As shown in the top panel of Fig. 3b, the largest dose of MG (30.0 mg/kg) showed partial substitution to the morphine stimulus resulting in only 55 % morphine-lever responding compared to vehicle (p < 0.05). However, the overall total number of responses showed no significant difference between the doses [F(4,28) = 2.1, n.s.]. The ED50 (95 % CI) of MG substitution to morphine was 7.61 (4.68–12.37) mg/kg.
7-Hydroxymitragynine substitution tests in morphine-trained rats
Figure 4 illustrates the results from substitution tests with 7-HMG (0.3, 1.0 and 3.0 mg/kg) in rats trained to discriminate morphine (5.0 mg/kg). The 7-HMG derivative produced a dose-dependent substitution [F(3,21) = 15.8, p < 0.0001] in which more than 80 % morphine-lever selection was obtained at the dose of 3.0 mg/kg (p < 0.0001). Furthermore, in a dose of 1.0 mg/kg 7-HMG also substituted for the morphine discriminative stimulus compared to vehicle (p < 0.0001). As shown in the bottom panel of Fig. 4, the substitution of 7-HMG to morphine was not associated with alterations in the total responses [F(3,21) = 1.63, n.s.]. The ED50 (95 % CI) of 7-HMG substitution was 0.60 (0.34–1.07) mg/kg. The relative potencies of 7-HMG to morphine and MG were 1.9 and 12.7 times, respectively, as shown in Table 1.
7-HMG substitution ( ) for morphine maintained drug discriminative behaviour in rats previously treated with 5.0 mg/kg morphine. Top panel shows the percentage of responses on the morphine-paired lever (mean ± SEM, n = 7). Bottom panel shows the total number of responses (mean ± SEM, n = 7). Black diamond suit represents baseline response of morphine training dose (5.0 mg/kg), and (
) for morphine maintained drug discriminative behaviour in rats previously treated with 5.0 mg/kg morphine. Top panel shows the percentage of responses on the morphine-paired lever (mean ± SEM, n = 7). Bottom panel shows the total number of responses (mean ± SEM, n = 7). Black diamond suit represents baseline response of morphine training dose (5.0 mg/kg), and ( ) represents baseline response of vehicle. ***p < 0.001 compared with the baseline response of vehicle
) represents baseline response of vehicle. ***p < 0.001 compared with the baseline response of vehicle
Cocaine substitution tests for mitragynine-trained rats
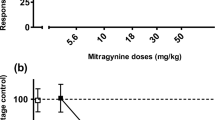
Table 2 shows that cocaine produced a significant level of partial substitution in rats trained on MG [F(3,15) = 14.3, p < 0.0001]. The highest dose of cocaine (10.0 mg/kg) produced significant substitution, i.e. about 65 % MG-lever response levels compared to vehicle (p < 0.05). However, cocaine in a dose of 5.0 mg/kg did not substitute for MG. Only the largest dose of cocaine (10.0 mg/kg) produced a significant reduction in the total lever-press responses compared to vehicle [F(3, 15) = 7.84, p < 0.002].
Cocaine substitution tests for morphine-trained rats
In two doses, cocaine (5.0 and 10.0 mg/kg, i.p.) did not substitute for morphine in rats that showed robust levels of stimulus control with morphine [F(3,18) = 89.7, p < 0.0001]. The percentage of substitution following cocaine was less than 20 % on the morphine lever, and no reductions were observed in total responses under these test conditions [F(3,18) = 1.85, n.s.].
Effect of naloxone pretreatment on discriminative stimulus effects of mitragynine and morphine
Naloxone was examined to investigate the role of opioid receptors in the discriminative stimulus effects of MG. Antagonism tests with a range of naloxone doses (0.1, 0.3 and 1.0 mg/kg) was conducted in both MG- and morphine-trained rats, and the results showed that 0.3 mg/kg naloxone produced optimal levels of blockade of the morphine discriminative stimulus (Fig. 5). As shown in Fig. 5, 0.3 mg/kg dose of naloxone showed a tendency towards a partial block of the MG stimulus, i.e. down to 43 % of MG-lever; however, this effect was not significant. Naloxone in a dose of 0.3 mg/kg fully blocked the morphine discriminative stimulus (p < 0.01) without producing any alterations in total responses.
a Effect of a range of naloxone doses (0.1, 0.3 and 1.0 mg/kg) on discriminative stimulus effects of 15.0 mg/kg MG (n = 6). Each bar represents the mean ± SEM of doses of naloxone pretreatment on vehicle discrimination (open bars) or on MG discrimination (closed bars). Top panel shows the percentage of responses on the drug-paired lever. Bottom panel shows the total number of responses. b Effect of a range of naloxone doses (0.1, 0.3 and 1.0 mg/kg) pretreatment on discriminative stimulus effects of 5.0 mg/kg morphine (n = 7). Each bar represents the mean ± SEM of doses of naloxone pretreatment on vehicle discrimination (open bars) or on morphine discrimination (closed bars). Top panel shows the percentage of responses on the drug-paired lever. Bottom panel shows the total number of responses. **p < 0.01, compared with the 0 mg/kg naloxone (vehicle) pretreatment on respective drugs conditions
Effect of 0.3 mg/kg naloxone pretreatment on discriminative stimulus of 3.0 mg/kg morphine-like effects of 7-HMG (n = 6). Each bar represents the mean ± SEM of 0 mg/kg naloxone/vehicle (open bars) or 0.3 mg/kg naloxone (closed bars) pretreatment on morphine-like effect of 7-HMG group. Top panel shows the percentage of responses on the drug-paired lever. Bottom panel shows the total number of responses. **p < 0.01 compared with the 0 mg/kg naloxone (vehicle) pretreatment on morphine-like effect of 7-HMG
Effect of naloxone pretreatment on discriminative stimulus effects of 7-Hydroxymitragynine
Of the naloxone doses tested, the 0.3 mg/kg dose of naloxone was selected to block the morphine-like discriminative stimulus effects of 7-HMG. As shown in Fig. 6, naloxone blocked the substitution effects of 7-HMG to the morphine discriminative stimulus (p < 0.01). It is worth noting that the decrease of morphine-like effects of 7-HMG after naloxone pretreatment was not associated with alterations in total responses.
Discussion
Drug discrimination procedures are valuable assays for studying behavioural and pharmacological effects of drugs (Overton 1988; Sevak et al. 2009; Stolerman et al. 1999; Su et al. 2008). The present study is the first to demonstrate that MG can serve as a discriminative stimulus in rats. MG produced a robust discriminative stimulus with at least 80 % accuracy by the majority of rats trained with 15.0 mg/kg dose. Given that the discriminative stimulus effect was characterised with only one dose, this may limit the full pharmacological characterisation.
As observed for morphine, MG showed a characteristic dose-dependent generalisation curve when a range of MG doses were tested. Given the similarities, cross-generalisation studies demonstrated MG to fully substitute for the morphine stimulus, suggesting similar subjective effects between the two drugs. This suggests that the effects could possibly be transduced via similar mechanisms that may involve, in part, the opioid receptor systems (Matsumoto 1996a; Boyer et al. 2008; Shamima et al. 2012; Stolt et al. 2014).
The subjective effects of psychoactive drugs are believed to play a role in their abuse potential (Preston and Bigelow 1991). Given that MG is the major constituent of kratom, the present study provides scientific evidence on the abuse potential of kratom since drugs that show substitution in drug discrimination models tend to exert similar subjective effects in humans (Sevak et al. 2009). The ED50 value of MG substitution for morphine was 7.61 mg/kg, which was higher than that of morphine substitution for MG (0.94 mg/kg). This suggests that MG can produce morphine-like subjective effects in humans but with lower abuse potential than morphine. Previous reports suggest that kratom has weaker, short-lasting effects and induces a milder withdrawal syndrome compared to morphine (Jansen and Prast 1988; Norakanphadung 1966). This is not surprising since kratom has been used as a substitution for opium addiction for decades (Ahmad and Aziz 2012; Burkill 1935; Vicknasingam et al. 2010; Wray 1907). In addition, kratom has become more popular in Thailand as a replacement for morphine to assist addicts during detoxification in treatment programmes (Norakanphadung 1966; Jansen and Prast 1988). From a clinical perspective, understanding the behavioural effects of substitution between the two drugs is relevant because cross-substitution among opiate addicts is a major concern. Recent surveys (Vicknasingam et al. 2010; Singh et al. 2014) have reported that almost 77 % of kratom users were associated with previous drug history, particularly morphine and heroin. The users also reported that kratom consumption is capable of reducing their intake of more expensive opiates and managing the opioid withdrawal symptoms. Besides, kratom itself is cheaper and easier to obtain than heroin (Vicknasingam et al. 2010). However, as the present study only employed the substitution between MG and morphine discriminative stimuli, further verification is required to support kratom abuse liability, as well as the use of kratom in the treatment of opioid dependence.
In the present study, the opioid-like effect of 7-HMG was also examined in rats trained to discriminate morphine from vehicle. The administration of the 7-HMG derivative showed a dose-dependent substitution for the morphine discriminative stimulus. Full substitution was achieved with a dose of 3.0 mg/kg 7-HMG, which is relatively smaller than the morphine training dose. 7-HMG produced potent substitution effects to the morphine discriminative stimulus that were about 2 times more potent than morphine. This data is in agreement with previous studies that showed a similar order of potency of between 7-HMG and morphine in the anti-nociceptive tests (Matsumoto et al. 2004). The present study also highlighted the relative potency between 7-HMG and MG; the derivative was about 13 times more potent than MG in substitution for morphine discriminative stimulus. The higher potency of 7-HMG in relation to MG has also been reported in anti-nociceptive tests (Matsumoto et al. 2004; Takayama et al. 2004). The potent action of 7-HMG is based on the activation of μ-opioid receptors and its morphine-like pharmacological properties (Matsumoto et al. 2005a). It was speculated that the additional hydroxyl group at its C7 position led to improved water solubility and greater formation of hydrogen bonding with target receptors and thus improves affinity of 7-HMG towards the opioid receptor. However, the actual mechanism underlying the opioid-like potency of 7-HMG is still unknown and requires further investigation. Since the 7-HMG derivative accounts for a minor constituent of M. speciosa (<2 %), the overall effect of kratom might, in part, be due to 7-HMG.
While MG was claimed to possess both opioid- and psychostimulant-like effects, (Assanangkornchai et al. 2007; Macko 1972; Ward et al. 2011; Grewal 1932a, b; Suwanlert 1975, Reanmongkol et al. 2007), further experiments utilising cocaine to test for substitution for MG were performed. Cocaine was found to partially substitute (>60 % drug-lever responding) for MG discriminative stimulus at larger substituted doses (10.0 mg/kg), suggesting some pharmacological similarities. This is in agreement with a previous study in which large doses (18.4 to 46.0 mg/kg) of MG produced stimulatory effects in cats that were qualitatively different from opiates (Macko et al. 1972). This finding also correlates with previous studies that reported that MG displays cocaine-like activities in cats (Grewal, 1932a, b, Macko et al. 1972). From a human perspective, kratom is often believed to have coca-like effects (Grewal 1932b; Suwanlert 1975; Macko 1972), but except for the increased ability to work, there is little evidence of psychostimulant-like effects, and the possible pharmacological mechanisms remain unclear. This is the first behavioural study to suggest a pharmacological similarity between MG and cocaine in a rodent model. It is important to note that the role of dopamine receptors has yet to be examined in the discriminative stimulus effects of MG. However, this cross-substitution is not surprising given that both MG and cocaine effects are attributed to actions on the ascending noradrenergic system, particularly ɑ-2 adrenergic receptors (Matsumoto et al. 1996a; Schmidt and Weinshenker 2014). These authors suggested that the stimulus effect of MG was due to the interaction between mitragynine and ɑ-2 adrenoreceptors. The involvement of adrenoreceptors merits further investigation. There are reports which suggest that the κ-opioid receptor may also be involved in mediating the effects exerted by MG (Shamima et al. 2012; Stolt et al. 2014; Taufik Hidayat et al. 2010; Thongpradichote et al. 1998). κ-opioid receptors have been reported to be involved in the modulation of some abuse-related effects in CNS stimulant (Negus and Mello 1999). However, the role of adrenergic and κ-opioid receptors in the mechanism of discriminative effects of MG remains unclear.
Pretreatment with the opioid receptor antagonist naloxone showed a tendency to partially block the MG discriminative stimulus effects. These results should be interpreted with caution due to the relatively small group size but, nevertheless, suggest that MG discriminative stimulus may be mediated via opioid receptors. Previous studies have reported that MG anti-nociceptive activity was blocked by naloxone (Takayama 2002; Sabetghadam et al. 2010; Sabetghadam et al. 2013), supporting an opioid receptor-mediated mechanism. The data also suggest that the discriminative stimulus effects of MG may involve other pathways in addition to actions on opioid receptors. In contrast to MG, naloxone pretreatment demonstrated a complete blockade of morphine discriminative stimulus. The reason for this discrepancy between the attenuation effects of MG and morphine may be due to the difference in the opioid receptor subtypes involved in their pharmacological activity. A study conducted by Thongpradichote (1998) demonstrated that the selectivity of MG for supraspinal opioid receptor subtypes was different from morphine in mice (Thongpradichote et al. 1998). The morphine-like effects of 7-HMG were also blocked by naloxone, which indicates the role of opioid receptors. This finding further suggests full agonist properties of 7-HMG on μ-opioid receptors since naloxone could antagonise the inhibitory action induced by 7-HMG on the electrically evoked contractions in the guinea-pig ileum (Matsumoto et al. 2004). This is the first study to demonstrate that 7-HMG has potent opioid receptor agonist effect that is sufficiently potent to substitute for the morphine discriminative stimulus effects.
In summary, the present study supports the hypothesis that pharmacological similarities exist between MG and morphine. The findings also provide additional support of the shared pharmacological and behavioural properties between MG and cocaine, but to a lesser extent as compared to morphine. Taken together, these findings are in accordance with numerous reports suggesting that MG possesses both opioid- and psychostimulant-like effects. This finding is interesting given that the chemical structure of MG is unrelated to those drugs (Jansen and Prast 1988; Shellard 1974). Thus, M. speciosa seems to be a unique plant that possesses both opioid- and psychostimulant-like drug effects. MG may share mechanisms of action with both drug classes. However, due to its diverse pharmacology (Matsumoto et al. 1996b; Taufik Hidayat et al. 2010; Stolt et al. 2014; Boyer et al. 2008; Shamima et al. 2012), the underlying pharmacological mechanisms of MG discriminative stimulus properties have not been fully determined. The resemblance between MG discriminative stimuli with the two drugs also contributes to defining the nature of the addictive properties of MG. Nevertheless, considering the subjective effects of the discriminative stimulus properties, it appears that MG is responsible for kratom abuse. Further behavioural studies investigating the putative addictive properties of MG, specifically the reinforcing properties using self-administration studies are currently in progress in our laboratories. The contribution of 7-HMG with respect to addictive potential also warrants further pharmacological investigation.
References
Ahmad K, Aziz Z (2012) Mitragyna speciosa use in the northern states of Malaysia: a cross-sectional study. J Ethnopharmacol 141:446–450
Apryani E, Hidayat MT, Moklas MAA, Fakurazi S, Farah Idayu N (2010) Effects of mitragynine from Mitragyna speciosa Korth leaves on working memory. J Ethnopharmacol 129:357–360
Assanangkornchai S, Muekthong A, Sam-Angsri N, Pattanasattayawong U, Takayama H (2007) The use of Mitragyna speciosa ("Krathom"), an addictive plant, in Thailand. Subst Use Misuse 42:2145–2157
Azizi J, Ismail S, Mordi MN, Ramanathan S, Said MI, Mansor SM (2010) In vitro and in vivo effects of three different Mitragyna speciosa korth leaf extracts on phase II drug metabolizing enzymes-glutathione transferases (GSTs). Molecules 15:432–441
Bartoletti M, Colantoni A, De Luca V, Gaiardi M (2010) Single and repeated baclofen treatment attenuates the discriminative stimulus effects of morphine in rats. Pharmacol, Biochem & Behav 97:279–283
Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragyna speciosa korth). Addiction 103:1048–1050
Burkill IH (1935) A dictionary of the Economic Products of the Malay Peninsula. II: 1480-1483
Chan KB, Pakiam C, Rahim RA (2005) Psychoactive plant abuse: the identification of mitragynine in ketum and in ketum preparations. Bull Narc 57(1–2):249–256
Fakurazi S, Rahman SA, Hidayat MT, Ithnin H, Moklas MA, Arulselvan P (2013) The combination of mitragynine and morphine prevents the development of morphine tolerance in mice. Molecules (Basel, Switzerland) 18:666–681
Farah Idayu N, TaufikHidayat M, Moklas MAM, Sharida F, NurulRaudzah AR, Shamima AR, Apryani E (2011) Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 18(5):402–407
Grewal KS (1932a) Observations on the pharmacology of mitragynine. J Pharmacol Exp Ther 46:251–271
Grewal KS (1932b) The effect of mitragynine on man. Br J Med Psychol 12:41–58
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Horsten S, Ismail NI, Jayabalan N, Hazim AI, Mansor SM, Muller CP (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37:138–151
Ishikawa H, Takayama H, Norio A (2002) Dimerization of indole derivatives with hypervalentiodines (III): a new entry for the concise total synthesis of rac- and meso-chimonanthines. Tetrahedron Lett 43:5637–5639
Janchawee B, Keawpradub N, Chittrakarn S, Prasettho S, Wararatananurak P, Sawangjareon K (2007) A high-performance liquid chromatographic method for determination of mitragynine in serum and its application to a pharmacokinetic study in rats. Biomed Chromatogr: BMC 21:176–183
Jansen KL, Prast CJ (1988) Ethnopharmacology of kratom and the Mitragyna alkaloids. J Ethnopharmacol 23:115–119
Macko E, Weisbach JA, Douglas B (1972) Some observations on the pharmacology of mitragynine. Arch Int de Pharmacodynamieet de Therapie 198:145–161
Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, Aimi N, Watanabe H (1996a) Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol 317:75–81
Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, Watanabe H (1996b) Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci 59:1149–1155
Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K (2004) Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci 74:2143–2155
Matsumoto K, Horie S, Takayama H, Ishikawa H, Aimi N, Ponglux D, Murayama T, Watanabe K (2005a) Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci 78:2–7
Matsumoto K, Yamamoto LT, Watanabe K, Yano S, Shan J, Pang PK, Ponglux D, Takayama H, Horie S (2005b) Inhibitory effect of mitragynine, an analgesic alkaloid from Thai herbal medicine, on neurogenic contraction of the vas deferens. Life Sci 78:187–194
Matsumoto K, Hatori Y, Murayama T, Tashima K, Wongseripipatana S, Misawa K, Kitajima M, Takayama H, Horie S (2006) Involvement of mu-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol 549:63–70
Negus SS, Mello NK (1999) Effects of kappa opioid agonists on the discriminative stimulus effects of cocaine in rhesus monkeys. Exp Clin Psychopharm 7:307–317
Norakanphadung P (1966) Pramuan Khuamru Ruang Yaseptit Hai Thot. Thanyarak Hospital, Bangkok
Overton DA (1988) Similarities and differences between behavioural control by drug-produced stimuli and by sensory stimuli. Psychopharmacology 4:176–198
Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, Aimi N, Sakai S (1994) A New Indole Alkaloid, 7 alpha-Hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand. Planta Med 60:580–581
Preston KL, Bigelow GE (1991) Subjective and discriminative effects of drugs. Behav Pharmacol 2:293–313
Reanmongkol W, Keaupradub N, Sawangjaroen K (2007) Effects of the extracts from Mitragyna speciosa korth. Leaves on analgesic and behavioral activities in experimental animals. J Sci Technol 29:39–48
Sabetghadam A, Ramanathan S, Mansor SM (2010) The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacogn Res 2:181–185
Sabetghadam A, Ramanathan S, Sasidharan S, Mansor SM (2013) Subchronic exposure to mitragynine, the principal alkaloid of Mitragyna speciosa, in rats. J Ethnopharmacol 146:815–823
Schmidt KT, Weinshenker D (2014) Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol 85:640–650
Schuster CR, Fischman MW, Johanson CE (1981) Internal stimulus control and subjective effects of drugs. NIDA Res Monogr 37:116–129
Sevak RJ, Stoops WW, Hays LR, Rush CR (2009) Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther 328:1007–1018
Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MA, Arulselvan P (2012) Antinociceptive action of isolated mitragynine from Mitragyna speciosa through activation of opioid receptor system. Int J Mol Sci 13:11427–11442
Shellard EJ (1974) The alkaloids of Mitragyna with special reference to those of Mitragyna speciosa, Korth. Bull Narc 26:41–55
Singh D, Muller CP, Vicknasingam BK (2014) Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend 139:132–137
Stolerman IP, Garcha HS (1989) Temporal factors in drug discrimination: experiments with nicotine. J Psychopharm (Oxford, England) 3:88–97
Stolerman IP, Naylor C, Elmer GI, Goldberg SR (1999) Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology 141:297–306
Stolt AC, Schroder H, Neurath H, Grecksch G, Hollt V, Meyer MR, Maurer HH, Ziebolz N, Havemann-Reinecke U, Becker A (2014) Behavioral and neurochemical characterization of kratom (Mitragyna speciosa) extract. Psychopharmacology 231:13–25
Su RB, Ren YH, Liu Y, Ding T, Lu XQ, Wu N, Liu ZM, Li J (2008) Agmatine inhibits morphine-induced drug discrimination in rats. Eur J Pharmacol 593:62–67
Suwanlert S (1975) A study of kratom eaters in Thailand. Bull Narc 27:21–27
Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (2002) Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. J Med Chem 45:1949–1956
Takayama H (2004) Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull 52:916–928
Taufik Hidayat M, Apryani E, Nabishah BM, Moklas MAA, Sharida F, Farhan MA (2010) Determination of mitragynine bound opioid receptors. Adv in Med Dent Sci 3:65–70
Thongpradichote S, Matsumoto K, Tohda M, Takayama H, Aimi N, Sakai S, Watanabe H (1998) Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life Sci 62:1371–1378
Tsuchiya S, Miyashita S, Yamamoto M, Horie S, Sakai S, Aimi N, Takayama H, Watanabe K (2002) Effect of mitragynine, derived from Thai folk medicine, on gastric acid secretion through opioid receptor in anesthetized rats. Eur J Pharmacol 443:185–188
Utar Z, Majid MI, Adenan MI, Jamil MF, Lan TM (2011) Mitragynine inhibits the COX-2 mRNA expression and prostaglandin E (2) production induced by lipopolysaccharide in RAW264.7 macrophage cells. J Ethnopharmacol 136:75–82
Vicknasingam B, Narayanan S, Beng GT, Mansor SM (2010) The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy 21:283–288
Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW (2011) Herbal medicines for the management of opioid addiction: safe and effective alternatives to conventional pharmacotherapy? CNS Drugs 25:999–1007
Watanabe K, Yano S, Horie S, Yamamoto LT (1997) Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci 60:933–942
Wing VC, Shoaib M (2012) Translating the smoking cessation properties of the antidepressant nortriptyline using reinforcing, discriminative and aversive stimulus effects of nicotine in rats. Psychopharmacology 219:847–857
Wray L (1907) “Biak”: an opium substitute. J Fed Malay States Mus 2:53–54
Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai S, Yano S, Shan J, Pang PK, Ponglux D, Watanabe K (1999) Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. Gen Pharmacol 33:73–78
Acknowledgments
This research received financial support from Higher Education Centre of Excellence (HiCoE) special funding (304/CDADAH/650527/K134), USM Research University Grant (RUT) [1001/CDADAH/855005], International Research Collaboration Fund (1002/CDADAH/910410) and MyBrain15 Scholarship from Ministry of Higher Education.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harun, N., Hassan, Z., Navaratnam, V. et al. Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology 232, 2227–2238 (2015). https://doi.org/10.1007/s00213-015-3866-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3866-5