Abstract
Rationale
Zolpidem is a short-acting, non-benzodiazepine hypnotic that acts as a full agonist at α1-containing GABAA receptors. Overall, zolpidem purportedly has fewer instances of abuse and dependence than traditionally used benzodiazepines. However, several studies have shown that zolpidem may be more similar to benzodiazepines in terms of behavioral tolerance and withdrawal symptoms.
Objectives
In the current study, we examined whether subchronic zolpidem or diazepam administration produced deficits in zolpidem’s locomotor-impairing effects, anxiety-like behaviors, and changes in GABAAR subunit messenger RNA (mRNA).
Methods
Mice were given subchronic injections of either zolpidem (10 mg/kg), diazepam (20 mg/kg), or vehicle twice daily for 7 days. On day 8, mice were given a challenge dose of zolpidem (2 mg/kg) or vehicle before open field testing. Another set of mice underwent the same injection regimen but were sacrificed on day 8 for qRT-PCR analysis.
Results
We found that subchronic zolpidem and diazepam administration produced deficits in the acute locomotor-impairing effects of zolpidem and increased anxiety-like behaviors 1 day after drug termination. In addition, we found that subchronic treatment of zolpidem and diazepam induced distinct but overlapping GABAAR subunit mRNA changes in the cortex but few changes in the hippocampus, amygdala, or prefrontal cortex. Levels of mRNA measured in separate mice after a single injection of either zolpidem or diazepam revealed no mRNA changes.
Conclusions
In mice, subchronic treatment of zolpidem and diazepam can produce deficits in the locomotor-impairing effects of zolpidem, anxiety-like withdrawal symptoms, and subunit-specific mRNA changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the imidazopyridine zolpidem has replaced benzodiazepines (BZs) as the most common prescription for patients suffering from insomnia (Benca 2005; Hoffman et al. 2007; IMS-Health 2011). The hypnotic effects of zolpidem and BZs are induced by enhancing the actions of the gamma-aminobutyric acid (GABA) at GABA type A receptors (GABAARs). These receptors are heteromeric complexes composed of multiple subunits (Barnard et al. 1998; Burt and Kamatchi 1991; Mehta and Ticku 1999). Whereas classical nonselective BZs can bind to GABAARs containing α1, α2-, α3-, or α5-subunits, zolpidem preferentially binds to receptors containing the α1-subunit (Low et al. 2000; Sanna et al. 2002), has limited affinity for the α2- and α3-subunits, and almost no affinity for the α5-subunit (Sanna et al. 2002). The sedative-hypnotic effects of BZs and zolpidem are mediated by GABAARs containing the α1-subunit, which are the largest subtype population, representing about 60 % of all GABAARs (McKernan and Whiting 1996).
While zolpidem is purportedly safe within clinical dose levels (Greenblatt and Roth 2012; Roehrs et al. 2012), numerous cases of zolpidem abuse and dependence have been reported in individuals using supratherapeutic doses (Hajak et al. 2003; Victorri-Vigneau et al. 2007). In humans, the prevalence of zolpidem abuse and dependence is higher in those who have a history of psychiatric disturbances and substance abuse, including BZ and alcohol abuse (Gericke and Ludolph 1994; Huang et al. 2007; Victorri-Vigneau et al. 2013). The development of tolerance after repeated treatments and the presence of withdrawal symptoms have been implicated as causal factors in BZ abuse and dependence (Lader 2011). However, there is conflicting evidence as to whether repeated zolpidem treatments can induce similar behavioral tolerance and withdrawal-like symptoms in rodent models that are traditionally used to elucidate the mechanisms underlying drug tolerance and dependence. Some studies have reported behavioral tolerance and withdrawal-like symptoms after prolonged zolpidem treatments in rodents (Auta et al. 2008; Murphy et al. 2011; Vlainic and Pericic 2009), while others have failed to show either tolerance (Ebert et al. 2008; Sanger and Zivkovic 1987, 1992) or withdrawal symptoms (Elliot and White 2000; Perrault et al. 1992). The degree to which repeated administration of zolpidem can produce neuroadaptive changes that may give rise to tolerance and dependency is uncertain. Past studies have identified plausible neuroadaptive mechanisms underlying BZs tolerance and withdrawal, including alterations in GABAAR subunit messenger RNA (mRNA) expression (Uusi-Oukari and Korpi 2010; Vinkers and Olivier 2012). Surprisingly, few studies have examined whether repeated zolpidem administration also alters GABAAR subunit mRNA expression.
In the current study, we examined whether a subchronic (7 days) treatment of either zolpidem or diazepam decreases the locomotor-impairing effects of zolpidem and/or produces anxiety-like symptoms in open field testing 1 day after treatment termination. Next, we investigated whether these treatment regimens induced changes in GABAAR subunit and GABAergic associated mRNAs by using quantitative RT-PCR.
Materials and methods
Animals
Male C57BL/6J mice about 3 months old and weighing about 25 g (Jackson Laboratories, Maine) were used in this study. Mice were housed in standard group cages (n = 4), allowed full access to food and water, and maintained on a 12-h light/dark cycle (light on from 7:00 a.m.). Experiments were performed during the light cycle and were approved by the UTHSC Institutional Review Board. All experiments followed the principles and standards of animal care outlined by the NIH publication “Guide for the Care and Use of Laboratory Animals” (Clark et al. 1997).
Drugs
Diazepam and zolpidem (Sigma, St Louis, MO) were suspended in saline with 0.3 % Tween-80 (vehicle). Drugs were administered intraperitoneally (i.p.) at a volume of 10 ml/kg. Mice were treated twice a day (8:00 am and 5:00 pm) for either 1 day (acute) or seven consecutive days (subchronic) with 5 mg/kg of zolpidem (ZOLP), 10 mg/kg of diazepam (DZP), or vehicle (VEH). Treatment doses were based on previous experiments designed to study the development of tolerance to diazepam or zolpidem (Ebert et al. 2008; Fernandes et al. 1999; Pugh et al. 1992; van Rijnsoever et al. 2004; Vinkers et al. 2012; Vlainic and Pericic 2009). On day 8, mice were administered with 2 mg/kg of zolpidem and were placed back in home cage for 2 min prior to testing. The test dose was selected from dose-response curves indicating the 50 % effective dose (ED50) for the locomotor-impairing effects of zolpidem in rats and mice ranging from 1.0 to 2.5 mg/kg (Griebel et al. 1999; Perrault et al. 1990). A 2-min latency between test drug injection and testing onset has been successfully used to identify the tolerance to the locomotor-impairing effects of the BZ midazolam (King et al. 1987), which, like zolpidem, is a relatively short acting and rapidly eliminated (Pieri et al. 1981). Animal weights were recorded before all drug injections. Daily injections and behavioral testing were conducted in distinct environments to prevent context-specific tolerance (Siegel 1989).
Behavioral testing and apparatus
Locomotor and exploratory activity was measured by examining open field behaviors during a 60-min test session (Vogel and Vogel 2002). Animals were treated with zolpidem (ZOLP), diazepam (DZP), or vehicle (VEH). On day 8, mice were given a test injection of zolpidem (ZOLP-ZOLP, n = 13; DZP-ZOLP, n = 13; VEH-ZOLP, n = 13) or vehicle (ZOLP-VEH, n = 8; DZP-VEH, n = 9; VEH-VEH, n = 9). Two minutes after test injections, mice were individually placed in open field chambers constructed from a clear polycarbonate and equipped with four 24-beam infrared arrays across the base of each chamber wall (MED Associates, Model ENV-520). Horizontal motor distance (in centimeters), vertical activity (rearing counts), time spent in the center and periphery zones, and number of zone transitions were automatically recorded and collected via a computer with the MED Associates’ Activity Monitor Data Analysis software. The central zone was defined as the compartment of the floor centrally located 8 cm from the perimeter of the chamber walls. Testing was conducted under standard test room lighting (100 lux), and each animal was tested once. Testing was conducted 18–24 h after the last treatment injection.
RT-PCR analysis
To investigate whether 7-day drug treatments induced changes in mRNA levels, mice were given injections following the same treatment regimen used in the behavioral studies and euthanized on day 8 without test injections or open field testing (ZOLP, n = 8; DZP, n = 9; VEH, n = 6). Similarly, changes in the mRNA expression after acute drug treatment were evaluated in mice euthanized 1 day after zolpidem, diazepam, or vehicle treatment regimen (ZOLP, n = 7; DZP, n = 6; VEH, n = 5).
Tissue isolation, RNA extraction, and RT-PCR analysis
Whole mouse brains were rapidly isolated and immediately frozen on crushed dry ice. Tissue isolation was performed by sectioning brains (300-μm thick) on a Leica cryostat, followed by dissection of the prefrontal cortex (mPFC), somatomotor cortex, basolateral amygdala, and the dorsal hippocampus (CA1, CA3, dentate gyrus) using pa stereomicroscope and a scalpel blade under RNAse-free conditions. After the collection, samples were stored in buffer RLT Lysis buffer (QUIAGEN) at −20 °C. Samples were then homogenized by sonication and RNA was isolated with the RNeasy protect mini kit (QIAGEN). RNA concentration and purity were determined using a microplate reader in conjunction with a Take3™ Micro-Volume plate (BioTek Instruments, Inc., Winooski, VT). The ratio of absorbance at 260 and 280 nm of samples were commonly in the range of 1.8–2.2. Samples with A260/A280 of less than 1.8 were considered to be contaminated by protein and excluded from further analyses. RNA integrity was determined using the Agilent Bioanalyzer 2100 (Agilent Technologies, UK) to generate an electropherogram and an RNA integrity number (RIN). RIN scores ranged between 5.3 and 9.4.
RNA was transcribed to cDNA using Transcriptor first-strand cDNA synthesis kit (Roche) following the manufacturer’s instructions. One reaction consisted of 2 μL Anchored-oligo(dT) primer (2.5 μM), 4 μL random hexamer primer (60 μM), 8 μL reaction buffer 5× (8 mM), 1 μL protector TNase inhibitor (20 U), 4 μL deoxynucleotide mix (1 mM), and 1 μL reverse transcriptase (10 U). Varying volumes of RNA (amounting to 100 ng) and PCR-grade water were added for a 20-μL reaction mixture. Complementary DNA (cDNA) was transcribed using an Eppendorf Mastercycler thermal cycler (Eppendorf) gradient PCR machine. Reverse transcription incubation conditions were 25 °C for 10 min, 55 °C for 30 min, and 85 °C for 5 min.
The relative expression levels of α1-5, β1-3, γ1-2 and δ subunits, GABAA clustering protein gephyrin, GABA receptor-associated protein (GABARAP), and GABA transporter 1 (GAT-1) were measured in mice given 7 days of treatment. Measurements for mice given acute administration were limited to α1-3, α5, β2, γ2, GABARAP, and GAT-1. Primer and probe combinations were designed using ProbeFinder software (version 2.45; Roche Applied Science). The oligonucleotide sequences used for the primers (Integrated DNA Technologies) and gene transcript IDs are shown in Table 1.
To determine PCR efficiencies, we generated standard curves for each target and reference genes (β-tubulin, β-actin, cyclin D, hypoxanthine phosphoribosyltransferase 1, ribosomal protein S19, and TATA-binding protein) by plotting the threshold value (Ct) versus the log of the amount of serial dilutions of cDNA (2 μg–0.2 ng). Separate standard curves were generated for each brain region analyzed. The amplification efficiencies of target and reference genes were within an acceptable slope range of −3.6 to −3.1, corresponding to efficiencies close to 100 %. TATA-binding protein was used as the endogenous reference gene and amplified in parallel with target genes. This reference gene showed stable expression across samples and between brain regions. PCR efficiencies between the target and reference genes were relatively equal. Real-time PCR was performed using the TaqMan detection method (for review, see Giulietti et al. 2001) and LightCycler® 480 System (Roche). Each reaction was performed in triplicate and consisted of 0.1 μL of UPL probe (10 μM), 0.1 μL of forward primer (20 μM), 0.1 μL of reverse primer (20 μM), 5 μL of LC 480 2X master mix (Roche), 2.7 μL of PCR grade water, and 2 μL of cDNA (10 ng). The PCR reaction consisted of initial incubations at 95 °C for 5 min followed by 50 amplification cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 10 s.
Data reduction and statistical analyses
To examine group differences, analyses were conducted on distance traveled, number of vertical counts, number of center zone entries, and percent of center zone time during each 10-min block using a three-way ANOVA with treatment (VEH, DZP, and ZOLP) and test (VEH and ZOLP) as between-subject factors and time (blocks 1–6) as a within-subject factor. Follow-up analyses were done with lower order ANOVAs and Bonferroni t tests where appropriate.
Calculations of standard curves, CT values, and quantification of mRNA expression levels were performed by Gene Scanning software (version 1.5.0) provided with the LightCycler® 480 Instrument (Roche Applied Science). The comparative CT method (ΔΔCT = ΔCT reference-ΔCT target) was used to compare expression levels of mRNAs between the control (VEH) and treatment (ZOLP and BZP) groups. For ease of presentation, data in tables and figures are expressed as relative fold change by applying the 2-ΔΔCT equation. Statistical comparisons were carried out for each analyzed region (cortex hippocampus, amygdala, and prefrontal cortex) and treatment protocol (1-day, 7-day), independently. Statistical analyses were performed by ANOVA with Dunnett's t test for pairwise comparisons against a single control (VEH) to maintain a 0.05 error rate for each analysis. Comparisons of ΔCT values indicated that the expression profile of GABAAR subunit mRNAs were similar to previously published report (Duncan et al. 1995; Heldt and Ressler 2007; Tietz et al. 1999; Wisden et al. 1992). In vehicle-treated animals, the relative abundance of mRNA was as follows: cortex, α1 > γ2 > β2 > α3 > α4 > β3 > δ > α5 > β1 > α2 > γ1; hippocampus, γ2 > α5 > β3 > α1 > β2 > α4 > β1 > α2 > α3 > δ > γ1; amygdala, γ2 > α3 > β2 > α1 > β3 > α4 > γ1 > β1 > α5 > α2 > δ; and PFC, α1 > α3 > γ2 > β2 > β3 > α2 > β1 > α5 > δ > α4 > γ1.
Results
Behavioral testing
The three-way ANOVA for locomotor activity revealed significant main effects of test, F(1, 59) = 61.68, p < 0.01, and time, Fs(5, 295) < 7.48, p < 0.01. The analysis also yielded significant interactions of test × time, F(5, 295) = 130.99, p < 0.01, treatment × time, F(10, 295) = 5.47, p < 0.01, and treatment × test × time, F(5, 295) = 4.91, p < 0.01. To examine the three-way interaction, groups receiving ZOLP and VEH during testing were analyzed separately using two-way ANOVAs with treatment and time as factors.
For mice receiving ZOLP during testing, the two-way ANOVA yielded significant main effects of treatment, F(2, 36) = 10.03, p < 0.01, time, F(5, 180) = 119.77, p < 0.01, and a reliable treatment × time interaction, F(10, 180) = 9.35, p < 0.01, signifying performance over time was differentially affected by drug treatment. Overall, ZOLP-ZOLP and DZP-ZOLP groups showed greater activity than the VEH-ZOLP group, ps < 0.05 (Fig. 1a). No differences were detected between ZOLP-ZOLP and DZP-ZOLP groups for overall activity (p > 0.05). Simple one-way ANOVAs at each level of time revealed group differences on blocks 1, 2, and 3, Fs(2, 36) > 12.30, ps < 0.01. Post hoc t tests revealed that the activity in ZOLP-ZOLP and DZP-ZOLP groups was significantly greater than the VEH-ZOLP group during blocks 1–3, ps < 0.01. No other differences were detected.
Decreased locomotor-impairing effects of zolpidem after subchronic zolpidem or diazepam treatment. Distance traveled in open field after test administration of 2 mg/kg of zolpidem (a) or vehicle (b) in mice previously given subchronic treatment of zolpidem (ZOLP), diazepam (DZP), or vehicle (VEH). Analyses of each 10-min block showed that differences between groups were most evident in the first half of the 60-min test session. Data are presented as mean ± sem. Black star denotes VEH-ZOLP < ZOLP-ZOLP and DZP-ZOLP groups (p < 0.05)
For the group receiving VEH during testing, the two-way ANOVA showed reliable main effects of treatment, F(2, 23) = 7.65, p < 0.01, and time, F(5, 115) = 318.57; p < 0.01. The treatment × time interaction was marginally significant, F(10, 115) = 1.96, p = 0.05. Overall, DZP-VEH and ZOLP-VEH groups showed less activity than VEH-VEH mice, ps < 0.05. No reliable differences were detected between DZP-VEH and ZOLP-VEH groups in terms of overall activity. One-way ANOVAs at each level of time identified significant treatment effects during the blocks 1, 2, 3, and 5, Fs(2, 23) < 3.52, ps < 0.05. Post hoc comparisons showed that VEH-VEH mice displayed more activity than DZP-VEH mice on blocks 1–3 and 5, and more activity than ZOLP-VEH mice on blocks 1 and 2 (Fig. 1b).
The VEH-VEH test group spent a greater percentage of time in the central zone, had a greater number of center zone entries, and a higher frequency of rearing. For percent of central zone time, the ANOVA yielded main effects of treatment, F(2, 23) = 5.27; p < 0.02, time, F(5, 115) = 8.36; p < 0.01, and a significant treatment × time interaction, F(10, 115) = 2.49; p = 0.01. Overall, ZOLP-VEH and DZP-VEH groups spent significantly less percentage of time in the center zone compared to VEH-VEH mice, ps < 0.01. One-way ANOVAs followed by Bonferroni t tests indicated significant differences during the second half of open field testing (Fig. 2a). For the number of center zone entries, the main effects of treatment, F(2, 23) = 8.20; p < 0.01, and time, F(5, 115) = 41.67; p < 0.01, were significant, as w the treatment × time interaction, F(10, 115) = 2.67; p < 0.01. ANOVAs followed by t tests indicated that ZOLP-VEH and DZP-VEH groups had fewer center zone entries during the blocks 1 and 2 (Fig. 2b). Analyses of rearing data showed reliable effects of time, F(5, 115) = 6.28; p < 0.01, and treatment, F(2, 23) = 41.67; p < 0.01. Overall, the frequency of rearing was lower in both ZOLP-VEH and DZP-VEH groups in comparison to the VEH-VEH group (Fig. 2c). Analyses and figures showing the effects of zolpidem treatment on the number of vertical counts, number of center zone entries, and percent of center zone time are given in online resource 1.
Changes in open field (OF) exploratory behaviors after cessation of subchronic zolpidem or diazepam treatment. Percent of center time (a), number of center zone entries(b), and frequency of rearing (c) in open field after test administration of vehicle (VEH) in mice previously given subchronic treatment of zolpidem (ZOLP-VEH), diazepam (DZP-VEH), or vehicle (VEH-VEH). Analyses were conducted on means of each 10-min block. Data are presented as mean ± sem. Black star denotes VEH-VEH significantly different than ZOLP-VEH and DZP-VEH groups (p < 0.05). Number sign denotes VEH-VEH significantly different than DZP-VEH group (p < 0.05). Asterisk denotes main effect of treatment, VEH-VEH > ZOLP-VEH and DZP-VEH groups (p < 0.05)
RT-PCR analysis: subchronic treatment
Cortex
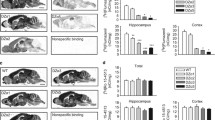
As illustrated by Fig. 3a, subchronic treatment of zolpidem and diazepam induced distinct and overlapping GABAAR subunit changes in the cortex. Both zolpidem and diazepam caused a decrease in the expression of α1-, α3-, β2-, and δ-GABAAR subunits in the cortex. Diazepam, but not zolpidem, induced significant decreases in expression of α4-, α5-, β1-, and γ2-GABAAR subunits after chronic treatment. These findings were identified by one-way ANOVAs, which showed significant group differences, Fs(2, 17) > 4.39, ps < 0.05. Follow-up Dunnett’s t tests revealed relative low expression levels of α1-, α3-, β2-, and δ-GABAAR subunits in ZOLP and DZP groups compared to VEH controls (ps < 0.05). Levels of α4-, α5-, β1-, and γ2-GABAAR subunits were significantly lower in DZP mice compared to VEH controls (ps < 0.05). In addition to subunits, group differences were identified for the GABAergic-associated genes gephyrin and GAT-1, Fs(2, 17) > 4.95, ps < 0.05. For both of these transcripts, expression levels were significantly lower in DZP mice compared to VEH controls (ps < 0.05).
Relative levels of mRNA in the somatomotor cortex (a) and dorsal hippocampus (b) following 7-day administration of zolpidem (ZOLP), diazepam (DZP), or vehicle (VEH). Histograms comparing levels of transcripts are expressed as mean percentage of VEH control (100 %). Statistical analyses were performed by ANOVA with Dunnett’s t test for pairwise comparisons against a VEH. Data are presented as mean + sem. Black star denotes VEH significantly different than ZOLP and DZP treatments (p < 0.05); Number sign denotes VEH significantly different than DZP treatment (p < 0.05)
Hippocampus
As shown by Fig. 3b, the expression profile observed in the hippocampus was different in comparison to that of the cortex. Overall, significant group differences were limited to expression levels of α5- and γ2-GABAAR subunits, Fs(2, 17) > 4.39, ps < 0.05. The expression of both of these transcripts was lower in the hippocampus of DZP- compared to VEH-treated mice, ps < 0.05.
Prefrontal cortex and amygdala
No significant group differences in mRNA transcript levels were identified in the amygdala or prefrontal cortex. Changes in amygdala or prefrontal cortex mRNA expression levels are given in online resource 2.
RT-PCR analysis: acute treatment
To examine whether mRNA expression changes seen in the subchronic treatment may be due to acute effects of drug administration, we measured the expression level in a subpopulation of genes after acute (1 day) zolpidem and diazepam administration. One-way ANOVAs revealed no significant group differences in candidate genes. The relative fold changes in mRNA expression after acute administration are presented in online resource 3.
Discussion
The current study reveals that mice previously exposed to zolpidem or diazepam treatment for 7 days are less sensitive to the locomotor-impairment of subsequent zolpidem treatment compared to those given prior vehicle injections. The reduced impairing effect of zolpidem does not appear to be due to increases in activity caused by cessation of subchronic treatments as zolpidem and diazepam groups showed mild decreases, rather than increases, in motor activity relative to vehicle-treated animals. Also, during a drug-free open field test 18–24 h after treatment termination, mice given either zolpidem or diazepam showed anxiety-like behaviors, including decreases in activity, rearing, and center zone entries. These mice also showed a progressive decrease percent of time in center zone, suggesting a gradual increase in anxiety during testing.
While tolerance has been widely reported in rodents following repeated BZs treatments (Fernandes et al. 1999; Galpern et al. 1991; Miller et al. 1989), there is conflicting evidence concerning the development of tolerance to zolpidem. Our findings are in agreement with Vlainic and Pericic (2009), who show that mice develop tolerance to the locomotor-impairing effects of zolpidem and diazepam after 10 days of treatment. Tolerance to zolpidem’s ataxic, hypothermic, and anticonvulsant effects have been observed in rodents and primates (Auta et al. 2008; Elliot and White 2000; Griffiths et al. 1992; Vlainic and Pericic 2009; Voss et al. 2003). Other studies have reported no tolerance after zolpidem administration in rodents (Ebert et al. 2008, 1992; Pesold et al. 1997). The detection of zolpidem tolerance in past studies is likely influenced by numerous factors; however, one major difference between ours and previous rodent studies was the latency between test drug injection and the onset of tolerance testing. In most studies, the time between zolpidem injection and behavioral testing onset is 15–30 min. In the current study, the time between the test injection and start of testing was 2 min. As seen in Fig. 1a, the motor-impairing effects of zolpidem are clearly evident during the first 30-min following drug injection, after which activity increased to levels seen in vehicle animals (Fig. 1b). Because zolpidem is relatively short acting and quickly metabolized, longer latencies between drug injection and the testing onset may prevent detection of tolerance.
The current study also identified diazepam cross-tolerance, as indicated by the diminished motor-impairing effects of zolpidem in diazepam-treated mice. While numerous studies have documented cross-tolerance between BZs in rodents (e.g., Cohen and Sanger 1994; File 1985; Marin et al. 1999), relatively few have examined cross-tolerance between zolpidem and BZs (Auta et al. 2008; Perrault et al. 1992). Vinkers et al. (2012) showed that mice chronically administered with diazepam displayed tolerance to diazepam’s hypothermic, locomotor-impairing and anxiolytic effects, while chronic zolpidem treatment resulted in the development of cross-tolerance to diazepam-induced hypothermic and anxiolytic-like effects but not to its locomotor-impairing effects. Point-mutated mice lacking diazepam binding at either α1- or α5- containing GABAARs failed to display any sedative tolerance (van Rijnsoever et al. 2004). These findings suggest that chronic BZ treatments causes cross-tolerance to zolpidem, whereas the detection of cross-tolerance to other BZs after chronic zolpidem administration is less likely. In addition, this implies that zolpidem-induced locomotor-impairing effects are mediated by α1-GABAARs, whereas the locomotor-impairing effects of diazepam and other nonselective BZ may be due to the activation of α1-GABAARs in combination with other GABAAR subtypes.
In nonhuman primates and rodents, chronic BZ administration can produce physical dependence as detected by the occurrence of withdrawal symptoms after treatment termination (Allison et al. 2002; File 1990; Griffiths and Weerts 1997; Lukas and Griffiths 1984; Yanagita and Takahashi 1973). Our results from diazepam-treated mice are consistent with previous studies showing anxiety-like behaviors in the elevated plus maze and open field when assessed 1–3 days after prolonged treatments (Acikmese et al. 2012; Allison et al. 2002; Divljaković et al. 2013; Steppuhn and Turski 1993; Ward and Stephens 1998). Animal studies investigating withdrawal-like symptoms following zolpidem treatment are varied. In nonhuman primates, spontaneous and flumazenil-induced withdrawal effects are reliably observed after chronic treatment with zolpidem (Weerts et al. 1998; Weerts and Griffiths 1998). In rodents, some studies have identified withdrawal-like symptoms (Ebert et al. 2008; Metten et al. 1998; Renger et al. 2004), while others have not (Elliot and White 2000; Perrault et al. 1992; VonVoigtlander and Lewis 1991). The distinctive withdrawal profile in rodents and primates may be due to differences in the distribution of α1-GABAAR subtypes and/or the binding characteristics of zolpidem (Benavides et al. 1993; Duncan et al. 1998; Schmid et al. 1995). Our study identified subchronic zolpidem treatment produced open field anxiety-like behavior after drug termination. Along with methodological differences between our and previous negative findings, it is possible that detection of anxiety-like behaviors may be more easily identified than other withdrawal-like symptom measured after prolonged zolpidem treatments (e.g., seizure thresholds). Interestingly, Murphy et al. (2011) have shown EPM anxiety-like behaviors in rats both during and after zolpidem administration. In the context of our study, these data may suggest that repeated zolpidem treatment produces an anxiogenic-like state that is still evident after treatment termination.
While it is possible that the decreases in activity were associated with the residual drug effects during testing, evidence from previous studies suggest this is not the case. In rats, the elimination half-life (t1/2) from the plasma is approximately 30 min for zolpidem (Benavides et al. 1988; Garrigou-Gadenne et al. 1989; Lau et al. 2002) and 70 min for diazepam and its active main metabolite desmethyldiazepam (Friedman et al. 1986; Markowitz et al. 2010; Walker et al. 1998). In the case of zolpidem, daily injections do not change t1/2 values or result in plasma or brain drug accumulation (Trenque et al. 1994). Repeated injection of zolpidem for up to 28 days is virtually undetectable 3–4 h after the last injection (Trenque et al. 1994), and the degradation of zolpidem does not lead to the formation of metabolites that can accumulate and sustain drug action (Diaz-Garcia et al. 1992; Drover 2004). Likewise, daily diazepam injections do not change t1/2 values (Walker et al. 1998), and most studies (Auta et al. 1994; Loscher and Schwark 1985; Smith and Darlington 1994), but not all studies (Fernandes et al. 1996), reveal no plasma accumulation of diazepam or its metabolite after long-term treatments (Auta et al. 1994). These data argue against dramatic changes in the pharmacokinetics as the basis for the detection of behavioral tolerance or anxiety-like behaviors.
In humans, the abrupt cessation of prolonged BZ treatments can be followed by unpleasant withdrawal symptoms including anxiety (e.g., Petursson 1994), which are believed to play a role in BZ abuse and dependence (Lader 2011). The abrupt cessation of supratherapeutic doses of zolpidem can also lead to a BZ-like withdrawal syndrome (Aragona 2000; Cubala and Landowski 2007; Huang et al. 2007; Kripke 2007; Sethi and Khandelwal 2005). It is hypothesized that zolpidem withdrawal and tolerance are due to long-term supratherapeutic dose activation of the α2- and α3-subunits along with α1-subunit containing GABAARs (Liappas et al. 2003). While zolpidem binds to α1-subunits with an affinity that is five- to tenfold greater than α2- and α3-subunits in vitro (Petroski et al. 2006; Sanna et al. 2002), the relatively high dose of zolpidem used in this study might be sufficient to activate these lower-affinity subunits in vivo and contribute to deficits in the locomotor-impairing effects of zolpidem and anxiety-like behaviors. Subchronic treatment of zolpidem and diazepam produced subunit- and region-specific reduction of GABAAR mRNAs. In Fig. 3a, there were trends in decreased mRNA levels for many subunits after subchronic zolpidem and diazepam treatments. Significant decreases in the expression of α1-, α3-, β2-, and δ-subunits in the cortex were identified after both treatments. There were also decreases in the cortical expression of α4-, β1-, and γ2-subunits with diazepam treatment. Group differences in other subunits may have become significant with higher or longer treatment regimens. Levels of mRNA were not affected in separate mice after a single injection of zolpidem or diazepam.
Past studies have revealed numerous GABAAR subunit mRNA changes that might contribute to tolerance development and withdrawal after long-term BZ treatment regimens (for review, see Uusi-Oukari and Korpi 2010). In rats, chronic diazepam treatments have been shown to alter α1-subunit mRNA expression in the cerebral cortex (Auta et al. 2008; Heninger et al. 1990; Impagnatiello et al. 1996; Longone et al. 1996; Pesold et al. 1997; Tietz et al. 1999), although this was not observed in all studies (Holt et al. 1996; Vinkers and Olivier 2012; Zhao et al. 1994). Likewise, Holt et al. (1997) reported that long-term zolpidem treatment decreases α1 mRNA in cerebral cortex after 14 days of treatment in rats. Decreased cortical α1-subunit mRNA in the current study concurs with many of these past observations. We also observed decreases in levels α5- and γ2-subunit mRNAs in the hippocampus after diazepam treatment. This latter finding is consistent with some reports (Wu et al. 1994; Zhao et al. 1994), but not all (Impagnatiello et al. 1996). The current study found no significant α1 mRNA changes in other areas. In rats, others have shown that diazepam treatment decreases α1-subunit mRNA in the hippocampus (Impagnatiello et al. 1996) and prefrontal cortex (Auta et al. 2008). However, in these studies, diazepam was administered three times daily for 14 days at increasing doses (5–20 mg/kg). Thus, more prolonged exposure and/or higher dose levels of diazepam may be necessary for prefrontal cortex changes. It is also important to note that rats have been historically used to investigate mRNA changes associated with tolerance and withdrawal after BZ treatments, while mice have been used in only a few studies (Fahey et al. 1999; Kang and Miller 1991). Neuroanatomical and behavioral differences between these two species may contribute to published discrepancies (Asan et al. 2005; Blanchard et al. 2001; Jardim et al. 1999).
Our study observed cortical decreases in α3- and δ-subunit mRNA after both treatments and α4-subunit mRNA after diazepam treatment. Holt et al. (1996) found that long-term treatment with diazepam increased α3 and α4 mRNA expression in the cerebral cortex, whereas other studies have found no such effects (see also, Arnot et al. 2001; Impagnatiello et al. 1996; Wu et al. 1994). An upregulation of α4 mRNA in the cortex has also been identified after long-term treatment with zolpidem (Holt et al. 1997). No previous studies have noted changes in δ mRNA after long-term diazepam or zolpidem exposure in vivo (Holt et al. 1997; Impagnatiello et al. 1996). Both α4- and δ-containing GABAARs are usually found in perisynaptic and extrasynaptic regions of the brain, including the cortex (Korpi et al. 2002; Pirker et al. 2000). These receptors are insensitive to BZs and zolpidem, indicating that changes in GABAAR mRNA levels are not necessarily dependent on direct drug-receptor interaction. In cultured cells, withdrawal from long-term treatment of diazepam and zolpidem has been shown to increase α4-subunit and decrease α1-subunit expression (Biggio et al. 2003; Follesa et al. 2001), suggesting that the molecular events that underlie withdrawal may involve a shift from BZ-sensitive to BZ-insensitive GABAAR subtypes.
While zolpidem preferentially binds to α1-GABAARs, it also possesses some agonistic action on α3-subunit containing receptors at high doses (Araujo et al. 1996; Sanna et al. 2002), which may mediate the decrease in α3 mRNA after zolpidem treatment. It is noteworthy that the anxiolytic effects of BZs are putatively mediated by α2- and α3-GABAAR subtypes (Atack et al. 2005; Crestani et al. 2001; Low et al. 2000). While no mRNA changes were detected in the amygdala, which is known to be involved in fear and anxiety (Davis 2000), changes in function of these receptors in the cortex may play a role in processing of sensory information that mediate anxiety-like behavior seen in vehicle-tested drug groups.
Alterations in mRNA levels of GABAAR subunits after continuous BZ exposure likely contribute to mechanisms underlying tolerance and withdrawal; however, the neuroadaptive changes associated with these alterations are unclear. Some studies observed decreased number of BZ binding sites after long-term BZ treatment (e.g., Miller et al. 1989). Receptor uncoupling, internalization, and degradation of GABAAR subunits have been proposed as mechanisms underlying BZ tolerance (Ali and Olsen 2001; Gravielle et al. 2005; Jacob et al. 2012; Vinkers and Olivier 2012). While these mechanisms are not fully understood, posttranslational processes, such as phosphorylation, are likely to play key roles (Bateson 2002; Kittler et al. 2000). Other studies have demonstrated that altered mRNA levels induced by chronic BZ exposure can correlate to their respective protein levels in vivo (Impagnatiello et al. 1996; Pesold et al. 1997). Thus, it is conceivable that mRNA changes seen in this study coincide with changes in the expression of their respective proteins. However, the relationship between changes in mRNA levels and corresponding protein level is not well characterized. While mRNA serves as an intermediate between DNA and protein, they are subject to posttranscriptional processing by RNA-binding proteins that bind to multiple sites on numerous RNAs to function in diverse time-dependent processes (Glisovic et al. 2008).
As reviewed elsewhere (Vinkers and Olivier 2012), alterations in other neurotransmitter systems have also been implicated as neuroadaptive mechanisms underlying the development of tolerance and withdrawal to BZ-like drugs, including an upregulation of the glutamatergic system (Allison and Pratt 2003). Increases in the expression of AMPA receptor subunit mRNAs and AMPA receptor ligand binding have been reported in a number of brain areas after BZ withdrawal (Allison et al. 2002, 2005). Electrophysiological data indicate that a single in vivo dose of zolpidem or diazepam can produce an increase in the AMPA/NMDA current ratio and activity of mesolimbic dopamine neurons (Heikkinen et al. 2009). A single high dose of diazepam (30 mg/kg) given to wild-type mice can also produce brain-region-dependent reduction in the expression level of mRNA transcripts involved in the regulation of synaptic plasticity such as c-Fos, CaMKII, and BDNF (Huopaniemi et al. 2004). Interestingly, many of the transcript changes induced by diazepam in wild-type mice are not altered in mice with α1-GABAARs that have been rendered diazepam-insensitive via point mutations, indicating that these transcript changes are specifically mediated by α1-containing GABAARs. Gene-targeted mice lacking the GluR-A subunit of AMPA-type glutamate (Zamanillo et al. 1999) receptors show less tolerance to a number of the acute behavioral effects of flurazepam (e.g., loss of righting reflex, walking beam impairments) after high-dose treatments (Aitta-aho et al. 2009). In contrast, greater flumazenil-induced withdrawal-like symptoms are seen after treatment termination. Together, these findings suggesting alterations in AMPA receptors are involved in mediating the processes involved in BZ tolerance and withdrawal.
In summary, this study found that subchronic zolpidem and diazepam administration can produce acute deficits in the locomotor-impairing effects of zolpidem and anxiety-like behaviors upon drug termination. In addition, our results demonstrate that zolpidem and diazepam treatments have differing effects on GABAAR subunit mRNA expression, mainly in the cortex, which may partially contribute to mechanisms underlying the behavioral phenomena. Elucidating the mechanisms behind zolpidem tolerance is necessary due to the ongoing usage of subunit-specific GABAergic drugs and the potential molecular and cellular mechanisms underlying adaptive changes of receptor function.
References
Acikmese B, Haznedar S, Hatipoglu I, Enginar N (2012) Evaluation of anxiolytic effect and withdrawal anxiety in chronic intermittent diazepam treatment in rats. Behav Pharmacol 23:215–219
Aitta-aho T, Vekovischeva OY, Neuvonen PJ, Korpi ER (2009) Reduced benzodiazepine tolerance, but increased flumazenil-precipitated withdrawal in AMPA-receptor GluR-A subunit-deficient mice. Pharmacol Biochem Behav 92:283–290
Ali NJ, Olsen RW (2001) Chronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem 79:1100–1108
Allison C, Pratt JA (2003) Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol Ther 98:171–195
Allison C, Claase LA, Pratt JA (2002) Diazepam withdrawal-induced anxiety and place aversion in the rat: differential effects of two chronic diazepam treatment regimes. Behav Pharmacol 13:417–425
Allison C, Pratt JA, Ripley TL, Stephens DN (2005) Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor autoradiography in mouse brain after single and repeated withdrawal from diazepam. Eur J Neurosci 21:1045–1056
Aragona M (2000) Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol 23:281–283
Araujo F, Tan S, Ruano D, Schoemaker H, Benavides J, Vitorica J (1996) Molecular and pharmacological characterization of native cortical γ-aminobutyric acidA receptors containing both α1 and α3 subunits. J Biol Chem 271:27902–27911
Arnot MI, Davies M, Martin IL, Bateson AN (2001) GABA(A) receptor gene expression in rat cortex: differential effects of two chronic diazepam treatment regimes. J Neurosci Res 64:617–625
Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch K-P, Schmitt A (2005) The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience 131:953–967
Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford K, McKernan RM, Dawson GR (2005) Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Br J Pharmacol 144:357–366
Auta J, Giusti P, Guidotti A, Costa E (1994) Imidazenil, a partial positive allosteric modulator of GABAA receptors, exhibits low tolerance and dependence liabilities in the rat. J Pharmacol Exp Ther 270:1262–1269
Auta J, Impagnatiello F, Kadriu B, Guidotti A, Costa E (2008) Imidazenil: a low efficacy agonist at alpha1- but high efficacy at α5-GABAA receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidem. Neuropharmacology 55:148–153
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–314
Bateson AN (2002) Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des 8:5–21
Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, Scatton B (1988) In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15-1788) in the mouse brain. Preferential affinity of zolpidem for the omega 1 (BZD1) subtype. J Pharmacol Exp Ther 245:1033–1041
Benavides J, Peny B, Ruano D, Vitorica J, Scatton B (1993) Comparative autoradiographic distribution of central ω (benzodiazepine) modulatory site subtypes with high, intermediate and low affinity for zolpidem and alpidem. Brain Res 604:240–250
Benca RM (2005) Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv 56:332–343
Biggio G, Dazzi L, Biggio F, Mancuso L, Talani G, Busonero F, Mostallino MC, Sanna E, Follesa P (2003) Molecular mechanisms of tolerance to and withdrawal of GABAA receptor modulators. Eur Neuropsychopharmacol 13:411–423
Blanchard DC, Griebel G, Blanchard RJ (2001) Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev 25:205–218
Burt DR, Kamatchi GL (1991) GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J 5:2916–2923
Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF (1997) Special Report: the 1996 guide for the care and use of laboratory animals. The National Academies Press, Bethesda, MD
Cohen C, Sanger DJ (1994) Tolerance, cross-tolerance and dependence measured by operant responding in rats treated with triazolam via osmotic pumps. Psychopharmacology (Berl) 115:86–94
Crestani F, Löw K, Keist R, Mandelli M-J, Möhler H, Rudolph U (2001) Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol 59:442–445
Cubala WJ, Landowski J (2007) Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 31:539–540
Davis M (2000) The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP (ed) The amygdala : A functional analysis. Oxford University Press, New York, pp 213–287
Diaz-Garcia JM, Oliver-Botana J, Fos-Galve D (1992) Pharmacokinetics of diazepam in the rat: influence of a carbon tetrachloride-induced hepatic injury. J Pharm Sci 81:768–772
Divljaković J, Milić M, Namjoshi OA, Tiruveedhula VV, Timić T, Cook JM, Savić MM (2013) βCCT, an antagonist selective for α1GABAA receptors, reverses diazepam withdrawal-induced anxiety in rats. Brain Res Bull 91:1–7
Drover DR (2004) Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives: zaleplon, zolpidem and zopiclone. Clin Pharmacokinet 43:227–238
Duncan GE, Breese GR, Criswell HE, McCown TJ, Herbert JS, Devaud LL, Morrow AL (1995) Distribution of [3H]zolpidem binding sites in relation to messenger RNA encoding the alpha 1, beta 2 and gamma 2 subunits of GABAA receptors in rat brain. Neuroscience 64:1113–1128
Duncan GE, Knapp DJ, Breese GR, Crews FT, Little KY (1998) Species differences in regional patterns of 3H-8-OH-DPAT and 3H-zolpidem binding in the rat and human brain. Pharmacol Biochem Behav 60:439–448
Ebert B, Anderson NJ, Cremers TI, Rasmussen S, Vogel V, Fahey JM, Sánchez C (2008) Gaboxadol—a different hypnotic profile with no tolerance to sleep EEG and sedative effects after repeated daily dosing. Pharmacol Biochem Behav 90:113–122
Elliot EE, White JM (2000) Precipitated and spontaneous withdrawal following administration of lorazepam but not zolpidem. Pharmacol Biochem Behav 66:361–369
Fahey JM, Pritchard GA, Grassi JM, Pratt JS, Shader RI, Greenblatt DJ (1999) In situ hybridization histochemistry as a method to assess GABA(A) receptor subunit mRNA expression following chronic alprazolam administration. J Psychopharmacol 13:211–218
Fernandes C, File SE, Berry D (1996) Evidence against oppositional and pharmacokinetic mechanisms of tolerance to diazepam's sedative effects. Brain Res Bull 734:236–242
Fernandes C, Arnot MI, Irvine EE, Bateson AN, Martin IL, File SE (1999) The effect of treatment regimen on the development of tolerance to the sedative and anxiolytic effects of diazepam. Psychopharmacology (Berl) 145:251–259
File SE (1985) Tolerance to the behavioral actions of benzodiazepines. Neurosci Biobehav Rev 9:113–121
File SE (1990) The history of benzodiazepine dependence: a review of animal studies. Neurosci Biobehav Rev 14:135–146
Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, Massa F, Desole MS, Carta M, Busonero F, Sanna E, Biggio G (2001) Increase in expression of the GABA(A) receptor alpha(4) subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res 92:138–148
Friedman H, Abernethy DR, Greenblatt DJ, Shader RI (1986) The pharmacokinetics of diazepam and desmethyldiazepam in rat brain and plasma. Psychopharmacology (Berl) 88:267–270
Galpern WR, Lumpkin M, Greenblatt DJ, Shader RI, Miller LG (1991) Chronic benzodiazepine administration. VII. Behavioral tolerance and withdrawal and receptor alterations associated with clonazepam administration. Psychopharmacology (Berl) 104:225–230
Garrigou-Gadenne D, Burke JT, Durand A, Depoortere H, Thenot JP, Morselli PL (1989) Pharmacokinetics, brain distribution and pharmaco-electrocorticographic profile of zolpidem, a new hypnotic, in the rat. J Pharmacol Exp Ther 248:1283–1288
Gericke CA, Ludolph AC (1994) Chronic abuse of zolpidem. JAMA 272:1721–1722
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386–401
Glisovic T, Bachorik JL, Yong J, Dreyfuss G (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582:1977–1986
Gravielle MC, Faris R, Russek SJ, Farb DH (2005) GABA induces activity dependent delayed-onset uncoupling of GABA/benzodiazepine site interactions in neocortical neurons. J Biol Chem 280:20954–20960
Greenblatt DJ, Roth T (2012) Zolpidem for insomnia. Expert Opin Pharmacother 13:879–893
Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ (1999) New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (omega) receptor subtypes. Psychopharmacology (Berl) 146:205–213
Griffiths RR, Weerts EM (1997) Benzodiazepine self-administration in humans and laboratory animals—implications for problems of long-term use and abuse. Psychopharmacology (Berl) 134:1–37
Griffiths RR, Sannerud CA, Ator NA, Brady JV (1992) Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther 260:1199–1208
Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W (2003) Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction 98:1371–1378
Heikkinen AE, Moykkynen TP, Korpi ER (2009) Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology 34:290–298
Heldt SA, Ressler KJ (2007) Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience 150:370–385
Heninger C, Saito N, Tallman JF, Garrett KM, Vitek MP, Duman RS, Gallager DW (1990) Effects of continuous diazepam administration on GABAA subunit mRNA in rat brain. J Mol Neurosci 2:101–107
Hoffman JM, Shah ND, Vermeulen LC, Schumock GT, Grim P, Hunkler RJ, Hontz KM (2007) Projecting future drug expenditures–2007. Am J Health Syst Pharm 64:298–314
Holt RA, Bateson AN, Martin IL (1996) Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology 35:1457–1463
Holt RA, Bateson AN, Martin IL (1997) Chronic zolpidem treatment alters GABA(A) receptor mRNA levels in the rat cortex. Eur J Pharmacol 329:129–132
Huang MC, Lin HY, Chen CH (2007) Dependence on zolpidem. Psychiatry Clin Neurosci 61:207–208
Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U (2004) Diazepam-induced adaptive plasticity revealed by α1 GABAA receptor-specific expression profiling. J Neurochem 88:1059–1067
Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, Guidotti A (1996) Modifications of gamma-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol 49:822–831
IMS-Health (2011) National (VONA) and total patient tracker (TPT). MS Health, Fairfield, CT
Jacob TC, Michels G, Silayeva L, Haydon J, Succol F, Moss SJ (2012) Benzodiazepine treatment induces subtype-specific changes in GABAA receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci USA 109:18595–18600
Jardim MC, Nogueira RL, Graeff FG, Nunes-de-Souza RL (1999) Evaluation of the elevated T-maze as an animal model of anxiety in the mouse. Brain Res Bull 48:407–411
Kang I, Miller LG (1991) Decreased GABAA receptor subunit mRNA concentrations following chronic lorazepam administration. Br J Pharmacol 103:1285–1287
King DA, Bouton ME, Musty RE (1987) Associative control of tolerance to the sedative effects of a short-acting benzodiazepine. Behav Neurosci 101:104–114
Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ (2000) Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci 20:7972–7977
Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H (2002) Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience 109:733–743
Kripke DF (2007) Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry 7:42
Lader M (2011) Benzodiazepines revisited—will we ever learn? Addiction 106:2086–2109
Lau CE, Sun L, Wang Q, Falk JL (2002) The effect of zolpidem on operant behavior and its relation to pharmacokinetics after intravenous and subcutaneous administration: concentration-effect relations. Behav Pharmacol 13:93–103
Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI, Nikolaou CK, Christodoulou GN (2003) Zolpidem dependence case series: possible neurobiological mechanisms and clinical management. J Psychopharmacol 17:131–135
Longone P, Impagnatiello F, Guidotti A, Costa E (1996) Reversible modification of GABAA receptor subunit mRNA expression during tolerance to diazepam-induced cognition dysfunction. Neuropharmacology 35:1465–1473
Loscher W, Schwark WS (1985) Development of tolerance to the anticonvulsant effect of diazepam in amygdala-kindled rats. Exp Neurol 90:373–384
Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U (2000) Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290:131–134
Lukas SE, Griffiths RR (1984) Precipitated diazepam withdrawal in baboons: effects of dose and duration of diazepam exposure. Eur J Pharmacol 100:163–171
Marin RH, Pérez MF, Duero DG, Ramirez OA (1999) Preexposure to drug administration context blocks the development of tolerance to sedative effects of diazepam. Pharmacol Biochem Behav 64:473–477
Markowitz GJ, Kadam SD, Boothe DM, Irving ND, Comi AM (2010) The pharmacokinetics of commonly used antiepileptic drugs in immature CD1 mice. Neuroreport 21:452–456
McKernan RM, Whiting PJ (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19:139–143
Mehta AK, Ticku MK (1999) Prevalence of the GABAA receptor assemblies containing alpha1-subunit in the rat cerebellum and cerebral cortex as determined by immunoprecipitation: lack of modulation by chronic ethanol administration. Brain Res Mol Brain Res 67:194–199
Metten P, Belknap JK, Crabbe JC (1998) Drug withdrawal convulsions and susceptibility to convulsants after short-term selective breeding for acute ethanol withdrawal. Behav Brain Res 95:113–122
Miller LG, Woolverton S, Greenblatt DJ, Lopez F, Roy RB, Shader RI (1989) Chronic benzodiazepine administration. IV. Rapid development of tolerance and receptor downregulation associated with alprazolam administration. Biochem Pharmacol 38:3773–3777
Murphy HM, Ihekoronze C, Wideman CH (2011) Zolpidem-induced changes in activity, metabolism, and anxiety in rats. Pharmacol Biochem Behav 98:81–86
Perrault G, Morel E, Sanger DJ, Zivkovic B (1990) Differences in pharmacological profiles of a new generation of benzodiazepine and non-benzodiazepine hypnotics. Eur J Pharmacol 187:487–494
Perrault G, Morel E, Sanger DJ, Zivkovic B (1992) Lack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidem. J Pharmacol Exp Ther 263:298–303
Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, Costa E (1997) Tolerance to diazepam and changes in GABAA receptor subunit expression in rat neocortical areas. Neuroscience 79:477–487
Petroski RE, Pomeroy JE, Das R, Bowman H, Yang W, Chen AP, Foster AC (2006) Indiplon is a high-affinity positive allosteric modulator with selectivity for α1 subunit-containing GABAA receptors. J Pharmacol Exp Ther 317:369–377
Petursson H (1994) The benzodiazepine withdrawal syndrome. Addiction 89:1455–1459
Pieri L, Schaffner R, Scherschlicht R, Polc P, Sepinwall J, Davidson A, Mohler H, Cumin R, Da Prada M, Burkard WP, Keller HH, Muller RK, Gerold M, Pieri M, Cook L, Haefely W (1981) Pharmacology of midazolam. Arzneimittelforschung 31:2180–2201
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101:815–850
Pugh SL, Boone MS, Emmett-Oglesby MW (1992) Tolerance, cross-tolerance and withdrawal in rats made dependent on diazepam. J Pharmacol Exp Ther 262:751–758
Renger JJ, Dunn SL, Motzel SL, Johnson C, Koblan KS (2004) Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res 1010:45–54
Roehrs TA, Randall S, Harris E, Maan R, Roth T (2012) Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol 26:1088–1095
Sanger DJ, Zivkovic B (1987) Investigation of the development of tolerance to the actions of zolpidem and midazolam. Neuropharmacology 26:1513–1518
Sanger DJ, Zivkovic B (1992) Differential development of tolerance to the depressant effects of benzodiazepine and non-benzodiazepine agonists at the omega (BZ) modulatory sites of GABAA receptors. Neuropharmacology 31:693–700
Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G (2002) Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABAA receptor subtypes. Eur J Pharmacol 451:103–110
Schmid L, Bottlaender M, Fuseau C, Fournier D, Brouillet E, Mazière M (1995) Zolpidem displays heterogeneity in its binding to the nonhuman primate benzodiazepine receptor in vivo. J Neurochem 65:1880–1886
Sethi PK, Khandelwal DC (2005) Zolpidem at supratherapeutic doses can cause drug abuse, dependence and withdrawal seizure. J Assoc Physicians India 53:139–140
Siegel S (1989) Pharmacological conditioning and drug effects. In: Goudie AJ, Emmett-Oglesby MW (eds) Psychoactive drugs: Tolerance and sensitization. Humana Press, Totowa, pp 115–180
Smith PF, Darlington CL (1994) The behavioural effects of long-term use of benzodiazepine sedative and hypnotic drugs: what can be learned from animal studies? N Z J Psychol 23:48–63
Smith R, Wright BT, Heldt SA (2011) Tolerance and withdrawal effects of zolpidem in mice given chronic zolpidem treatment Society for Neuroscience. Neuroscience Meeting Planner, Washington, DC
Steppuhn KG, Turski L (1993) Diazepam dependence prevented by glutamate antagonists. Proc Natl Acad Sci USA 90:6889–6893
Tietz EI, Huang X, Chen S, Ferencak Iii WF (1999) Temporal and regional regulation of α1, β2 and β3, but not α2, α4, α5, α6, β1 or γ2 GABAA receptor subunit messenger RNAs following one-week oral flurazepam administration. Neuroscience 91:327–341
Trenque T, Bustany P, Lamiable D, Legros S, Choisy H (1994) Pharmacokinetics and brain distribution of zolpidem in the rat after acute and chronic administration. J Pharm Pharmacol 46:611–613
Uusi-Oukari M, Korpi ER (2010) Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev 62:97–135
van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F (2004) Requirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci 24:6785–6790
Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P (2007) Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol 64:198–209
Victorri-Vigneau C, Feuillet F, Wainstein L, Grall-Bronnec M, Pivette J, Chaslerie A, Sébille V, Jolliet P (2013) Pharmacoepidemiological characterisation of zolpidem and zopiclone usage. Eur J Clin Pharmacol 1–8
Vinkers CH, Olivier B (2012) Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABAA receptor modulators? Adv Pharmacol Sci. doi:10.1155/2012/416864
Vinkers CH, van Oorschot R, Nielsen EO, Cook JM, Hansen HH, Groenink L, Olivier B, Mirza NR (2012) GABA(A) receptor alpha subunits differentially contribute to diazepam tolerance after chronic treatment. PLoS ONE 7:e43054. doi:10.1371/journal.pone.0043054 PONE-D-11-24285
Vlainic J, Pericic D (2009) Effects of acute and repeated zolpidem treatment on pentylenetetrazole-induced seizure threshold and on locomotor activity: comparison with diazepam. Neuropharmacology 56:1124–1130
Vogel HG, Vogel WH (2002) Drug discovery and evaluation: pharmacological assays. Springer, New York
VonVoigtlander PF, Lewis RA (1991) A rapid screening method for the assessment of benzodiazepine receptor-related physical dependence in mice. Evaluation of benzodiazepine-related agonists and partial agonists. J Pharmacol Methods 26:1–5
Voss J, Sanchez C, Michelsen S, Ebert B (2003) Rotarod studies in the rat of the GABAA receptor agonist gaboxadol: lack of ethanol potentiation and benzodiazepine cross-tolerance. Eur J Pharmacol 482:215–222
Walker MC, Tong X, Brown S, Shorvon SD, Patsalos PN (1998) Comparison of single- and repeated-dose pharmacokinetics of diazepam. Epilepsia 39:283–289
Ward BO, Stephens DN (1998) Sensitisation of withdrawal signs following repeated withdrawal from a benzodiazepine: differences between measures of anxiety and seizure sensitivity. Psychopharmacology 135:342–352
Weerts EM, Griffiths RR (1998) Zolpidem self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Behav Pharmacol 9:285–297
Weerts EM, Ator NA, Grech DM, Griffiths RR (1998) Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther 285:41–53
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Wu Y, Rosenberg HC, Chiu TH, Ramsey-Williams V (1994) Regional changes in [3H]zolpidem binding to brain benzodiazepine receptors in flurazepam tolerant rat: comparison with changes in [3H]flunitrazepam binding. J Pharmacol Exp Ther 268:675–682
Yanagita T, Takahashi S (1973) Dependence liability of several sedative-hypnotic agents evaluated in monkeys. J Pharmacol Exp Ther 185:307–316
Zamanillo D, Sprengel R, Hvalby Ø, Jensen V, Burnashev N, Rozov A, Kaiser KMM, Köster HJ, Borchardt T, Worley P, Lübke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284:1805–1811
Zhao TJ, Chiu TH, Rosenberg HC (1994) Reduced expression of gamma-aminobutyric acid type A/benzodiazepine receptor gamma 2 and alpha 5 subunit mRNAs in brain regions of flurazepam-treated rats. Mol Pharmacol 45:657–663
Acknowledgments
Support was provided by NIH (MH-086727), NARSAD, and a UTHSC base grant. Some of these results were reported at the meetings of the Society for Neuroscience (Smith et al. 2011). We would like to thank Jordan Marie Ross for her assistance with manuscript preparation and Regina Smith for assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 302 kb)
Online Resource 2
mRNA expression levels in the amygdala (BLA) and prefrontal cortex (PFC) after sub-chronic treatment (VEH, DZP, or ZOLP). (PDF 301 kb)
Online Resource 3
mRNA expression levels in the cortex, hippocampus, amygdala (BLA) and prefrontal cortex (PFC) after acute (1-day) administration (VEH, DZP, or ZOLP). (PDF 254 kb)
Rights and permissions
About this article
Cite this article
Wright, B.T., Gluszek, C.F. & Heldt, S.A. The effects of repeated zolpidem treatment on tolerance, withdrawal-like symptoms, and GABAA receptor mRNAs profile expression in mice: Comparison with diazepam. Psychopharmacology 231, 2967–2979 (2014). https://doi.org/10.1007/s00213-014-3473-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3473-x