Abstract
Rationale
BMS-820836 is a novel antidepressant that selectively inhibits the reuptake of serotonin, norepinephrine, and dopamine.
Objective
This Phase I study assessed safety, tolerability, and pharmacokinetics of multiple daily doses of BMS-820836 in healthy subjects. Central serotonin transporter (SERT) and dopamine transporter (DAT) occupancy were assessed using positron emission tomography and [11C]MADAM or [11C]PE2I, respectively.
Methods
Fifty-seven healthy volunteers were enrolled in this double-blind, placebo-controlled, ascending multiple-dose study (ClincalTrials.gov identifier: NCT00892840). Eight participants in seven dose cohorts received oral doses of BMS-820836 (0.1–4 mg) or placebo for 14 days to assess safety, tolerability, and pharmacokinetics. Additionally, SERT and DAT occupancies were evaluated in 4–8 subjects per cohort at 8 h post-dose on Day 10 and 24 h post-dose on Day 15 at anticipated steady-state conditions.
Results
Most adverse events were mild to moderate; there were no serious safety concerns. Median maximum concentrations of BMS-820836 were observed at 4.0–5.5 h post-dose; estimated elimination half-life was 44–74 h. About 80 % striatal SERT occupancy was achieved after multiple doses of 0.5 mg BMS-820836 at both 8 and 24 h post-dose. Striatal DAT occupancy ranged between 14 % and 35 % at 8 h post-dose with a slight decline at 24 h post-dose.
Conclusions
Multiple daily doses of up to 4 mg BMS-820836 appeared to be generally safe and well tolerated in a healthy population. SERT and DAT occupancies were in a range associated with therapeutic efficacy of antidepressants. Together with the pharmacokinetic profile of BMS-820836, the occupancy data support once-daily administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Converging lines of evidence indicate that depression and other mood disorders may be associated with a heterogeneous dysregulation of monoaminergic systems (i.e., those involving dopamine [DA], norepinephrine [NE], and serotonin [5-HT]) (Garlow and Nemeroff 2004; Millan 2009; Nikolaus et al. 2012). However, current antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs), modulate primarily 5-HT and NE pathways. While these currently available therapies demonstrate efficacy, almost 50 % of depressed patients may not achieve a full response to therapy (Fava 2003; Nemeroff 2007; Trivedi et al. 2006b), suggesting that alternative approaches are needed.
The triple reuptake inhibitor BMS-820836 has been shown to be an inhibitor of 5-HT, NE, and DA neuronal reuptake transporters, SERT, NET, and DAT, respectively (Li et al. 2012; Risinger et al. 2014). Compared with the already established therapy of combined SERT and NET inhibition in the form of SNRIs, BMS-820836 adds the component of DAT inhibition. Dopamine neurotransmission is involved in motivation, pleasure, and reward, all of which can be impacted in major depression (Dunlop and Nemeroff 2007). Multiple studies have suggested that there may be benefits to combining drugs that have DAT inhibition properties — such as bupropion (Bodkin et al. 1997; Hirschfeld et al. 2002; Marshall and Liebowitz 1996; Trivedi et al. 2006a) or methylphenidate (Lavretsky et al. 2003) — with established therapies like SSRIs or SNRIs. Beneficial effects were observed across a number of efficacy parameters, including onset of action (Lavretsky et al. 2003) and response rates (Bodkin et al. 1997; Marshall and Liebowitz 1996; Trivedi et al. 2006a) in ordinary and treatment-resistant patients. Although a minimum SERT blockade of 80 % has been indicated as a condition in order to achieve clinical efficacy for SSRI treatment (Meyer et al. 2001, 2004; Meyer 2007; Voineskos et al. 2007), the most favorable occupancy levels at NET and DAT need still to be further evaluated. Moreover, due to extensive interaction between monoaminergic pathways (Damsa et al. 2004; Lane 1998; Morelli et al. 2011; Nikolaus et al. 2012), it is unknown whether the ranges or threshold occupancies required for efficacy differ between mono-, dual-, and triple-reuptake inhibiting drugs. In preclinical testing, BMS-820836 demonstrated a dose-dependent reduction of immobility in the mouse tail suspension test at the minimum effective dose of 0.3 mg/kg, which yielded SERT, NET, and DAT occupancies of 86 %, 76 %, and 28 % measured ex vivo, respectively (Li et al. 2012). In a Phase I single ascending-dose study of BMS-820836 in healthy subjects, mean striatal SERT occupancies were 19 ± 9 % and 82 ± 8 % between 7 and 48 h after single oral doses of 0.5 and 3 mg BMS-820836, respectively; similarly, the mean striatal DAT occupancy was 19 ± 9 % for 3 mg BMS-820836 (Risinger et al. 2014).
This Phase I study was primarily designed to assess the safety, tolerability, and pharmacokinetics of BMS-820836 following multiple oral doses of BMS-820836 in healthy subjects. Further, use of positron emission tomography (PET) was incorporated in order to provide an early assessment of therapeutic potential of BMS-820836 and to guide future dose selection. The PET radioligands [11C]MADAM (Chalon et al. 2003; Halldin et al. 2005; Larsen et al. 2004; Lundberg et al. 2007) and [11C]PE2I (Chalon et al. 1999; Emond et al. 1997; Guilloteau et al. 1998; Halldin et al. 2003) were selected to determine the occupancy of BMS-820836 at SERT and DAT, respectively. NET occupancy was not determined due to the lack of a validated PET-radioligand at the time of study initiation. Finally, the relationship between the plasma BMS-820836 concentrations and estimated transporter occupancies was also explored.
Methods
Study design
This was a randomized, placebo-controlled, double-blind, multiple ascending-dose study in healthy subjects. Eight subjects were assigned to each of seven independent cohorts. Each dose cohort was randomized 6:2 to receive BMS-820836 or matching placebo. BMS-820836 was dosed at <0.5, 0.5, 1, 2, 3, and 4 mg for males (cohorts 1–6) and at 1 mg for females (cohort 7) as an oral daily dose for 14 days.
The starting dose of 0.5 mg BMS-820836 was based on the results of the Phase I single ascending-dose study of BMS-820836 in healthy subjects (Risinger et al. 2014). The design allowed for the investigation of doses <0.5 mg in cases of safety or tolerability issues at higher doses and/or relatively high occupancies at SERT or DAT for the 0.5-mg dose. At this early stage of drug development, the study was restricted mainly to males; however, to explore differences between the sexes, one dose cohort was assigned to females. For the female cohort, the BMS-820836 dose used was in the range of doses previously identified as being safe and well tolerated, as well as having demonstrated adequate central occupancies in male subjects.
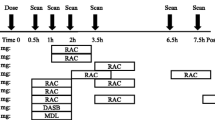
SERT and DAT occupancies were assessed in subsets of the male cohorts through the use of PET, and subjects received either the radioligand [11C]MADAM or [11C]PE2I for SERT and DAT investigations, respectively. Subjects were randomly assigned to the PET cohorts, with the restriction that only one placebo-dosed subject was included per PET cohort. The active treatment/placebo ratios varied by PET cohorts depending on the radioligand used and the number of subjects (Table 1).
Subjects were admitted to the clinical facility on Day −2 and remained in the clinical facility until furlough on Day 20. Subjects returned to the clinical facility on Day 27 (±2 days) for study discharge.
Titration was implemented in the 3- and 4-mg dose cohorts to improve tolerability. Dosing was initiated at 1 mg once daily with dose up-titration by 1 mg every 3 days up to the target doses of 3 and 4 mg, respectively. Based on pharmacokinetic simulations, it was projected that with use of this titration scheme, subjects in the 3-mg dose cohort would attain 97 % of plasma steady-state of BMS-820836 by Day 10. Subjects in the 4-mg dose cohort were projected to reach 76 % and 93 % of steady-state of BMS-820836 on Days 10 and 14, respectively.
The study was conducted in accordance with the ethical principles that have their origin in the current Declaration of Helsinki, and was consistent with International Conference on Harmonisation Good Clinical Practice and applicable regulatory requirements. The study was performed with approval from the Swedish Medical Products Agency, the local Independent Ethics Committee, and the local Radiation Protection Committee. After complete description of the study to the subjects, written informed consent was obtained. This trial is registered with ClinicalTrials.gov (ID: NCT00892840).
Participants: inclusion/exclusion criteria
The study included 49 men (cohorts 1–6) and 8 women (cohort 7) aged 21–55 years, with a body mass index of 18–32 kg/m2. In the study population, the mean age ± standard deviation was 25 ± 3 and 47 ± 12 years at screening for males and females, respectively. Subjects were screened within 30 days of dosing to determine study eligibility. Main exclusion criteria included a history of or current treatment for psychiatric disorders or drug abuse, and a recent history of gastrointestinal disease, cholecystectomy, and any significant acute or chronic mental illness. Based on a screening visit, subjects were excluded if there was any evidence of organ dysfunction or any significant deviation from normal for vital signs, electrocardiogram (ECG), and clinical laboratory determinations (blood and urine). Women who were pregnant or breastfeeding were not eligible. Female subjects of childbearing potential were required to use an adequate method of contraception throughout the study and for 8 weeks after the last dose of study drug. The progress of eligible subjects from screening to study completion and analysis is described in Online Resource 1 (Fig. S1).
Prior to dosing, all eligible subjects had brain magnetic resonance imaging (MRI) examinations (T1-IR, T2-weighted, and FLAIR) using a 1.5-Tesla scanner (Philips Healthcare, Best, The Netherlands) for anatomical information and to exclude subjects with clinically significant structural brain abnormalities or abnormalities impairing the ability to interpret the PET data.
Safety and tolerability
During the in-clinic stay, subjects had physical examinations, vital sign measurements, ECGs, and clinical laboratory evaluations at pre-specified times pre- and post-dose. Subjects were closely monitored for adverse events (AEs) throughout the study, and were not discharged until the investigator determined that ongoing AEs were resolved. Blood and urine samples were obtained at screening, Day −2 (baseline), post-dose on Days 2, 8, 14, and 20, and at study discharge. Physical examinations were performed at the screening visit, pre-dose on Day 1, and prior to discharge. On Days 1–14, vital sign measurements, including supine and orthostatic blood pressure and heart rate, respiratory rate, and temperature, were assessed at approximately 6 h post-dose, which was expected to coincide with maximum plasma BMS-820836 concentrations.
Additional vital sign measurements were required on Days 15–20 and on Day 27. Blood pressure and heart rate were recorded continually at 30-min intervals from approximately 30 min pre-dose to 15 h post-dose on Days −1, 1, 5, and 14 by an automated ambulatory monitor (ABPM; Spacelabs Healthcare; Snoqualmie, WA, USA).
Pharmacokinetics
BMS-820836 exerts its mechanism of action on DAT, SERT, and NET as the parent compound and through its major N-desmethyl metabolite, BMS-821007, which also has substantial affinity with the same monoamine systems (Li et al. 2012; Risinger et al. 2014). Therefore, serial venous blood samples were collected on Days 1 and 14, and trough venous blood samples (minimum concentration [C min]) were collected pre-dose on Days 5, 8, 10, and 12 to characterize the pharmacokinetics of BMS-820836 and its pharmacologically active metabolite BMS-821007. Venous blood samples were also collected immediately before and after each PET scan to determine the mean plasma BMS-820836 and BMS-821007 concentrations during scanning. Plasma samples were analyzed for BMS-820836 and BMS-821007 by validated liquid chromatography–tandem mass spectrometric (LC-MS/MS) assays.
The maximum observed plasma concentration (C max) and time of maximum observed plasma concentration (T max) were directly recorded from the observed pharmacokinetic data. Steady-state conditions were checked by visual inspection of the trough plasma concentrations obtained pre-dose on various study days. The area under the concentration–time curve (AUC) in one dosing interval (AUC[TAU]) and plasma half-life (T 1/2) were calculated for Days 1 and 14 using non-compartmental analysis in Kinetica version 5.0 (Thermo Electron Corporation, Philadelphia, PA, USA). The accumulation index was determined as the ratio of AUC(TAU) at steady-state (Day 14) to AUC(TAU) after the first dose (Day 1). The dose proportionality of the pharmacokinetic parameters BMS-820836 C max and AUC(TAU) on study Day 14 was assessed by a power model: y = A × Doseβ (Gough et al. 1995), where y is either C max or AUC(TAU). Following logarithmic transformation, A is the intercept and β is the slope, where β = 1 indicates perfect dose proportionality. The dose proportionality assessment only included dose panels from 0.1 to 2 mg once daily in the male subjects; the 1-mg female cohort and the 3- and 4-mg titration cohorts were excluded to avoid confounding factors of sex and titration. A simple E max model was used to estimate the relationships between BMS-820836 plasma concentrations and SERT or DAT occupancy (Phoenix WinNonlin 6.2; Pharsight, Cary, NC, USA).
Positron emission tomography
Assigned participants underwent three PET scans: one scan 4 to 6 days prior to dosing and two scans at anticipated plasma steady-state conditions following daily dosing of BMS-820836. For logistical reasons, the first post-dosing scan was conducted on Day 10 or 11 (8 h post-dose) and the second post-dose scan was performed on Day 15 (24 h post-dose).
Radioligands were synthesized following reported methods for [11C]MADAM (Tarkiainen et al. 2001) and [11C]PE2I (Halldin et al. 2003; Hirvonen et al. 2008). The mean specific radioactivity of the delivered tracer batches was 95 and 75 GBq/μmol for [11C]MADAM and [11C]PE2I, respectively. The PET investigations were performed on an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN, USA), enabling the acquisition of 63 contiguous planes of data with a size of 2.46 mm each. A 10-min transmission scan was performed in two-dimensional mode prior to tracer injection for attenuation correction. Following the transmission scan, either [11C]MADAM or [11C]PE2I (in both cases about 250–350 MBq) was administered intravenously as a bolus injection via a venous cannula in the arm of the subject. Simultaneously, a dynamic emission scan sequence was started in three-dimensional mode. The emission scans consisted of 22 time-frames with progressive frame duration (4 × 60, 2 × 120, 4 × 180, and 12 × 300 s) and total duration of 80 min. Dynamic images were reconstructed using ordered subset expectation maximization, using six iterations and eight subsets, a 4-mm Hanning post-filter, and application of corrections for deadtime, tissue attenuation, decay, scatter, and randoms.
The PET data analyses were performed by two experienced raters who were blinded to the treatment allocation, following a standard protocol and cross-checking the results. For each subject, the dynamic PET images were realigned (1) within scans to adjust for subject movements during scanning, and (2) between scans to adjust for different positions in the scanner on the three occasions. The T1-IR MRI scan, which provided anatomical information, was co-registered with the realigned PET scans using a summation image to enable a proper delineation of the regions of interest (ROIs) in the PET data.
The considered ROIs were cerebellum and striatum, comprising putamen and nucleus caudatus, which were all delineated bilaterally, in four consecutive slices, for both [11C]MADAM and [11C]PE2I examinations. In contrast to DAT, SERT is widely distributed throughout the brain, and the single ascending-dose study of BMS-820836 (Risinger et al. 2014) demonstrated that the approximate 80 % SERT occupancy observed in the striatum, as the primary ROI, was also obtained in cortical and subcortical regions. Based on those results, it was decided to focus on striatal SERT occupancy, which would also be a marker for levels of SERT occupancy throughout the brain.
After outlining of the ROIs, regional time–activity (TAC) data were generated in each dynamic scan representing the levels of radioactivity over time, and were adjusted for the subject’s body weight and administered dose of radioactivity. These steps were conducted using VOIager software (GE Healthcare, Uppsala, Sweden).
Tracer kinetic analyses of the TAC data were implemented using a simplified reference tissue model (Hirvonen et al. 2008; Lammertsma and Hume 1996; Lundberg et al. 2005), with striatum as target region and cerebellum as reference region. Previous studies have shown that cerebellum does not have specific binding of [11C]MADAM (Halldin et al. 2005; Lundberg et al. 2005) or [11C]PE2I (Hall et al. 1999; Halldin et al. 2003). The model parameters were estimated by a nonlinear least squares method using in-house MATLAB routines. The percentage of striatal SERT and DAT occupancies was calculated as the relative difference between the estimated binding potential in the baseline scan (BPbase) and the post-dose scans (BPpd):
Results
Safety and tolerability
The most common AEs are listed in Table 2. Most reported AEs were mild to moderate in intensity (95 %) as judged by the investigator. However, three out of 57 subjects did not complete the protocol due to severe AEs; one subject was replaced. In the 3-mg titration cohort, one subject, who received 1 mg BMS-820836 as part of the dose titration, discontinued on Day 2 due to persistent dry mouth, tremor, and cold sweat. The subject was replaced, as termination occurred at an early stage and there were no significant safety issues in a prior 1-mg cohort. In the same dose cohort, a placebo-treated subject terminated the study on Day 14 due to suicidal ideation. Finally, in the 4-mg titration cohort, one subject discontinued on Day 7 (after the first dose of 3 mg BMS-820836) due to persistent oropharyngeal discomfort and hypersensitivity.
The mean differences in supine blood pressure and heart rate between baseline and Day 14 are depicted in Fig. 1. Generally, supine blood pressure increased during BMS-820836 treatment, but no consistent dose–response pattern was observed. A dose–response relationship was observed in the mean change from baseline in supine heart rate, with the greatest increases noticed for 1–4 mg BMS-820836. A similar result, although less distinct, was observed in orthostatic change in heart rate for 2–4 mg doses, but without any substantial change in orthostatic blood pressure compared with placebo (Online Resource 2 [Fig. S2]). Similar effects were observed with the ABPM data. No dose-related patterns were observed in heart rate adjusted QT interval.
There were no AEs related to laboratory evaluations during the study. There were no clinically meaningful changes over time in any hematology, serum chemistry, or urinalysis parameters.
Pharmacokinetics
A summary of the pharmacokinetic parameters for BMS-820836, on Days 1 and 14, is presented in Table 3. Median T max values ranged from 4.0 to 5.5 h among dose cohorts on Days 1 and 14. C max and AUC(TAU) were slightly less than dose-proportional in the male subjects following 0.1–2 mg once daily. There was approximately a 2.7- to 4.6-fold accumulation, determined by AUC(TAU), by Day 14, following 0.1–2 mg once daily. The mean apparent elimination T 1/2 ranged from 44 to 74 h. There were no apparent sex differences in the pharmacokinetics of 1 mg BMS-820836 in the study. BMS-820836 reached steady-state around Day 10 following 0.1–2 mg once daily and for 50 % of the subjects following 3-mg titration. For the 4-mg dose cohort, steady-state was not attained on Day 10, but was nearly achieved on Day 14 (Online Resource 3 [Fig. S3]).
The AUC(TAU) of the pharmacologically active metabolite BMS-821007 was approximately 12 % of BMS-820836 AUC(TAU) by Day 14. A similar result was found for all investigated doses, and for male and female subjects in the 1-mg dose cohorts (data not shown). The C max and AUC(TAU) of BMS-821007 increased slightly less than proportionally to dose, and the median T max ranged from 5.1 to 9.0 h for the different dose cohorts. The mean T 1/2 ranged between 157 and 276 h, which was approximately 3- to 4-fold longer than that of the parent compound. In all dose cohorts, BMS-821007 did not appear to have attained steady-state by Day 14. Based on simulation, steady-state is projected to be achieved around Day 30 of daily dosing and not to exceed 20 % of the parent compound.
SERT and DAT occupancies
In total, 43 male subjects were enrolled in various PET cohorts; 41 of these subjects were included in the data presented. The replaced subject (from the 3-mg dose cohort), as indicated previously, was not considered, as this subject had no post-dosing scans. In the 4-mg dose cohort, one subject was excluded because drug administration was terminated on Day 7, which made the subject’s final PET scan on Day 10 not comparable with those from other subjects. Further, in the 2-mg dose cohort two subjects had no 24-h PET assessment due to technical issues in tracer production.
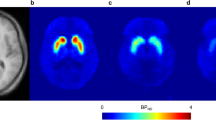
PET images illustrating [11C]MADAM uptake (prior to dosing and at expected steady-state conditions after multiple doses of 0.5 mg BMS-820836), mean striatal SERT occupancies for various dose cohorts, and the relationship between BMS-820836 plasma concentrations and striatal SERT occupancies are depicted in Fig. 2. The mean striatal SERT occupancy was 79 % for 0.5 mg BMS-820836 at both 8 and 24 h post-dosing. Having achieved a SERT occupancy around 80 %, we decided to investigate doses of 0.25 and 0.1 mg BMS-820836 in one dose cohort. For these doses, the mean striatal SERT occupancies ranged between 65 % and 70 % and also remained at a similar level at 24 h post-dosing (Online Resource 4 [Table S1]). The relationship between plasma BMS-820836 concentrations and striatal SERT occupancies indicated that occupancies were approaching a plateau at the doses tested, with an estimated maximum SERT occupancy of 79 % at steady-state conditions.
Serotonin transporter occupancy. Left: uptake of [11C]MADAM at the level of basal ganglia, expressed relative to uptake in cerebellum, for a single subject prior to dosing (baseline) as well as at 8 and 24 h post-dose at anticipated steady-state conditions following multiple doses of 0.5 mg BMS-820836. Right: striatal SERT occupancy following once-daily dosing of 0.1–0.5 mg BMS-820836. Top: SERT occupancy at 8 and 24 h post-dose. Bottom: BMS-820836 plasma concentration versus SERT occupancy using the individual data and a simple E max model. Note: the estimated parameters (CV) were: E max=79 % (7), EC50 = 105 pg/ml (54), and E 0 = 8 % (39). CV coefficient of variation, E 0 the effect of the concurrent therapy based on placebo treatment, EC 50 the BMS-820836 concentration at the half of maximum SERT occupancy, E max maximum SERT occupancy at steady-state conditions, SD standard deviation, SERT serotonin transporter
Similar results are presented for DAT in Fig. 3. The mean striatal DAT occupancies ranged between 14 % and 35 % following multiple doses of 0.5–4.0 mg BMS-820836 (Online Resource 5 [Table S2]). Mean striatal DAT occupancy of 35 % was obtained for both 3 and 4 mg BMS-820836 at 8 h post-dose, which declined to approximately 30 % at 24 h post-dose. Based on modeling of the relationship between BMS-820836 plasma concentrations and DAT occupancies, the maximum DAT occupancy was estimated to be 68 % at steady-state conditions.
Dopamine transporter occupancy. Left: uptake of [11C]PE2I at the level of basal ganglia, expressed relative to uptake in cerebellum, for a single subject prior to dosing (baseline) as well as at 8 and 24 h post-dose at anticipated steady-state conditions following multiple doses of 3 mg BMS-820836. Right: striatal DAT occupancy following once-daily dosing of 0.5–4 mg BMS-820836. Top: DAT occupancy at 8 and 24 h post-dose. Bottom: BMS-820836 plasma concentration versus DAT occupancy using the individual data and a simple E max model. Note: the estimated parameters (CV) were: E max (CV%) = 68 % (43), EC50 = 20,639 pg/ml (72), and E 0 = 12 % (16). CV coefficient of variation, DAT dopamine transporter, E 0 effect of the concurrent therapy based on placebo treatment, EC 50 the BMS-820836 concentration at the half of maximum DAT occupancy, E max maximum DAT occupancy at steady-state conditions, SD standard deviation
Discussion
This paper describes the first clinical study in which multiple doses of the novel triple reuptake inhibitor BMS-820836 were evaluated simultaneously with respect to safety, tolerability, and pharmacokinetics, as well as DAT and SERT occupancy. Overall, there were no major safety concerns for doses up to 4 mg BMS-820836 in these healthy subjects. The SERT and DAT occupancy results were consistent with the preclinical occupancy profile of BMS-820836 (Li et al. 2012) and the SERT occupancy was in the range associated with efficacious antidepressants (Meyer et al. 2004). Relatively low doses of BMS-820836 (≤0.5 mg/day) appeared to saturate SERT at a level of approximately 70–80 %. Previously, it was demonstrated in the single-dose study that approximate 80 % striatal SERT occupancy corresponded well with SERT occupancies achieved for cortical and other subcortical regions (Risinger et al. 2014). Hence, it is anticipated that in the current study, close to 80 % SERT occupancy was achieved throughout the brain after multiple doses of 0.5 mg BMS-820836 at both 8 and 24 h post-dose. A near-linear relationship between dose level and mean DAT occupancy was observed for BMS-820836 doses of 1–3 mg at both 8 and 24 h post-dose, comprising occupancy values of 20–35 %. The pharmacokinetic and occupancy profiles of BMS-820836 revealed a long plasma half-life for BMS-820836 and its pharmacologically active metabolite BMS-821007, as well as a long duration of brain occupancies, which supported once-daily dosing. Altogether, these results demonstrated an important mechanism of action of BMS-820836 in humans and justified further investigation of BMS-820836 in Phase II clinical studies.
One of the primary aims was to evaluate the safety profile in healthy subjects after multiple doses of BMS-820836. Only three out of 57 subjects terminated drug administration prematurely, and one of these three was treated with placebo. Most AEs were mild to moderate in intensity and were consistent with those of current antidepressants. Several of the observed AEs, such as decreased appetite, euphoric mood, restlessness, insomnia, and tremor, could potentially be related to a substantial blockade of DAT and consequently enhanced amounts of endogenous DA. However, we cannot attribute these AEs entirely to alterations of the dopaminergic system, as BMS-820836 is a triple reuptake inhibitor and there is broad evidence of interdependency between monoaminergic systems (Damsa et al. 2004; Lane 1998; Morelli et al. 2011; Nikolaus et al. 2012). Nonetheless, the apparent marked dose-related increase in euphoria, when starting doses were increased from 0.5 to 2 mg BMS-820836, is not usually seen with SERT and/or NET inhibitors.
The descriptive heart and blood pressure data showed an increase in heart rate proportional to doses of BMS-820836, which is consistent with the mechanism of action of the drug, whereas no dose-response relationship was observed for blood pressure. Cardiovascular effects have been previously associated with inhibition of NET. Sibutramine, an anti-obesity agent mainly acting on SERT and NET, showed an increased risk of cardiovascular side effects (James et al. 2010) and was recently withdrawn from the US and Canadian markets. Although NET occupancy was not assessed in this study, in vitro and in vivo preclinical experiments show substantial inhibition of NET (Li et al. 2012). This Phase I study was not powered to detect significant differences in heart rate and blood pressure, and the current findings need to be investigated further.
The pharmacokinetics following 3- and 4-mg titration schemes confirmed that plasma steady-state conditions were not attained by Day 10 for the 4-mg dose cohort and were accomplished only in part for the 3-mg cohort. As a consequence, for these doses, the DAT occupancies at 8 h post-dose may have been underestimated. Based on Day 14 pharmacokinetics and the assessed relationship between plasma BMS-820836 concentrations and DAT occupancy, we projected an underestimation of approximately 5–10 % and 15–20 % of the mean occupancy at 8 h post-dose for the 3- and 4-mg dose cohorts, respectively. Although near steady-state conditions were reached for BMS-820836, these conditions were not reached for the active metabolite BMS-821007 due to its estimated long plasma half-life. Further, steady-state of plasma pharmacokinetics does not necessarily imply similar conditions for brain kinetics. It was expected that half-life of BMS-820836 would be greater in the brain than in plasma, as BMS-820836 is a typical lipophilic compound (data on file), which tends to persist in the brain. This was consistent with the relative slow decline in occupancy, especially SERT occupancy, from 8 to 24 h post-dose. Brain penetration of triple monoamine inhibitors may also provoke a cascade of neurochemical events that affect monoamine transporter availabilities. Altogether, this may have consequences for the occupancy levels (especially DAT) in subsequent patient studies of longer duration.
There is an emerging consensus that after SSRI or SNRI treatment, SERT occupancy of approximately 80 % is required to increase central serotonin levels to the degree that most therapeutic effects can occur (Meyer et al. 2001, 2004; Meyer 2007; Voineskos et al. 2007). In clinical treatment studies, Meyer and co-workers demonstrated that for five established SSRIs and SNRIs (citalopram, fluoxetine, paroxetine, sertraline, and venlafaxine), the striatal SERT occupancy was near 80 %. This level was already accomplished at minimum therapeutic doses (Meyer et al. 2001, 2004; Meyer 2007), which was consistent with our findings. For DAT, therapeutic effects are expected at relatively low levels of DAT inhibition. For bupropion, a DA-NE reuptake inhibitor that has been used favorably in pharmacotherapy with SSRIs or SNRIs, striatal DAT occupancies were generally low, varying from 14 % to 26 %, after repeated therapeutic doses in healthy volunteers (Learned-Coughlin et al. 2003) and depressed patients (Argyelan et al. 2005; Meyer et al. 2002). Knowledge is limited regarding an optimum NET inhibition in humans, in part due to lack of a generally accepted PET radioligand. However, the optimal occupancy profile required for a triple reuptake inhibitor might be different from mono- and dual reuptake-inhibiting drugs, such as SSRIs and SNRIs.
The occupancy results can be compared with the Phase I single-ascending dose study of BMS-830836 (Risinger et al. 2014) and with clinical reports from at least two other triple reuptake inhibitors in early drug development (Comley et al. 2013; DeLorenzo et al. 2011). In these studies, DAT and SERT occupancies were assessed using PET and the radioligands [11C]PE2I and [11C]DASB, respectively, in healthy volunteers, following single doses of the novel compounds. After single oral doses of 3 mg BMS-820836, the estimated DAT and SERT occupancies were in the range where therapeutic effects are expected to occur (Risinger et al. 2014), whereas the current study demonstrated that these occupancy levels can be achieved for relatively low multiple oral doses of BMS-830836. For the novel compound SEP-255289 (DeLorenzo et al. 2011), mean DAT occupancies ranged between 33 % and 49 % for three different single doses of SEP-255289, resulting in an estimated maximum DAT occupancy of 85 %, whereas the SERT occupancies were generally below 20 %, despite in vitro potencies similar to DAT. For another novel compound (Comley et al. 2013), well-tolerated single doses of GSK1360707 were able to produce high SERT occupancies and moderate DAT occupancies, up to approximately 70 % and 60 %, respectively. Both SERT and DAT occupancies showed a similar linear proportionality with GSK1360707 plasma concentrations, whereas a level of saturation was not reached.
This Phase I study was an important step in the drug development process of BMS-820836. Certainly, the current study has some limitations, but due to the nature of the drug development process, questions need to be answered in consecutive Phase II studies, in male and female patients, using adequately powered samples of the target population, and having longer durations of treatment. One limitation was that this study was performed mainly in males, whereas safety and tolerability may differ between the sexes. Hospitalization of subjects was determined as necessary, but it may have affected some outcome parameters as a result of continuous care and social processes. In the 3- and 4-mg dose cohorts, titration was implemented to extend tolerability, which interfered with the establishment of steady-state conditions. As a consequence, the maximum tolerable dose and DAT occupancy were not established. Further, there was no clinical interview by a psychiatrist to confirm that the enrolled subjects were free from any psychiatric disorder. However, during the screening procedure, subjects were interviewed with regard to their medical history; subjects could be excluded on the basis of any sound medical, psychiatric, and/or social reason as determined by the investigator. Despite these minor limitations, the study design allowed consistent data acquisition for all dose cohorts.
In conclusion, the results of this Phase I study demonstrate an important mechanism of action of BMS-820836, a triple reuptake inhibitor, on the monoamine targets in healthy subjects. Levels of SERT and DAT occupancy were in a range associated with therapeutic efficacy of current antidepressants, following generally safe and well-tolerated multiple doses of up to 4 mg BMS-820836. Further evaluation is required in Phase II trials to determine overall antidepressant efficacy, safety, tolerability, and occurrence of side effects, in large cohorts of patients with major depressive disorder.
Disclosures
Ming Zheng, Feng Luo, Matthew Cahir, Sanjay Keswani, Wendy Hayes, and Zubin Bhagwagar are employees of Bristol-Myers Squibb. Roger Lane is a former employee of Bristol-Myers Squibb and a current employee of Isis Pharmaceuticals, Inc. Robert Risinger is a former employee of Bristol-Myers Squibb and a current employee of Alkermes, plc. David Burt is a former employee of Bristol-Myers Squibb and a current employee of GlaxoSmithKline. Lieuwe Appel and Gunnar Antoni report no financial relationships with commercial interests. This clinical Phase I trial was initiated and supported by Bristol-Myers Squibb. Bristol-Myers Squibb was involved in the original concepts and systematic review of existing trial evidence, the design, the choice of investigators, the control of allocation schedule, the conduct of the trial, the collection and monitoring of data, the analysis and interpretation, and the writing and approval of the report. The experiments described in this paper comply with the current laws of the country in which they were performed.
References
Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z, Pavics L (2005) Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 89:115–123
Bodkin JA, Lasser RA, Wines JD Jr, Gardner DM, Baldessarini RJ (1997) Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry 58:137–145
Chalon S, Garreau L, Emond P, Zimmer L, Vilar MP, Besnard JC, Guilloteau D (1999) Pharmacological characterization of (E)-N-(3-iodoprop-2-enyl)-2beta-carbomethoxy-3beta-(4′-methylphenyl) nortropane as a selective and potent inhibitor of the neuronal dopamine transporter. J Pharmacol Exp Ther 291:648–654
Chalon S, Tarkiainen J, Garreau L, Hall H, Emond P, Vercouillie J, Farde L, Dasse P, Varnas K, Besnard JC, Halldin C, Guilloteau D (2003) Pharmacological characterization of N, N-dimethyl-2-(2-amino-4-methylphenyl thio)benzylamine as a ligand of the serotonin transporter with high affinity and selectivity. J Pharmacol Exp Ther 304:81–87
Comley RA, Salinas CA, Slifstein M, Petrone M, Marzano C, Bennacef I, Shotbolt P, Van der AJ, Neve M, Iavarone L, Gomeni R, Laruelle M, Gray FA, Gunn RN, Rabiner EA (2013) Monoamine transporter occupancy of a novel triple reuptake inhibitor in baboons and humans using positron emission tomography. J Pharmacol Exp Ther 346:311–317
Damsa C, Bumb A, Bianchi-Demicheli F, Vidailhet P, Sterck R, Andreoli A, Beyenburg S (2004) "Dopamine-dependent" side effects of selective serotonin reuptake inhibitors: a clinical review. J Clin Psychiatry 65:1064–1068
DeLorenzo C, Lichenstein S, Schaefer K, Dunn J, Marshall R, Organisak L, Kharidia J, Robertson B, Mann JJ, Parsey RV (2011) SEP-225289 serotonin and dopamine transporter occupancy: a PET study. J Nucl Med 52:1150–1155
Dunlop BW, Nemeroff CB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337
Emond P, Garreau L, Chalon S, Boazi M, Caillet M, Bricard J, Frangin Y, Mauclaire L, Besnard JC, Guilloteau D (1997) Synthesis and ligand binding of nortropane derivatives: N-substituted 2beta-carbomethoxy-3beta-(4′-iodophenyl)nortropane and N-(3-iodoprop-(2E)-enyl)-2beta-carbomethoxy-3beta-(3′,4′-disubstituted phenyl)nortropane. New high-affinity and selective compounds for the dopamine transporter. J Med Chem 40:1366–1372
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659
Garlow SJ, Nemeroff CB (2004) The neurochemistry of depressive disorders: clinical studies. In: Charney DS, Nestler EJ (eds) Neurobiology of mental illness, 2nd edn. Oxford University Press, New York, pp 440–460
Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey L, McKellar J (1995) Assessment of dose proportionality: report from the Statisticians in the Pharmaceutical Industry/Pharmacokinetics UK Joint Working Party. Drug Inf J 29:1039–1048
Guilloteau D, Emond P, Baulieu JL, Garreau L, Frangin Y, Pourcelot L, Mauclaire L, Besnard JC, Chalon S (1998) Exploration of the dopamine transporter: in vitro and in vivo characterization of a high-affinity and high-specificity iodinated tropane derivative (E)-N-(3-iodoprop-2-enyl)-2beta-carbomethoxy-3beta-(4′-methylphenyl)nortropane (PE2I). Nucl Med Biol 25:331–337
Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J, Farde L, Sedvall G (1999) Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage 9:108–116
Halldin C, Erixon-Lindroth N, Pauli S, Chou YH, Okubo Y, Karlsson P, Lundkvist C, Olsson H, Guilloteau D, Emond P, Farde L (2003) [(11)C]PE2I: a highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging 30:1220–1230
Halldin C, Lundberg J, Sovago J, Gulyas B, Guilloteau D, Vercouillie J, Emond P, Chalon S, Tarkiainen J, Hiltunen J, Farde L (2005) [(11)C]MADAM, a new serotonin transporter radioligand characterized in the monkey brain by PET. Synapse 58:173–183
Hirschfeld RM, Montgomery SA, Aguglia E, Amore M, Delgado PL, Gastpar M, Hawley C, Kasper S, Linden M, Massana J, Mendlewicz J, Moller HJ, Nemeroff CB, Saiz J, Such P, Torta R, Versiani M (2002) Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry 63:826–837
Hirvonen J, Johansson J, Teras M, Oikonen V, Lumme V, Virsu P, Roivainen A, Nagren K, Halldin C, Farde L, Hietala J (2008) Measurement of striatal and extrastriatal dopamine transporter binding with high-resolution PET and [11C]PE2I: quantitative modeling and test-retest reproducibility. J Cereb Blood Flow Metab 28:1059–1069
James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL (2010) Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 363:905–917
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158
Lane RM (1998) SSRI-induced extrapyramidal side-effects and akathisia: implications for treatment. J Psychopharmacol 12:192–214
Larsen AK, Brennum LT, Egebjerg J, Sanchez C, Halldin C, Andersen PH (2004) Selectivity of (3)H-MADAM binding to 5-hydroxytryptamine transporters in vitro and in vivo in mice; correlation with behavioural effects. Br J Pharmacol 141:1015–1023
Lavretsky H, Kim MD, Kumar A, Reynolds CF III (2003) Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J Clin Psychiatry 64:1410–1414
Learned-Coughlin SM, Bergstrom M, Savitcheva I, Ascher J, Schmith VD, Langstrom B (2003) In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry 54:800–805
Li Y-W, Lelas S, Liu S, Raybon J, Pieschl R, Langdon S, Orie A, Wright R, Molski T, Lodge N, Molino B, Sinz M, Adams S, Olson R, Macor J, Sargent B, Houston J, Zaczek R (2012) Preclinical pharmacological characterization of BMS-820836, a novel triple monoamine reuptake inhibitor. Biol Psychiatry 71:299S
Lundberg J, Odano I, Olsson H, Halldin C, Farde L (2005) Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J Nucl Med 46:1505–1515
Lundberg J, Christophersen JS, Petersen KB, Loft H, Halldin C, Farde L (2007) PET measurement of serotonin transporter occupancy: a comparison of escitalopram and citalopram. Int J Neuropsychopharmacol 10:777–785
Marshall RD, Liebowitz MR (1996) Paroxetine/bupropion combination treatment for refractory depression. J Clin Psychopharmacol 16:80–81
Meyer JH (2007) Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci 32:86–102
Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S (2001) Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry 158:1843–1849
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S (2002) Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 163:102–105
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S (2004) Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 161:826–835
Millan MJ (2009) Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics 6:53–77
Morelli E, Moore H, Rebello TJ, Gray N, Steele K, Esposito E, Gingrich JA, Ansorge MS (2011) Chronic 5-HT transporter blockade reduces DA signaling to elicit basal ganglia dysfunction. J Neurosci 31:15742–15750
Nemeroff CB (2007) Prevalence and management of treatment-resistant depression. J Clin Psychiatry 68(Suppl 8):17–25
Nikolaus S, Hautzel H, Heinzel A, Muller HW (2012) Key players in major and bipolar depression—a retrospective analysis of in vivo imaging studies. Behav Brain Res 232:358–390
Risinger R, Bhagwagar Z, Luo F, Cahir M, Miler L, Mendonza A, Meyer JH, Zheng M, Hayes W (2014) Evaluation of safety and tolerability, pharmacokinetics and pharmacodynamics of BMS-820836 in healthy subjects: a placebo-controlled, ascending single-dose study. Psychopharmacology 231:2299–2310
Tarkiainen J, Vercouillie J, Emond P, Sandell J, Hiltunen J, Frangin Y, Guilloteau D, Halldin C (2001) Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. J Labelled Comp Radiopharm 44:1013–1023
Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ (2006a) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354:1243–1252
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M (2006b) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40
Voineskos AN, Wilson AA, Boovariwala A, Sagrati S, Houle S, Rusjan P, Sokolov S, Spencer EP, Ginovart N, Meyer JH (2007) Serotonin transporter occupancy of high-dose selective serotonin reuptake inhibitors during major depressive disorder measured with [11C]DASB positron emission tomography. Psychopharmacology (Berl) 193:539–545
Acknowledgements
The current staff at the PET Centre, as well as the former staff at GE Healthcare Uppsala Imanet and Quintiles AB (Phase I), Uppsala, Sweden, are acknowledged for their various contributions related to this study. Special thanks to Wolfgang Kühn, MD, and Jakob Björk, PhD, Quintiles AB, Uppsala, Sweden, for their contributions as principal investigator and project manager, respectively. The authors are grateful to Brian Atkinson, PhD, a former employee of Bristol-Myers Squibb, for providing writing and editorial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. M. Zheng and L. Appel contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 661 kb)
Rights and permissions
About this article
Cite this article
Zheng, M., Appel, L., Luo, F. et al. Safety, pharmacokinetic, and positron emission tomography evaluation of serotonin and dopamine transporter occupancy following multiple-dose administration of the triple monoamine reuptake inhibitor BMS-820836. Psychopharmacology 232, 529–540 (2015). https://doi.org/10.1007/s00213-014-3688-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3688-x