Abstract
Rationale
During a smoking quit attempt, a single smoking lapse is highly predictive of future relapse. While several risk factors for a smoking lapse have been identified during clinical trials, a laboratory model of lapse was until recently unavailable and, therefore, it is unclear whether these characteristics also convey risk for lapse in a laboratory environment.
Objectives
The primary study goal was to examine whether real-world risk factors of lapse are also predictive of smoking behavior in a laboratory model of smoking lapse.
Methods
After overnight abstinence, 77 smokers completed the McKee smoking lapse task, in which they were presented with the choice of smoking or delaying in exchange for monetary reinforcement. Primary outcome measures were the latency to initiate smoking behavior and the number of cigarettes smoked during the lapse. Several baseline measures of smoking behavior, mood, and individual traits were examined as predictive factors.
Results
Craving to relieve the discomfort of withdrawal, withdrawal severity, and tension level were negatively predictive of latency to smoke. In contrast, average number of cigarettes smoked per day, withdrawal severity, level of nicotine dependence, craving for the positive effects of smoking, and craving to relieve the discomfort of withdrawal were positively predictive of number of cigarettes smoked.
Conclusions
The results suggest that real-world risk factors for smoking lapse are also predictive of smoking behavior in a laboratory model of lapse. Future studies using the McKee lapse task should account for between subject differences in the unique factors that independently predict each outcome measure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine dependence is a chronic, relapsing disorder. Despite several FDA approved treatments, the majority of smokers who attempt to quit will ultimately relapse and return to regular smoking behavior within the first year following a quit attempt (Hughes et al., 2004; Wileyto et al., 2004; Jorenby et al., 2006). Numerous risk factors for relapse have been identified (e.g., see Garvey et al., 1992; Doherty et al., 1995; Shiffman et al., 1996b; Ockene et al., 2000; Piasecki et al., 2000; Piasecki et al., 2003), and developing treatments to effectively target these many factors remain a high research priority. In early abstinence, one of the most reliable predictors of relapse is the occurrence a single smoking lapse, which is generally defined as smoking at least a puff of a cigarette (Marlatt and George 1984; Brandon et al., 1990; Kenford et al., 1994). Because as much as 95 % of smokers who experience a lapse will progress to relapse (Garvey et al., 1992; Kenford et al., 1994), the first lapse has been theorized to represent the transition from abstinence to regular smoking; yet, the mechanisms mediating this progression remain unclear (Shiffman et al., 1996b; Shiffman et al., 2006). Characterizing the factors that predispose individuals to a smoking lapse may aid in the development of treatments targeted to prevent a lapse and, by proxy, preclude the progression from smoking lapse to relapse.
In several smoking cessation studies, a number of stable, individual traits as well as dynamic, context-dependent influences have been identified as increasing risk for a smoking lapse. A series of reports by Shiffman and colleagues have identified contextual changes in mood and motivation, proximal to the lapse event itself, that promote a lapse into smoking, such as increases in negative affect, nicotine withdrawal, and urge to smoke (Shiffman et al., 1996a; Shiffman et al., 1997b; Shiffman et al., 2002; Shiffman and Waters 2004). Additionally, individuals who possess certain traits, including high anxiety, low distress tolerance, and a high severity of nicotine dependence, are also more at risk to lapse in early abstinence (Shiffman et al., 1996b; Shiffman et al., 1997a; Brown et al., 2005; Zvolensky et al., 2009). These individual traits are often predictive of the type and magnitude of the proximal changes in mood and motivation that in turn precede a smoking lapse (Shiffman et al., 1996b; Shiffman et al., 1997a). Accordingly, it has been postulated that the interplay between individual traits and contextual, proximal factors mediate the occurrence of a smoking lapse, and neither family of risk factors should be viewed in isolation of the other (Shiffman et al., 1997a).
Despite the well-established role of an initial smoking lapse in the relapse process, a laboratory model of smoking lapse was unavailable until recently (McKee 2009). As human laboratory models of addictive behaviors represent a crucial bridge between animal models and clinical trials in medication development (McKee 2009; Ray et al., 2010), the design of a laboratory model of lapse is a critical step toward characterizing and targeting mechanisms associated with a smoking lapse and relapse. The McKee smoking lapse task (McKee 2009; McKee et al., 2012) represents a unique paradigm that provides measures of latency to first lapse (i.e., ability to resist smoking) after a period of abstinence and subsequent smoking behavior after the lapse occurs. In this paradigm, participants are first exposed to a known risk factor for smoking relapse (e.g., nicotine deprivation and alcohol) and subsequently presented with their preferred brand of cigarettes, a lighter, and an ashtray. These latter items act as a smoking cue and provide the participant with an opportunity to smoke, both of which are highly related to the occurrence of a smoking lapse (Shiffman et al., 1996a; McKee 2009). Participants are then informed that they can begin a cigarette self-administration session or delay smoking in exchange for a fixed amount of monetary reinforcement. If the participant decides to smoke, they then begin the self-administration session in which they are again given the choice to smoke their preferred brand of cigarettes or receive a fixed monetary reinforcement for each cigarette that is not smoked. In previous studies employing this task, known precipitants of lapse and relapse, such as a stress exposure, alcohol ingestion, and nicotine deprivation, effectively reduce the ability to resist smoking, while pharmacotherapies for smoking cessation improve heavy smokers’ ability to abstain (McKee et al., 2006; 2011; 2012; McKee 2009).
To date, nearly all studies that identified predictors for smoking lapse were conducted with treatment seeking smokers in a clinical trial setting. In one recent exception, a laboratory study administering the McKee lapse task after various durations of nicotine deprivation failed to find a relationship between task performance and several participant characteristics, such as sex, motivation to quit, level of nicotine dependence, and income (McKee et al., 2012). Yet, no laboratory studies employing the McKee lapse task have been a priori designed with the sole intention to investigate the predictive validity of a combination of individual and context-dependent risk factors for smoking lapse. Therefore, the primary goal of the current study is to examine whether several previously identified real-world risk factors of smoking lapse are also predictive of lapse behavior in non-treatment seeking smokers participating in a relatively novel laboratory smoking lapse paradigm. A secondary and exploratory study goal is to examine the relationship between the two outcome measures of the lapse paradigm, namely latency to smoking lapse and number of cigarettes smoked after lapse. If we find that certain risk factors uniquely predict one outcome measure but not the other, then this may suggest that the McKee lapse task (McKee 2009; McKee et al., 2012) provides distinct and separate laboratory indices of real-world smoking behavior and the relapse process.
Methods
Participants
Non-treatment seeking daily cigarette smokers were recruited from the greater Los Angeles area through flyers, print, and online advertisements. Inclusion criteria consisted of the following: (1) aged between 18 and 55 years; (2) smoked 10 or more cigarettes per day; (3) had fewer than 3 months of smoking abstinence in the past year; (4) no self-reported use of cocaine, methamphetamine, heroin, or other illicit drugs (other than marijuana) in the previous 60 days, as verified by a negative urine toxicology; (5) not pregnant, as verified by a negative pregnancy screen; and (6) no self-reported lifetime history of psychotic disorders.
Screening procedures
Interested individuals called the laboratory and completed an initial telephone-screening interview in order to assess general eligibility requirements. Eligible participants were invited to the laboratory for an in-person session in which they read and signed an informed consent form and completed a battery of personality and substance use questionnaires and interviews, as described in the “Measures” section below. Participants were asked to abstain from drinking alcohol for 24 h prior to the in-person screening visit. After providing written informed consent, urine cotinine levels were measured to verify regular smoking (>100 ng/mL), and breathalyzers ensured a breath alcohol concentration (BrAC) of 0.000 g/dL. Expired carbon monoxide (CO) levels were collected at the screen in order to later verify overnight abstinence prior to the experimental session. Toxicology screens and pregnancy tests were then performed. Individuals who did not meet the thresholds for regular smoking, had a detectable BrAC, or tested positive for drug use or pregnancy were excluded from participation. Any individuals who were excluded following the in-person session were offered referrals for smoking cessation treatment in the community. Participants were compensated $20 for the screening session.
Smoking lapse task procedures
Following the in-person screening, eligible participants were scheduled to return to laboratory following 12 h of abstinence from cigarette smoking (or any form of tobacco products) and 24 h of abstinence from drinking alcohol. These visits took place either from 10 AM to 1 PM or 2 PM to 5 PM and took approximately 3 h to complete. Upon arrival to the laboratory, nicotine abstinence was immediately verified with expired CO levels (parts per million; PPM), as determined by a cut-off of half of the participant’s screening session CO concentration or <10 PPM. Two participants initially failed to remain abstinent for the 12 h prior to visit and were rescheduled for a later date. While CO levels can only determine recent smoking behavior, <1 % of the participants (n = 1/77) reported using alternate forms of nicotine administration (e.g., chewing tobacco and snuff) within the 7 days preceding the experimental session. Therefore, we are confident that there are no tobacco use-related confounds in the presented results. Breathalyzers ensured a breath alcohol concentration of 0.000 g/dL, and urine was tested for pregnancy in women. After verification of abstinence and non-pregnancy, participants completed several baseline personality, mood, and smoking-related questionnaires (described below in the “Measures” section). Following completion of these measures, participants completed McKee’s (2009) smoking lapse task. Eight cigarettes of the participants’ preferred brand were placed in front of them with a lighter and an ashtray. They were then instructed that over the next 50 min, they had the option to initiate a cigarette self-administration session at any point or to delay initiation in exchange for monetary reinforcement. If participants chose to delay, they were awarded $0.20 for each 5-minute increment that they were able to resist smoking. Once participants chose to end the delay period in order to smoke or resisted smoking for the entire 50 minute delay period, they then participated in a 60-minute cigarette self-administration session, in which they were given the choice to either smoke their preferred brand of cigarettes or receive monetary reinforcement for cigarettes not smoked. Participants were given $1.60 at the beginning of the self-administration session and lost $0.20 for each cigarette that they smoked. Thus, the primary outcome measures are (1) the latency to initiate smoking during the delay period and (2) the number of cigarettes smoked during the 60-minute self-administration period. Participants could not keep any unsmoked cigarettes at the end of the session and were compensated $60 for completing the experimental session in addition to receiving any money earned during the smoking relapse task.
Measures
During the screening session, demographic information was collected, including age, sex, ethnicity, and education. Intent to quit smoking was measured using a four-item questionnaire asking how strongly the participant identified with wanting to quit today (the day of the session) and within the next 3 months, 6 months, and year (Likert range: 1–7; 1 = strongly disagree; 4 = neutral; 7 = strongly agree). In addition, multiple self-report measures evaluating smoking history and behavior, binge drinking behavior, mood, and individual personality traits were administered during the screening session or at baseline of the experimental session. Several of these measures, as described below, were chosen to be examined as predictive factors for performance on the lapse paradigm due to their previously being identified in the literature as real-world risk factors for smoking lapse. For analytic and descriptive purposes, measures were grouped into one of two families, termed proximal or distal. Proximal measures referred to those expected to be context dependent and, therefore, sensitive to the effects of the overnight abstinence. Distal measures were those expected to be more stable and trait based and less sensitive to the effects of overnight abstinence.
Proximal measures
The following measures were administered at baseline immediately prior to the smoking lapse task: the Wisconsin Smoking Withdrawal Scale measured nicotine withdrawal symptoms (WSWS; Welsch et al., 1999), and the Questionnaire of Smoking Urges-Brief (QSU-Brief) measured urge to smoke and craving (Cox et al., 2001; Toll et al., 2004; Toll et al., 2006). The two factors of the QSU-Brief, which capture craving for the positive effects of smoking and craving to relieve the discomfort of nicotine withdrawal (Cox et al., 2001), were analyzed. In order to assess mood following 12 h of abstinence, the short version of the Profile of Mood States (POMS) was administered at baseline prior to the smoking lapse task and the Depression and Tension subscales were analyzed in this study.
Distal measures
The following smoking measures were collected during the in-person screening: the Fagerstrom Test of Nicotine Dependence (FTND) is a 6-item questionnaire and is a reliable measure that is commonly used to determine nicotine dependence (Heatherton et al., 1991) and the Timeline Follow-Back (TLFB; Sobell and Sobell 1985) is a calendar assisted interview used to assess quantity and frequency of cigarette use over the past 30-days with average cigarettes per day being the primary variable of interest from this measure. Participants also completed a questionnaire developed in our laboratory in order to assess binge-drinking behavior during the individual’s heaviest period of lifetime use. In order to capture binge-drinking frequency, the item “How many times would you have 5 or more drinks on a single drinking occasion during your heaviest drinking period?” was scored on a 6-point Likert scale ranging from “never” to “daily.”
To capture individual personality traits, the following measures were given during the screening session: the Distress Tolerance Scale, a reliable and valid self-report measure of emotional distress tolerance (Simons and Gaher 2005), and The State-Trait Anxiety questionnaire (Spielberger 1983), which measures trait level anxiety. The Center for Epidemiological Studies Depression Scale (CES-D; NIMH) was administered prior to the smoking lapse task in order to assess for depressive symptomatology over the week preceding the experimental session (Radloff 1977).
Data analytic strategy
All analyses were conducted in SAS version 9.3 for Windows. All predictor variables were Z-score transformed. General linear regressions were conducted to examine the effect of proximal and distal variables on number of cigarettes smoked during the self-administration period. To analyze the effect of proximal and distal risk factors on latency to smoke in the delay period, a series of univariate Cox proportional hazard regressions were conducted. This strategy was employed as a sizeable number of participants (n = 24) delayed smoking for the full 50 min of the delay period, precluding the assignment of a valid latency time for these participants. Furthermore, analyses with a general linear model were contraindicated by the extreme non-normality of the latency variable. A Cox proportional hazard modeling approach is able to accurately model these features of our data. Furthermore, all models assessed for non-proportionality of Hazard functions through the addition of a time-varying covariate (predictor variable × square-root-latency). When this time-varying covariate was a significant predictor, suggesting non-proportionality, it was retained in the final models allowing for a more accurate assessment of the predictor variables of interest.
All analyses were conducted with continuous predictor variables; however, to interpret significant latency effects graphically, predictor variables were median-split and separate Kaplan–Meier survival curves were generated for “high” and “low” participants on each predictor variable. Type 1 error correction was implemented to reduce alpha inflation. In light of the two families of predictors being assessed (i.e., proximal and distal), we set an alpha threshold of p < 0.025 for significance (Dar et al., 1994) and p ≤ 0.05 for nominal significance. In order to remain conservative regarding possible assumption violation for the Cox modeling procedure and because non-proportionality was not a theoretically meaningful effect in the current investigation (and thus was not interpreted as substantive effects), alpha threshold for our measure of non-proportionality was set to p < 0.10. No demographic variables (e.g., age, sex, education, race) were significant predictors of latency to smoke (ps > 0.10) or number of cigarettes smoked (ps > 0.07), and their inclusion did not alter the significance of the results presented. Thus demographic variables were removed from the final models presented herein.
In order to further explore the relationship between the two main outcome variables in the context of proximal and distal predictors, several additional analyses were performed. Pearson correlation was computed between the two outcome variables of interest as an initial assessment of the independence of these outcome variables. Secondly, given the non-normality of the latency variable, an ANOVA was conducted, wherein, smoking behavior during the delay period was coded as a three-level categorical variable (i.e., smoked immediately, smoked during the delay period but not immediately, or did not smoke), and the number of cigarettes smoked in the ad-lib period was the outcome variable. Next, given that a substantial portion of participants (33 %) smoked immediately during the task, the original analyses were repeated after excluding participants who smoked immediately. Additionally, to assess unique effects of predictor variables on ad-libitum smoking while accounting for the effect of delay latency, general linear regressions were repeated for significant predictors of the number of cigarettes smoked with latency to smoke added as a covariate. Finally, using the previously identified significant individual predictors for each main outcome, multivariate general linear and Cox-proportional regressions were performed with each family of proximal or distal variables included as predictor variables.
Statistical power was estimated using the program G*Power 3.1 for Windows. With a final sample size of n = 77, power to detect a small effect (i.e., ρ = 0.10, α = 0.025, two-tailed bivariate correlation test) was very small (1-β = 0.085). Power to detect a medium sized effect (ρ = 0.30) was substantially improved (1-β = 0.67). Finally, ability to detect statistically large effects (ρ = 0.50) was essentially 100 % (1-β = 0.99).
Results
Sample characteristics
A total of 487 people were screened over the phone to participate in the study. Of those, 131 completed an in-person screening visit, and 77 people completed the experimental lapse paradigm. Demographic, smoking-related, personality, and mood characteristics are displayed in Table 1. On the day of the experimental session, approximately 99 % of the sample reported that they were not planning on quitting smoking (i.e., intent to quit score of 4 or less), and 71 % of the sample reported an income of less than $30,000.
Latency to smoke
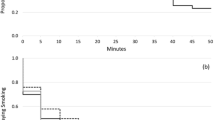
The relationship between the risk factors and latency to smoke is summarized in Table 2. On average, participants delayed 22.29 min (range: 0–50) before smoking a cigarette. Several proximal mood- and smoking-related factors were predictive of the latency to initiate smoking behavior. Cox proportional analyses revealed a significant effect of craving to relieve the discomfort of nicotine withdrawal on latency to smoke (χ 2 = 5.73, p < 0.025, HR = 1.41; Fig. 1), such that increased craving to relieve the discomfort of nicotine withdrawal was associated with significantly shorter latency to initiate smoking in the delay period. A similar pattern of results was observed for withdrawal (χ 2 = 4.12, p = 0.04, HR = 1.34), with those reporting greater withdrawal symptoms displaying nominally shorter latency to smoke. Higher tension levels were also nominally associated with a shorter latency to initiate smoking (χ 2 = 3.70, p = 0.05, HR = 1.30). Neither POMS-depression (p = 0.28) nor craving for the positive effects of smoking (p = 0.13) was significantly predictive of latency to smoke. No distal individual trait or smoking variables were significant predictors of latency to smoke (CESD-depression: p = 0.21; trait anxiety: p = 0.14; distress tolerance: p = 0.11; cigarettes per day: p = 0.89; nicotine dependence: p = 0.52; binge drinking frequency: p = 0.27).
Kaplan–Meier survival curve depicting latency to smoke in the delay period predicted by severity of craving to relieve the discomfort of nicotine withdrawal. Analyses examined craving to relieve the discomfort of nicotine withdrawal as a continuous variable. However, for ease of presentation, survival curves are displayed for “high” and “low” responders according to a median split. Non-proportionality was modeled via inclusion of a time-varying covariate (withdrawal severity × sqrt-latency)
The distribution of the latency to smoke measure was non-normal, with 33 % of participants choosing to smoke immediately, 37 % smoking at some point during the delay period (but not immediately), and 30 % abstaining for the entire delay period. Smoking immediately during the task may not be generalizable to real-world lapse behavior during a quit attempt; thus, latency analyses were repeated excluding participants who immediately lapsed. In support of the utility of the McKee paradigm as a lapse analog, all predictors identified in the full sample remained significant in the subset of participants who delayed or refrained from smoking during the paradigm. Specifically, craving to relieve the discomfort of nicotine withdrawal (Χ 2 = 5.55, p < 0.025, HR = 4.34; craving to relieve the discomfort of nicotine withdrawal × sqrt-latency: Χ 2 = 3.58, p = 0.06, HR = 0.76), withdrawal severity (Χ 2 = 6.43, p < 0.025, HR = 5.02; withdrawal × sqrt-latency: Χ 2 = 5.48, p = 0.02, HR = 0.69), and tension (Χ 2 = 7.12, p < 0.025, HR = 4.20; tension × sqrt-latency: Χ 2 = 5.09, p = 0.024, HR = 0.73) were significantly and negatively predictive of latency to smoke in these participants.
Number of cigarettes smoked in self-administration period
Results for number of cigarettes smoked are summarized in Table 2. Participants averaged 1.77 (SD = 0.97, range: 0–5) cigarettes smoked during the self-administration period. Several proximal smoking factors were again predictive of smoking behavior. Severity of nicotine withdrawal (β = 0.30, SE = 0.01, p < 0.01, R 2 = 0.098) and craving for the positive effects of smoking (β = 0.28, SE = 0.11, p < 0.01, R 2 = 0.087) were significantly positively predictive, and craving to relieve the discomfort of nicotine withdrawal was nominally predictive (β = 0.24, SE = 0.11, p = 0.03, R 2 = 0.059) of number of cigarettes smoked. Neither proximal indicator of negative mood was significantly predictive (tension: p = 0.53, depression: p = 0.56).
In contrast to latency to smoke, distal smoking-related variables were significantly related to the number of cigarettes smoked. Both average cigarettes per day (β = 0.37, SE = 0.12, p < 0.01, R 2 = 0.11) and FTND score (β = 0.29, SE = 0.11, p < 0.025, R 2 = 0.085) were significantly positively predictive of number of cigarettes smoked. Comparatively, distal indicators of depression, anxiety, and distress tolerance were not significantly predictive of ad-lib smoking (CESD-depression: p = 0.52; trait anxiety: p = 0.22; distress tolerance: p = 0.66; binge drinking frequency: p = 0.11).
Additional analyses of the relationship between outcome measures
As latency to smoke and number of cigarettes smoked were significantly correlated (r = −0.46, p < 0.0001), additional analyses were performed to explore this relationship and determine whether the proximal and distal variables identified above were uniquely predictive of task performance. Latency to smoke was predictive of number of cigarettes smoked (F(2) = 9.46, p < 0.001), such that those who smoked immediately averaged 2.2 cigarettes, those who delayed between 1 and 49 min smoked 1.9 cigarettes, and subjects who delayed for the full 50 min smoked 1.2 cigarettes. Yet, ∼80 % of the participants who refrained from smoking for the entire delay period still decided to smoke during the self-administration period at some point. Furthermore, cigarettes per day, FTND score, and craving for the positive effects of smoking remained predictive of number of cigarettes smoked during the self-administration period (p’s = 0.004, 0.018, 0.033, respectively) after including latency to smoke as a covariate (all p’s < 0.001). Withdrawal severity (p = 0.06) and craving to relieve the discomfort of nicotine withdrawal (p = 0.34) lost significance after the addition of the covariate, which appears to be a result of the shared variance between these two predictors and latency to smoke (i.e., only withdrawal severity and craving to relieve the discomfort of nicotine withdrawal were significantly predictive of both latency to smoke and number of cigarettes smoked).
Finally, families of individual significant predictors of task performance (proximal or distal) were analyzed in separate multivariate models. The model including the previously identified proximal predictors of latency to smoke (i.e., withdrawal severity, craving to relieve the discomfort of nicotine withdrawal, and tension) was nominally predictive (p < 0.05), but none of the individual factors reached statistical significance. A similar pattern was observed for number of cigarettes smoked: overall the models of proximal (i.e., withdrawal, craving to relieve the discomfort of nicotine withdrawal, and craving for the positive effects of smoking) and distal factors (i.e., cigarettes per day and nicotine dependence) were significantly predictive of smoking behavior during the self-administration period (p’s < 0.01, R 2 = 0.12, 0.13, respectively), but no individual predictor reached significance. Because all of the individual predictors within each family of variables were significantly intercorrelated (r’s > 0.49; p’s < 0.001) and each of the three multivariate models was significantly predictive of task performance, the lack of significance of the individual predictors within each model can be confidently attributed to the multicollinearity of the predictor variables.
Discussion
The present study examined whether previously identified real-world risk factors for smoking lapse and relapse also predict behavioral outcomes in a controlled laboratory lapse paradigm after overnight abstinence. The study also explored the relationship between the two outcome measures of the paradigm, latency to initiate smoking and the number of cigarettes smoked after lapse, and how they may relate to real-world smoking behaviors and the relapse process. We found that the McKee lapse task (2009) was sensitive to several previously identified risk factors for smoking lapse, but the relationship between risk factors and smoking behavior was highly dependent on the outcome being measured. The results provide support for the McKee lapse task as a valid laboratory model of smoking lapse that is susceptible to factors that convey risk in smoking cessation studies, but may also suggest that this paradigm provides two distinct proxy measures of real-world smoking behavior.
Latency to initiate smoking during the delay period was associated with proximal risk factors (i.e., measures sensitive to overnight abstinence), including withdrawal, craving, and tension, but was not related to any of the a priori distal factors (i.e., measures relatively insensitive to overnight abstinence). Participants who reported high levels of withdrawal, tension, or craving for the relief of negative affect after 12 h of abstinence were more likely to initiate smoking earlier during the delay period, which may suggest that the primary motivation for initiating smoking was alleviation of the negative symptoms related to acute abstinence. This notion is supported by the findings of several smoking cessation studies which demonstrated that a rapid increase in withdrawal symptoms, negative affect, and craving and the subsequent desire to relieve this aversive symptomatology is the most reliable predictor of a smoking lapse (Shiffman et al., 1996a; Shiffman et al., 1997b; Shiffman et al., 2002; Shiffman and Waters 2004).
In comparison to the latency to initiate smoking, the number of cigarettes smoked after the lapse was related to a unique combination of proximal and distal risk factors. Individuals who were heavier smokers, had greater severity of nicotine dependence, had high levels of craving for the positive effects of smoking, reported heightened levels of withdrawal, or desired to smoke to reduce withdrawal symptoms tended to smoke more cigarettes after a lapse or during the self-administration session. Thus, in contrast to the observed results for latency to initiate smoking, urge for the hedonic effects of smoking, possibly to counteract the aversive effects of withdrawal, may predict the number of cigarettes smoked after a lapse occurs. In support of these findings, several studies, including one using the McKee lapse task, have demonstrated that a period of abstinence can increase the hedonic satisfaction derived from smoking (Perkins et al., 1994; Fant et al., 1995; McKee et al., 2012), and that those who report the greatest hedonic response to the first lapse tend to smoke the most cigarettes during the lapse (Shiffman et al., 2006). As number of cigarettes smoked per day and level of nicotine dependence was also predictive of the number of cigarettes smoked in lab, one possible interpretation for this relationship could be that heavier smokers require more cigarettes to feel the hedonic effects of smoking during a lapse. However, as hedonic satisfaction from smoking was not collected in the current study, we note that the preceding argument may be an extrapolation from the reported results. Therefore, we suggest that future laboratory work clarifies the relationship between the aforementioned risk factors, hedonic response to smoking, and smoking behavior during a lapse.
While the latency to initiate smoking and the number of cigarettes smoked after lapse were significantly related, with individuals who were faster to lapse also smoking more cigarettes, there was a clear distinction in the factors that predicted each outcome. This may suggest that the McKee lapse task provides distinct and separate laboratory indices of real-world smoking behavior and the relapse process. For example, the interaction between level of nicotine dependence, negative effect, and smoking urge has been shown to be positively predictive of a smoking lapse occurring without being related to the number of cigarettes smoked during the lapse episode (Shiffman et al., 1996b; Shiffman et al., 1997a). Conversely, hedonic response is positively related to the number of cigarettes smoked during a lapse, which in turn is highly predictive of progression to the next lapse and, ultimately, relapse (Shiffman et al., 2006). Taken together, these results may indicate that a smoking lapse and smoking behavior during the lapse are predictive of future lapses and progression to relapse through separate pathways and risk factors. Of the 11 risk factors examined in the current study, only withdrawal severity and craving to relieve the discomfort of nicotine withdrawal were predictive of both latency to initiate smoking and number of cigarettes smoked. As several diffuse proximal and distal smoking-related factors may converge during acute abstinence to influence the severity of withdrawal (Shiffman et al., 1997a), the motivation and craving to alleviate these negative effects may in turn manifest itself in various measures of smoking-related behavior (i.e., withdrawal severity is affected by both dynamic and static factors and, therefore, may mediate the relationship between latency to smoke and number of cigarettes smoked). Thus, the McKee paradigm may provide two unique laboratory measures of real-world smoking behavior during the relapse process by capturing the time immediately preceding the first lapse and the severity of the lapse itself, as measured by the latency to initiate smoking and the number of cigarettes smoked, respectively.
Although the present study has several strengths, such as a novel approach to validate a human laboratory model and determine its ability to capture the effects of real-world risk factors, some limitations should also be noted. The monetary values that were used as alternate reinforcers in the lapse paradigm were slightly lower than those recommended by McKee (2009) relative to the length of the abstinence period. This was done to better match the compensation to usual incentives of local studies. However, the average latency to lapse in the current study (∼22 min) approached the target length of delay period (i.e., 25 min; McKee 2009), suggesting that the monetary amount was still effective in providing incentive to not smoke. Additionally, the study population consisted mostly of moderate smokers (∼15 cigarettes per day) with a low degree of nicotine dependence (FTND score ∼4). Therefore, the results should be confirmed in a sample of heavy smokers with higher levels of nicotine dependence to ensure generalizability, as this group of smokers may be in the greatest need of effective pharmacotherapy. The smokers in the study were also non-treatment seeking, which could feasibly limit the study’s real-world validity. However, others have found that monetary compensation is an effective alternate reinforcer in providing motivation to resist smoking (Perkins et al., 2006; Gilbert et al., 1999), and McKee et al. (2012) found that treatment seeking status did not affect performance on this lapse task. Furthermore, the smoking cues (i.e., cigarettes and lighter) that are presented during the McKee paradigm may limit the ability of this task to identify risk factors that are predictive of lapse in the real-world. While the presence of cigarettes is necessary to test smoking lapse in the laboratory (i.e., it is impossible for a lapse to occur without a cigarette being present) and were purposely included as smoking cues in the original design of the McKee task, cigarettes and other smoking cues are not always immediately on hand during a quit attempt. Finally, subjective measures of mood, craving, and withdrawal were only collected at baseline. As these characteristics have been previously shown to change across the delay period (McKee et al., 2012), collecting multiple measures throughout the procedure would have allowed the investigation of how changes in subjective state over time relate to latency to smoke and number of cigarettes smoked.
The current results suggest that several real-world predictors of smoking lapse can influence behavioral outcomes in a novel laboratory smoking lapse paradigm using non-treatment seeking smokers. These results have several implications for future research studies using this paradigm, such as indicating that these experiments should account for between subject differences in withdrawal symptoms, craving, and negative affect after overnight abstinence, as well as current smoking behavior and severity of nicotine dependence, when interpreting results in both outcome measures. Additionally, the latency to smoke measure had an unexpected, non-normal distribution with approximately one third of the sample lapsing immediately and one third resisting smoking for the entire delay period. As the distribution of this measure has not been reported in previous publications describing this task, it is unclear if its observed shape is specific to the current study or a common feature across studies. While the sizable number of participants who choose to immediately smoke may be comparable to reports of early relapse in self-quitters (Hughes et al., 1992; 2004), and the identified risk factors were predictive of the number of cigarettes smoked independent of latency to smoke during the delay period, future studies using the McKee lapse task should report the distribution of this measure and examine individual differences in those who immediately lapse vs. those who abstain for the duration of the task.
In sum, the McKee lapse task appears to effectively translate smoking lapse from the real-world to the laboratory, which lends support for its use as a model for medication development. Future studies using this task should examine whether a medication’s ability to reduce withdrawal, craving, and negative affect is related to its efficacy in increasing the latency to initiate smoking and/or whether medications that reduce the hedonic value associated with smoking after lapse may be most effective in reducing the number of cigarettes smoked and, thus, the severity of the lapse event itself.
References
Brandon TH, Tiffany ST, Obremski KM, Baker TB (1990) Postcessation cigarette use: the process of relapse. Addict Behav 15:105–114
Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ (2005) Distress tolerance and early smoking lapse. Clin Psychol Rev 25:713–733
Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16
Dar R, Serlin RC, Omer H (1994) Misuse of statistical tests in three decades of psychotherapy research. Journal of consulting and clinical psychology 62:75–81
Doherty K, Kinnunen T, Militello FS, Garvey AJ (1995) Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacol 119:171–178
Fant RV, Schuh KJ, Stitzer ML (1995) Response to smoking as a function of prior smoking amounts. Psychopharmacol 119:385–390
Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B (1992) Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addict Behav 17:367–377
Gilbert DF, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA (1999) Effects of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Exp Clin Psychopharmacol 7:174–181
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Shea P, Solomon LJ, Flynn BS (1992) Smoking cessation among self-quitters. Health Psychol 11:331–334
Hughes JR, Keely J, Naud S (2004) Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 99:29–38
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63
Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB (1994) Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA 271:589–594
Marlatt GA, George WH (1984) Relapse prevention: introduction and overview of the model. Br J Addict 79:261–273
McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SM (2006) Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacol 189:201–210
McKee SA (2009) Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 14, 99–107
McKee, S. A., Sinha, R., Weinberger, A. H., Sofuoglu, M., Harrison, E. L R., Lavery, M., Wanzer, J. (2011) Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol 25:490–502
McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S (2012) Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res 14:1362–1371
Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC, Hollis JF (2000) Relapse and maintenance issues for smoking cessation. Health Psychol 19:17–31
Perkins KA, Epstein LH, Grobe J, Fonte C (1994) Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav 47:107–112
Perkins KA, Stitzer M, Lerman C (2006) Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacol 184:628–636
Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB (2003) Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Exp Clin Psychopharmacol 11:276–285
Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB (2000) Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol 109:74–86
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Applied psychological measurement 1:385–401
Ray LA, Hutchison KE, Tartter M (2010) Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des 16:2149–2158
Shiffman S, Waters AJ (2004) Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol 72:192–201
Shiffman S, Ferguson SG, Gwaltney CJ (2006) Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacol 184:608–618
Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M (1996a) First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol 64:366–379
Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ (1996b) Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol 64:993–1002
Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD (1997a) Individual differences in the context of smoking lapse episodes. Addict Behav 22:797–811
Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M (1997b) A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol 106:104–116
Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M (2002) Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol 111:531–545
Simons JS, Gaher RM (2005) The Distress Tolerance Scale: development and validation of a self-report measure. Motiv Emot 29:83–102
Spielberger, C. D. (1983) Manual for the State-Trait Anxiety Inventory STAI (form Y) ("self-evaluation questionnaire").
Toll BA, Katulak NA, McKee SA (2006) Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief). Addict Behav 31:1231–1239
Toll BA, McKee SA, Krishnan-Sarin S, O’Malley SS (2004) Revisiting the factor structure of the questionnaire on smoking urges. Psychol Assess 16:391–395
Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB (1999) Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol 7:354–361
Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, Hawk L, Lerman C (2004) Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine Tob Res 6:357–366
Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D (2009) Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine Tob Res 11:323–331
Funding
This study was supported by grant 20XT-0154 from the Tobacco-Related Disease Research Program (TRDRP).
Competing interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roche, D.J.O., Bujarski, S., Moallem, N.R. et al. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology 231, 2889–2897 (2014). https://doi.org/10.1007/s00213-014-3465-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3465-x