Abstract
Rationale
Phosphodiesterases (PDEs) play an important role in the regulation of intracellular signaling mediated by cyclic adenosine monophosphate (cAMP). Recently, several PDE inhibitors were assessed for their possible cognitive enhancing properties. However, little is known about the effect of PDE3 inhibitors on memory function.
Objectives
We examined how the PDE3 inhibitor cilostazol affects C57BL/6 J mice as they perform various behavioral tasks. After behavioral assessment, brains of the mice were analyzed immunohistochemically to quantify the phosphorylation of cAMP-responsive element binding protein (CREB), a downstream component of the cAMP pathway.
Results
Oral administration of cilostazol significantly enhanced recollection of the exact platform location in the Morris water maze probe test. Cilostazol also improved context-dependent long-term fear memory, without affecting short-term memory. No apparent effect was observed in cue-dependent fear memory. The results suggest that cilostazol selectively improves hippocampus-dependent long-term memory in these tasks. Cilostazol also significantly increased the number of phosphorylated-CREB-positive cells in hippocampal dentate gyrus.
Conclusions
These results suggest that cilostazol may exert its beneficial effects on learning and memory by enhancing the cAMP system in hippocampus, where it increases intracellular cAMP activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activation of 3′:5′-cyclic adenosine monophosphate (cAMP) plays essential roles in synaptic plasticity (Kandel 2001; Mayford and Kandel 1999). Studies in Aplysia (Bartsch et al. 1995; Goelet et al. 1986), Drosophila (Davis 1996; Yin et al. 1995), and rodent species (Brightwell et al. 2007; Kida 2012) confirm that the cAMP–protein kinase A (PKA)–cAMP response element-binding protein (CREB) signaling pathway is involved in synaptic plasticity of memory from invertebrates to mammals. The elevation of intracellular cAMP concentration (Fujioka et al. 2004) and subsequent gene regulation by CREB (Florian et al. 2006; Ota et al. 2008) are hypothesized to mediate neural plasticity and higher cognitive function. Intracellular cAMP concentration is regulated by balancing its synthesis via adenylate cyclase and its degradation via phosphodiesterases (PDEs) (Burgers et al. 1979; Goldberg et al. 1980). Eleven families of PDEs (PDE1–PDE11) have been identified based on deduced amino acid sequences, pharmacological function, and substrate specificity (Lakics et al. 2010; Lugnier 2006; Xu et al. 2011). PDE1, 2, 3, 10, and 11 can hydrolyze both cAMP and 3′:5′-cyclic guanosine monophosphate (cGMP). Meanwhile, PDE4, 7, and 8 specifically hydrolyze cAMP, and PDE5, 6, and 9 hydrolyze cGMP (Lugnier 2006). PDE inhibitors elevate cellular cAMP and/or cGMP concentration by inhibiting their hydrolysis. The cAMP signaling pathway is suggested to be involved in synaptic plasticity in addition to nitric oxide-cGMP intracellular signaling pathway (Lu and Hawkins 2002). Thus, PDE inhibitors have been studied as a possible therapeutic intervention for cognitive disorders (Blokland et al. 2006; Reneerkens et al. 2009) via their cyclic nucleotides enhancing property.
Recent studies demonstrate that certain PDE specific inhibitors improve or enhance memory and cognitive functions in rodent models. Examples include inhibitors of PDE2 (bay 60-7550; Boess et al. 2004), PDE4 (rolipram; Bach et al. 1999; Gong et al. 2004; Monti et al. 2006; Nagakura et al. 2002; Rutten et al. 2007), PDE5 (sildenafil; Boccia et al. 2011; Cuadrado-Tejedor et al. 2011; Puzzo et al. 2009), PDE7 (S14; Perez-Gonzalez et al. 2013), and PDE9 (bay 73-6691; van der Staay et al. 2008). To date, little is known whether PED3 specific inhibitor have similar effects.
Cilostazol is a selective PDE3 inhibitor that was originally prescribed as an anti-platelet agent (O’Donnell et al. 2009). Recent studies report that repeated administration of cilostazol significantly ameliorates cognitive impairment induced by intracerebral infusion of the amyloid peptide fragment Aβ25-35 (Hiramatsu et al. 2010; Park et al. 2011). Within the central nervous system (CNS), PDE3 is widely expressed throughout the brain including cerebellum, frontal cortex, hypothalamus, and hippocampus (Lakics et al. 2010; Xu et al. 2011). Given the well-established safety (Pratt 2001) and the distribution of PDE3 in the CNS (Lakics et al. 2010; Xu et al. 2011), cilostazol is an ideal candidate for therapeutic improvement of cognitive impairment (Arai and Takahashi 2009; Hiramatsu et al. 2010; Park et al. 2011). The effects of cilostazol on cognitive functions have not been characterized in detail. Careful examination of cilostazol effects may lead to the identification of a therapeutic agent for treating cognitive disorders based on PDE inhibition. In the present study, we investigated how the selective PDE3 inhibitor cilostazol affects learning ability in mice.
Materials and methods
Subjects
Experimentally naive male C57BL/6J mice (CLEA Japan Inc., Tokyo) were used. Mice were housed in groups of four or five per cage in a solid aluminium cage (17 × 30 × 11 (WDH) cm, Tokiwa Kagaku Inc., Tokyo) with wood-chip bedding. Mice assigned to the behavioral test battery group were fed ad libitum for 4 h after daily behavioral testing. For the next 20 h, however, they had no access to feed. Mice assigned for the fear conditioning were fed ad libitum. All mice had free access to drinking water. The vivarium was maintained at 23 ± 1 °C and 50 ± 5 % humidity, under a 12-h light–dark cycle (light on at 7:00 AM). Number of subjects used in this study was summarized in Table 1.
All experiments were approved by the animal experiment committee of the Tokyo Metropolitan Institute of Gerontology and carried out according to its guidelines.
Apparatus
Apparatuses for the open field test, the forced swim test, the Morris water maze task, and the fear conditioning task, were purchased from O’Hara & Co., Ltd. (Tokyo, Japan). In the open field test, a square arena (50 × 50 cm) with high wall (50 cm) is used. The arena was illuminated by overhead LED lights and the mice behaviors were monitored via CCD camera and analyzed automatically by Image OFCR (O’Hara & Co., Ltd) to yield the immobile time, the center time, the travel distance, and the number of rearing. Image OFCR is software based on NIH Image (a public domain program developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/).
In the Morris water maze task, the circular pool (100 cm in diameter) was filled with water to a depth of 30 cm. The submerged platform was placed 1 cm below the water. The water was maintained at 23 ± 1 °C and made opaque by nontoxic white ink.
In the fear conditioning, two different chambers were used; a shocking chamber and a chamber for the cue-dependent fear memory test. The size (17 × 11 × 12 (WDH) cm) of the two chambers was the same; however, they were different in their tactile, texture, and color. In addition, odor stimulus was used in the cue-dependent fear memory test to further discrimination of experimental context.
Automated software (Time Morris water maze, forced swim, and fear conditioning; O’Hara & Co., Ltd) were utilized to control the experimental devices and to analyze the data.
Drug and drug administration
Cilostazol [6-[−4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2-(1H)-quinolinone] was administered either through a gavage tube or by mixing it in the feed (CRF-1; Oriental Yeast Ltd., Tokyo, Japan). Body weight of individual mouse was measured on each day before the experiment to determine the drug dose to be administered.
For gavage administration, cilostazol was suspended in distilled water containing 0.5 % carboxymethyl cellulose. The concentration of cilostazol in suspension was adjusted according to body weight (BW) to achieve 30 and 100 mg/kg BW. Cilostazol suspension or vehicle was orally administered 30 min prior to the behavioral experiment.
For administration by feed, the amount of cilostazol-containing feed was calculated based on body weight to achieve 30, 60, and 100 mg/kg BW. Then, the weighed cilostazol-containing feed or control feed was provided for individually housed mouse. Because of the time required for a mouse to completely eat the feed varied from 15 to 45 min, the behavioral experiments were conducted 1 h after the feed was supplied. Preliminary experiment revealed that gavage administration of saline for more than seven consecutive days altered the mice behaviors including enhanced anxiety in the open field test and delay in the acquisition of the Morris water maze task (data not shown). The 3-day gavage saline administration, on the other hand, had no apparent effects on the fear conditioning task that was completed in 3 days. Based on these observations, gavage administration was used only for the fear conditioning group. The mixed-in-feed administration was used for the behavioral test battery group that required long-term cilostazol administration.
Pharmacokinetic assay
Cilostazol were administered either by mixing it in the feed or by gavage. The dose was 30, 60, or 300 mg/kg BW for cilostazol in feed, and 30 or 100 mg/kg BW cilostazol for gavage. At 0.5, 1, 2, 3, 4, or 24 h from the start of presenting the cilostazol-containing feed or the gavage administration, mice were deeply anesthetized with isoflurane, and blood was collected from the caudal vena cava. Analysis on 0.5 h after the mixed-in-feed administration was not conducted because the time point occurred during feeding time. Blood samples were allowed to clot for 30 min at room temperature before centrifugation for 15 min at 3,000×g to collect serum. The cilostazol concentration in serum was measured using a high-performance liquid chromatography system (LC-20 AD; Shimadzu Corp., Kyoto, Japan), according to a method reported previously (Akiyama et al. 1985). Sera from control feed- or vehicle-administered mice were served as standards.
Behavioral experiments
Mice were habituated with handling for 3 days. Then, the mice were divided into the groups for behavioral analysis in such a way that the mean body weight among the groups was similar. All behavioral experiments were conducted between 9:00 AM and 17:00 PM. The experiments were carried out in a blind manner so that the experimenters did not know which mice received cilostazol.
Behavioral test battery group
Mice were sequentially subjected to the open field test, the forced swim test, and the Morris water maze task.
Open field test
Spontaneous motor activity, exploratory behavior, and emotional responses in a novel environment were assessed, as described previously (Kojima et al. 2008). Briefly, a mouse was placed in the field and allowed to explore for 15 min. Behavior was assessed under dark-lit conditions (28 lx) on the first day, followed by bright-lit conditions (150 lx) on the second day. Distance traveled, number of rearings, time spent in the center of the field, and immobile time were measured (Kojima et al. 2008).
Forced swim test
A mouse was placed for 6 min in a cylinder (10 cm in diameter and 25 cm in height) filled with water (23 ± 1 °C). In this test, immobility during the last 4 min was regarded as an index of depression (Lucki et al. 2001).
Morris water maze task
After the completion of the forced swim test, mice were tested in the Morris water maze to examine spatial memory (Morris et al. 1982). The task was carried out as previously described (Yanai et al. 2012). Briefly, each mouse was allowed to swim for a maximum of 60 s to escape to a platform submerged 1 cm below the water surface. Four trials were conducted each day for 14 days. One day after acquisition training was completed, a probe test without the platform was conducted. On the next day of the probe test, the mouse was subjected to cued training. In this task, the location of the platform was made visible by attaching a prominent black flag to the platform.
Fear conditioning group
Separate groups of mice were tested either in the fear conditioning task or in analgesia tests. For the latter group, hotplate and electrical footshock sensitivity tests were conducted.
Pavlovian fear conditioning task
Mice were tested in the fear conditioning task (Phillips and LeDoux 1992) to examine conditioned fear to either context or cue, according to procedures detailed in previous studies (Borlikova and Endo 2009; Kojima et al. 2008; Yanai et al. 2012). Briefly, mice were individually placed in a shocking chamber on the first day. After an exploratory period of 60 s, the conditioned stimulus (CS; 10 kHz, 70 dB pure tone) was presented for 3 s. The CS co-terminated with an unconditioned stimulus (US; 0.30 mA scrambled electrical footshock for 0.5 s). Mice were returned to their holding cages 50 s after the single conditioning trial. After the conditioning, mice were divided into two groups: cue-dependent fear memory group and context-dependent fear memory group. Conditioned freezing to either cue or context were examined 1 h, 24 h, and 7 days after the conditioning; i.e., each mouse was repeatedly tested three times for either cue- or context-dependent fear memory.
For the cue-dependent fear memory group, mice were placed in a new chamber and the tone was presented for 60 s without the footshock. For the context-dependent fear memory group, mice were placed in the original shocking chamber. In both tests, cumulative time of freezing behavior during a 60-s period was measured as an index of fear (Borlikova and Endo 2009; Kojima et al. 2008; Yanai et al. 2012).
Analgesia tests
Mice were subjected to the hotplate test, followed by the electrical footshock sensitivity test. In the hotplate test, pain sensitivity was assessed by measuring the latency to lick a paw after the mouse was placed on a 55 °C hotplate (Muromachi Kikai, Tokyo, Japan). On the next day of the hotplate test, sensitivity to electrical footshock was examined, by measuring the threshold intensity to evoke a paw flick and vocalization. A mouse was placed in the shocking chamber, and a series of scrambled electrical foot shocks were delivered, starting at an intensity of 0.01 mA and increasing by the increment of 0.01 mA. The inter-stimulus interval was 15 s.
Immunohistochemistry
Mice subjected to context-dependent fear memory testing were processed for further immunohistochemical analysis of the brain. Thirty minutes after the final testing, mice were deeply anesthetized with isoflurane and transcardially perfused with phosphate-buffered saline (PBS) followed by 4 % paraformaldehyde in PBS. The brains were removed from the skull and cryoprotected in 30 % sucrose. Sections (30 μm) were immunostained with anti-phosphorylated CREB antibody (phosphorylation of Ser133; Upstate/Millipore, Billerica, MA, USA). Immunoreactions were visualized using an ABC kit and 3,3′-diaminobenzidine (Vector Laboratories). To quantify phosphorylated CREB-immunoreactive cells in each section, we took images of four to six randomly chosen areas of each hippocampal region and analyzed the images with Image J software. Quantification of phosphorylated CREB-immunoreactive cells was carried out for CA1, CA3, and dentate gyrus (DG) of the hippocampal formation, and basolateral amygdala (BLA).
Statistical analysis
All data were expressed as means ± SEM. Statistical differences among groups were determined by one-way or two-way analysis of variance (ANOVA) as mentioned in the text. All analyses were performed using SPSS software (IBM, Tokyo, Japan). Statistical significance was set at P < 0.05. When the main effect or interaction was statistically significant, a Tukey–Kramer multiple comparison test or test for the simple main effect was conducted.
Results
Cilostazol concentration in serum
A high concentration of cilostazol was observed in the serum at 0.5 and 1 h after gavage administration, and the concentration decreased by approximately half in 4 h (Fig. 1a), suggesting that the half-life of cilostazol is shorter in mice compared to those in humans (approximately 6 h; Yoo et al. 2010). Two-way ANOVA confirmed the statistical significance of the main effect of dose (F(1, 59) = 16.04, p < 0.001) and time after administration (F(5, 59) = 25.37, p < 0.001). The serum cilostazol concentrations for both doses (30 and 100 mg/kg BW) remained above the clinically effective concentration (0.76 μg/ml; Takase et al. 2007) for 3 h, concentrations that reduce platelet coagulation through PDE3 inhibition.
Pharmacokinetic analysis of cilostazol administered by two methods. Sera from cilostazol-administered mice were analyzed as described in the “Materials and methods”. a For gavage administration, the suspensions of cilostazol were administered either at 30 or 100 mg/kg BW. b For mixed-in-feed administration, feed containing cilostazol was given to mice to achieve doses of 30, 60, and 300 mg/kg BW. Cilostazol concentration in serum remained above the clinically effective concentration (0.76 μg/ml) for 2 h after administration, regardless of administration method. Five to seven mice were used for each concentration and time point. Error bars indicate SEM
A similar pharmacokinetic pattern was observed for administration of cilostazol in the animals’ feed (Fig. 1b). Two-way ANOVA revealed a significant main effect of dose (F(2, 71) = 84.32, p < 0.001), time after administration (F(4, 71) = 46.70, p < 0.001), and an interaction between dose and time after administration (F(8, 71) = 10.73, p < 0.001) was observed. Tukey–Kramer multiple comparison tests revealed that serum cilostazol concentration resulting from a dose of 300 mg/kg BW was significantly higher than that resulting from doses of 30 and 60 mg/kg BW, except 24 h after the administration. Serum cilostazol concentration differences between the 30 and 60 mg/kg BW doses were marginally significant at 1 h after administration (p = 0.074). The serum cilostazol concentration observed for the 300 mg/kg BW was moderately higher than the clinically effective concentration (Takase et al. 2007). Since higher concentrations of cilostazol may cause headache, diarrhea, and nausea in patients (Ahn et al. 2001; Dawson et al. 1998), behavioral experiments using the 300 mg/kg BW cilostazol were not conducted in this study.
We assumed that cilostazol concentration in serum remained above the clinically effective concentration during behavioral examinations completed within 2 h after cilostazol administration, either by gavage or through feed. The cilostazol concentrations were consistently higher in the gavage group compared to the mixed-in-feed group (two-way ANOVA: F(1, 49) = 56.52, p < 0.001) for the 30 mg/kg BW dose. In gavage administration, absorption might be better because cilostazol was thoroughly pulverized and suspended in solution. On the other hand, in the feed administration, effective absorption of cilostazol might be hindered by the feed.
Behavioral test battery group
Open field test
In the open field test, the two cilostazol-treated groups and the control group behaved similarly on all indices; cilostazol had no significant observable effects (Fig. 2). In the open field test, the number of rearing and travel distance reflect locomotor activity, whereas immobile time and time spent in the center of the field reflect anxiety levels (Walsh and Cummins 1976). On the second day (bright-lit condition), locomotive indices, number of rearing (Fig. 2a), and distance traveled (Fig. 2b) were significantly decreased compared to those measured on the first day (dark-lit condition; number of rearing: F(1, 15) = 6.90, p < 0.05; distance traveled: F(1, 15) = 32.18, p < 0.001). With regard to anxiety level indices, all groups of mice showed significantly increased immobility on the second day (Fig. 2c, F(1, 15) = 49.61, p < 0.001). For the time spent in the center partition, the main effect of light condition was significant (F(1, 15) = 57.12, p < 0.001), and we observed a significant interaction between groups and light condition (Fig. 2d; F(2, 15) = 4.50, p < 0.05). The significant simple main effect of light condition was observed for the control and 60 mg/kg BW cilostazol group (p < 0.001), however, the simple main effect of the 30 mg/kg BW cilostazol group was marginally significant (p = 0.068). In summary, these results obtained from the open field test suggest that cilostazol did not have an apparent effect on locomotor activity, but it might reduce anxiety-like behavior at low concentrations.
Open field test. Effect of ambient light and cilostazol on locomotor activity and anxiety-related behavior in a novel environment were analyzed. Mice were fed cilostazol-containing feed to achieve doses of 30 mg/kg BW (n = 6) or 60 mg/kg BW (n = 6). Feeding was carried out 1 h before testing. The control group (n = 6) received standard rodent feed. Locomotion (i.e., number of rearing (a) and distance traveled (b)) and anxiety-related behavior (i.e., immobile time (c) and time spent in the center partition (d)) were examined. Error bars indicate SEM
Forced swim test
Mice displayed vigorous movement when they were initially placed into water. However, they gradually became immobile and afloat. Mean ± SEM immobile times during a 240-s observation period were 177.2 ± 43.4, 194.2 ± 40.5, and 184.5 ± 47.0 s for the control group, 30 mg/kg BW cilostazol group, and 60 mg/kg BW cilostazol group, respectively. Cilostazol did not significantly affect immobile time (one-way ANOVA: F(2, 15) = 0.23, n.s.), suggesting that cilostazol has no effect on depression-like behavior.
Morris water maze task
In the acquisition of spatial memory in the Morris water maze, the two cilostazol-treated groups and the control group acquired the task similarly over 14 days of training (Fig. 3a), as indicated by the significant main effect of training days in decreased escape latency (two-way ANOVA: F(13, 195) = 44.22, p < 0.001). Although the significant main effect of cilostazol administration on escape latencies was not significant overall, cilostazol significantly affected mean swim speed on day 1 (Table 2; one-way ANOVA: F(2, 15) = 4.01, p < 0.05). Post hoc tests revealed that the difference between the control group and the two cilostazol-treated groups of mice were marginally significant (control and 30 mg/kg BW cilostazol, p = 0.056; control and 60 mg/kg BW cilostazol, p = 0.079). Because the mean swim speed of three groups was statistically equivalent on day 14 (one-way ANOVA: F(2, 15) = 1.12, n.s.), cilostazol-induced changes in swimming ability could not account for the tendency toward faster swim speeds measured during the early stage of training. To examine in detail the cilostazol-induced changes in swim speed on day 1, further analysis was conducted on immobile time. The mean (±SEM) percentages of immobility time on day 1 were 47.4 ± 5.7 % for the control group, 21.9 ± 7.0 % for the 30 mg/kg BW cilostazol group, and 24.8 ± 8.1 % for the 60 mg/kg BW cilostazol group. Cilostazol had a significant effect on the percentage of immobility on day 1 (one-way ANOVA: F(2, 15) = 3.93, p < 0.05). Post hoc tests revealed that the difference between the control group and the two cilostazol-treated groups was marginally significant (control and 30 mg/kg BW cilostazol, p = 0.054; control and 60 mg/kg BW cilostazol, p = 0.091).
Spatial memory in the Morris water maze. Effect of cilostazol on spatial learning and memory were analyzed. Mice were given either 30 mg/kg BW (n = 6) or 60 mg/kg BW (n = 6) of cilostazol mixed in feed 1 h prior to the behavioral experiments. For the control group (n = 6), standard rodent feed was administered. a Mean escape latency to the submerged platform during spatial acquisition training. b Mean escape latency to the visible platform during cued training. c Mean number of platform crossings (annuls crossings) during the probe test, which was carried out 1 day after the completion of spatial acquisition training. d Mean time spent in the training quadrant during the probe test. †p < 0.10, *p < 0.05 compared with control mice. Error bars indicate SEM
In the cued training for the Morris water maze task, cilostazol had no effect on escape latency (Fig. 3b), indicating that cilostazol did not demonstrably alter vision and swimming ability required to navigate the Morris water maze. In the probe test (Fig. 3c), however, the two cilostazol-treated groups demonstrated significantly greater number of crossings of the platform’s location than the control group (one-way ANOVA: F(2, 15) = 5.53, p < 0.05). Tukey–Kramer multiple comparison tests revealed that the number of crossings in the 60 mg/kg BW cilostazol group was significantly greater than that of the control group (p < 0.05). The difference between the control group and the 30 mg/kg BW cilostazol group was marginally significant (p = 0.072). Although cilostazol had a significant effect on the number of platform location crossings, it had no significant effect on the time spent in the training quadrant for the different groups (Fig. 3d). Exact recollection of the platform’s location is crucial for superior performance in the number of platform crossings, therefore, the result may suggest that cilostazol improves the preciseness of spatial memory.
Fear conditioning group
Pavlovian fear conditioning task
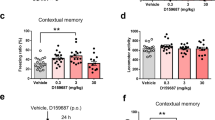
In the cue-dependent fear memory test, the two cilostazol-treated groups and the control group showed a similar degree of conditioned freezing throughout the experiments (Fig. 4a). A mixed design two-way ANOVA revealed that cilostazol did not significantly affect conditioned freezing (F(2, 24) = 1.30, n.s.). On the other hand, freezing duration demonstrated to be statistically significant main effect of time after conditioning (F(2, 48) = 2.95, p < 0.001). Tukey–Kramer multiple comparison tests revealed that conditioned freezing was significantly lower when mice were tested 7 days after conditioning compared to when they were tested 1 and 24 h after conditioning (ps < 0.001). These results suggest that cilostazol does not affect the decline of cue-dependent fear memory for up to 7 days.
Classical fear conditioning task. Effect of cilostazol on cue- and context-dependent fear memory was analyzed. Mice were administered 30 or 100 mg/kg BW of cilostazol by gavage 30 min before the behavioral experiments. For the control group, the same volume of vehicle was administered. After conditioning, 60 mice were divided into either a cue-dependent fear memory group (n = 27) or a context-dependent fear memory test group (n = 33). a Conditioned freezing to a tone was examined 1 h, 24 h, and 7 days after conditioning. Each group consisted of nine mice. b Conditioned freezing to context was examined 1 h, 24 h, and 7 days after conditioning. Twelve, ten, and 11 mice were assigned to the 30 mg/kg BW of cilostazol, 100 mg/kg BW of cilostazol, and control groups, respectively. ***p < 0.001 compared with control mice. Error bars indicate SEM
With respect to context-dependent fear memory, no significant differences were observed in tests conducted 1 and 24 h after conditioning. When mice were tested 7 days after conditioning, the control group showed a similar decline in conditioned freezing as in the cue-dependent fear memory test. However, conditioned freezing in the two cilostazol-treated groups was significantly greater than in the control group (Fig. 4b). A mixed design two-way ANOVA revealed a significant main effect of time after conditioning (F(2, 60) = 6.54, p < 0.01) and an interaction between cilostazol administration and time after conditioning (F(4, 60) = 5.39, p < 0.001). Tests for simple main effect showed that cilostazol had a significant effect on freezing observed on day 7 (F(2, 90) = 7.24, p < 0.01). These tests also showed a simple main effect of time after conditioning in the control group (F(2, 60) = 16.20, p < 0.001). These results suggest that cilostazol specifically improved or maintained context-dependent 7-day long-term fear memory without affecting 1-h short-term memory and 24-h long-term memory.
Analgesia tests
Mice in the two cilostazol-treated groups and control group behaved similarly in both analgesia tests (Table 3), as indicated by the lack of statistical significance (one-way ANOVA; latency to paw flick in hotplate test, F(2, 21) = 0.12, n.s.; current intensity to evoke paw flick in electrical footshock sensitivity test, F(2, 21) = 0.00, n.s.; current intensity to evoke vocalization in electrical footshock sensitivity test, F(2, 21) = 0.54, n.s.). These results suggest that cilostazol did not demonstrably alter pain sensitivity, regardless of the type of stimuli.
Immunohistochemical analysis
Thirty minutes after the last test in the contextual fear conditioning task, the mice were euthanized and their brains were processed for immunohistochemical analysis (Fig. 5a–d). Cilostazol did not affect the number of phosphorylated CREB-immunoreactive cells in the BLA, or the CA1, and CA3 fields of the hippocampal region (Fig. 5e–g). In the DG, however, the number of phosphorylated CREB-immunoreactive cells was greater in the two cilostazol-treated groups compared to the control group (Fig. 5h); this finding was statistically significant (one-way ANOVA: F(2, 19) = 6.52, p < 0.01). Post hoc tests confirmed that cilostazol significantly increased the number of phosphorylated CREB-immunoreactive cells in the DG compared to the control group (ps < 0.05). These results may suggest that cilostazol elevated the concentration of intracellular cAMP, which in turn led to enhanced CREB phosphorylation by PKA. Further examination is necessary to assess phosphorylation caused by other kinases activated indirectly by cilostazol.
Number of phosphorylated CREB-immunoreactive cells in subregions of the hippocampal formation and basolateral amygdala. The mice were processed for immunohistochemical analysis 30 min after 7-day context-dependent fear memory testing. a Image of Nissl-stained coronal section was used to identify the BLA from other subnuclei of the amygdala. White and black rectangles represent areas of the hippocampal formation and amygdala, respectively, that were analyzed. Scale bar is 1 mm. Phosphorylated CREB-positive cells were identified using an antibody that specifically recognized the phosphorylated form of CREB. The immunoreaction was visualized using an ABC kit and diaminobenzidine. b, c Higher-magnification images of the amygdala (b) and hippocampal region (c) in a tissue section processed for immunohistochemical staining. Scale bars are 200 μm. d Representative images of phosphorylated CREB-immunoreactive cells in the BLA and CA1, CA3, and DG of the hippocampal formation of the control (upper row) and the cilostazol-administered mice (lower; 100 mg/kg BW of cilostazol). Scale bars are 20 μm. e–h Quantification of phosphorylated CREB-immunoreactive cells in BLA (e) and CA1 (f), CA3 (g), and DG (h) of the hippocampal formation. *p < 0.05 compared with control mice. Error bars indicate SEM
Discussion
In the present study, we investigated the effect of the selective PDE3 inhibitor cilostazol on learning and memory using several different behavioral tasks in mice. We demonstrated that cilostazol improved long-term memory, which was correlated with an increase in phosphorylated CREB-positive cells in the DG. Importantly, swimming ability in the water maze task was unaffected by the cilostazol doses we used, and locomotor activity, anxiety state, and pain sensitivity were also similarly unaffected.
In the open field test, mice administered cilostazol behaved like control mice with regard to locomotion (Fig. 2a–b) and anxiety state (Fig. 2c–d), suggesting that cilostazol has no apparent effect on locomotor activity and anxiety level. In the forced swim test, we found that cilostazol does not alter depression-like behavior; therefore, it is reasonable to conclude that cilostazol has no antidepressant effects. This is in contrast to a recent study in which it was reported that cilostazol significantly reduces depression-like behavior in the forced swim and tail suspension tests (Patel et al. 2012). This disparity may be due to differences in experimental methods used in the two studies, such as how cilostazol was administered and the mouse strains used. Further studies are warranted to assess the antidepressant qualities of cilostazol.
In the Morris water maze task, the acquisition of spatial memory was equivalent across groups in their escape latencies (Fig. 3a). Cilostazol, however, significantly increased mean swim speed on day 1. This observation may suggest that cilostazol administration have influence on the motivation to escape from the water by reducing floating behavior that was frequently observed before mice learned to escape to the platform. In the probe trial, for mice given the two doses of cilostazol, the number of platform crossings significantly increased compared to mice in the control group (Fig. 3c). Exact recollection of the platform’s location is crucial for superior performance in the probe test, not just general recollection of which quadrant the platform was located during training. The former is demonstrated by the number of platform location crossings, the latter by swimming unpurposefully throughout the training quadrant. Our results suggest that mice administered cilostazol recalled the exact position of the platform during training and searched for it with high accuracy. Cilostazol therefore may improve the preciseness of spatial memory retention and/or recollection. Although platform crossings and quadrant time both are indices frequently used to assess spatial memory, platform crossings could better reflect recollection of more accurate spatial memory (i.e., precise location of the platform versus approximate location in the experimental environment). These findings might be consistent with the argument that the quadrant time may overestimate the spatial ability because the quadrant time may not be the complete reflection of the goal-directed searching behavior (Blokland et al. 2004). More selective indices such as the time spent in the circular zone centered on the platform (Blokland et al. 2004) and mean proximity to the platform (Gallagher et al. 1993) might provide us a better understanding of the accuracy of spatial memory. Detailed analysis of the current experiment is necessary using these methods in the future.
Because cilostazol potentially enhances the accuracy of spatial memory, cilostazol might enhance this fundamental component of memory. Similar to our results, deleting Kvβ1.1, an auxiliary potassium channel subunit (Murphy et al. 2004), or ryanodine-sensitive receptor subtype 3 (Futatsugi et al. 1999) improves performance in the probe test, without affecting acquisition training. Notably, these genetically modified mice also exhibit facilitation in long-term potentiation, a type of long-term synaptic plasticity that is considered to be one of the major cellular mechanisms underlying learning and memory (Bliss and Collingridge 1993; Bliss and Lomo 1973). Further examination is required to identify memory components and cellular mechanisms involved in enhancing the accuracy of memory. Also, we cannot rule out the possibility that, under our experimental conditions, cilostazol may increase the tendency to perseverate in the original spatial location acquired. We need to use different tasks, such as reversal water maze task (Yanai et al. 2004) or matching-to-place task (Steele and Morris 1999), to determine whether our findings are due to enhanced spatial memory or due to increased perseverance.
In classical fear conditioning, cilostazol did not have an effect on context-dependent fear when assessed 1 and 24 h after conditioning (Fig. 4). Cilostazol, however, significantly enhanced context-dependent fear memory examined 7 days after conditioning (Fig. 4b). Cilostazol may enhance the retention or recollection of context-dependent long-term memory. Because mice in the two cilostazol-administered groups and control group performed similarly in analgesia tests (Table 3), it is suggested that cilostazol did not demonstrably alter pain sensitivity, regardless of the type of stimuli. Therefore, the enhanced conditioned fear memory to context observed in the two groups of mice administered cilostazol was not due to differences in pain sensitivity.
The hippocampus and the amygdala have been reported to have a dissociable role in fear conditioning tasks (LeDoux 1995; Phillips and LeDoux 1992); furthermore, amygdala lesions impair both cued and contextual fear conditioning, whereas hippocampus lesions impair contextual conditioning without adversely affecting cued conditioning (Anagnostaras et al. 1999; Maren et al. 1997). On the basis of these observations, cilostazol may exert a significant influence on hippocampal activity. Indeed, we hypothesize that cilostazol may exert its beneficial effects by enhancing the cAMP–PKA system in the hippocampal region by maintaining increased levels of cAMP induced by cellular activities (Cone et al. 1999).
Taken together, our results suggest that cilostazol selectively improves hippocampal-dependent long-term memory. Our observations are consistent with those of previous studies showing that inhibition of other PDEs enhances hippocampal-dependent processes: PDE2 (Boess et al. 2004), PDE4 (Bach et al. 1999; Gong et al. 2004; Monti et al. 2006; Nagakura et al. 2002), PDE5 (Boccia et al. 2011; Cuadrado-Tejedor et al. 2011; Puzzo et al. 2009), PDE7 (Perez-Gonzalez et al. 2013), and PDE9 (van der Staay et al. 2008). PDE4 and PDE5 inhibition also improved performance on an object recognition task (Rutten et al. 2007; Prickaerts et al. 2004) that requires perirhinal cortex and prefrontal cortex to be intact, in addition to the hippocampus (Warburton and Brown 2010). Unlike with what is known about inhibitors of PDE4 and PDE5, little is known about the functional relationship between cilostazol administration and extra-hippocampal brain regions. Further study is required to determine how cilostazol affects tasks that involve extra-hippocampal functions.
The observation that cilostazol improved hippocampus-dependent memories and that the cAMP–PKA–CREB pathway plays a critical role in cognitive function (Bartsch et al. 1995; Brightwell et al. 2007; Davis 1996; Florian et al. 2006; Fujioka et al. 2004; Goelet et al. 1986; Kandel 2001; Kida 2012; Ota et al. 2008; Yin et al. 1995) prompted us to examine the phosphorylation of CREB as a downstream component of the cAMP pathway. Cilostazol significantly increased the number of cells positive for phosphorylated CREB in the DG (Fig. 5h), without affecting CREB phosphorylation in BLA, CA1, and CA3 (Fig. 5e–g). Classically described, sensory information is first delivered to the DG for processing in the hippocampus (Amaral and Lavenex 2006). Therefore, the cilostazol-induced increase in CREB phosphorylation may significantly affect information processing through subsequent regions of the hippocampal neuronal circuitry. Some neurons in the DG act as place cells, which fire when an animal is in a specific location (Jung and McNaughton 1993; Leutgeb et al. 2007; Neunuebel and Knierim 2012). An increase in phosphorylated CREB in the DG could result in the activation of place cells, thereby enhancing context-dependent fear memory. In addition, a recent study found that the DG is involved in formation of memory (Ramirez et al. 2013). Similar to our results, the PDE4 inhibitors rolipram and RS 67333 were found to increase the phosphorylation of CREB in hippocampus (Li et al. 2009). The location of the increased phosphorylated CREB in the hippocampus, however, was not identified because these authors used immunoblotting of whole hippocampus samples for the analysis.
Recently, mRNA expression profiles for 11 PDEs have been reported (Lakics et al. 2010). PDE3 is widely distributed throughout the brain including hippocampus, however, mRNA expression of PDE3 is apparently low compared to that of PDE4 (Lakics et al. 2010), which is the most studied therapeutic target for the cognitive disorders among PDEs. Considering the lower PDE3 expression together with the memory improvement by PDE3 inhibitor cilostazol, the mRNA amount may not necessarily reflect its importance for neuronal functions. Instead, cilostazol administration might enhance the cellular function to increase the CREB phosphorylation, in the certain region of the brain, dentate gyrus which is the gate of information flow into the hippocampus. This may lead to the cognitive enhancement by cilostazol. In addition to the potentiation of intracellular signal transduction pathways in neuronal circuitry caused by the direct effect of cilostazol, other indirect mechanisms might underlie the cognitive enhancement resulting from cilostazol administration to cells. For example, cilostazol significantly increases cerebral blood flow (Kwon et al. 2005). This may play an important role in learning and memory function (Mori et al. 2002; Moser et al. 2012; Tota et al. 2012). This increased blood flow may also explain the findings that cilostazol improved performance in the Morris water maze task in an ischemia-induced model of vascular dementia (Lee et al. 2007).
A significant detail regarding PDE inhibitors in cognitive enhancement is that their mechanism of action differs from that of the acetylcholinesterase inhibitor donepezil, which is currently the most often used drug to treat dementia (Dooley and Lamb 2000; Pepeu and Giovannini 2009; Yuede et al. 2007). Combination therapy comprising two or more drugs has been demonstrated to be an effective strategy for treating some types of diseases (Croom and Dhillon 2011). However, this strategy is yet to be applied in the treatment of cognitive impairment. Cilostazol and donepezil in combination enhances, to some extent, cognitive functions in animal models (Lee et al. 2007), and in moderate Alzheimer’s disease patients (Arai and Takahashi 2009). In addition, PDE inhibitor and acetylcholinesterase inhibitor are shown to affect differently on memory process; PDE inhibitor improves memory consolidation and acetylcholinesterase inhibitor enhances acquisition of memory, respectively (Prickaerts et al. 2005). Hence, concurrent administration of cilostazol with other dementia drugs, such as donepezil, may offer a new pharmacological approach for treating cognitive disorders.
References
Ahn CW, Lee HC, Park SW, Song YD, Huh KB, Oh SJ, Kim YS, Choi YK, Kim JM, Lee TH (2001) Decrease in carotid intima media thickness after 1 year of cilostazol treatment in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 52:45–53
Akiyama H, Kudo S, Shimizu T (1985) The absorption, distribution and excretion of a new antithrombotic and vasodilating agent, cilostazol, in rat, rabbit, dog and man. Arzneimittelforschung 35:1124–1132
Amaral D, Lavenex P (2006) Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J (eds) The hippocampus book. Oxford University Press, New York, pp 37–114
Anagnostaras SG, Maren S, Fanselow MS (1999) Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci 19:1106–1114
Arai H, Takahashi T (2009) A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: pilot follow-up study. Am J Geriatr Psychiatry 17:353–354
Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER (1999) Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A 96:5280–5285
Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER (1995) Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 83:979–992
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Blokland A, Geraerts E, Been M (2004) A detailed analysis of rats’ spatial memory in a probe trial of a Morris task. Behav Brain Res 154:71–75
Blokland A, Schreiber R, Prickaerts J (2006) Improving memory: a role for phosphodiesterases. Curr Pharm Des 12:2511–2523
Boccia MM, Blake MG, Krawczyk MC, Baratti CM (2011) Sildenafil, a selective phosphodiesterase type 5 inhibitor, enhances memory reconsolidation of an inhibitory avoidance task in mice. Behav Brain Res 220:319–324
Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G (2004) Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47:1081–1092
Borlikova G, Endo S (2009) Inducible cAMP early repressor (ICER) and brain functions. Mol Neurobiol 40:73–86
Brightwell JJ, Smith CA, Neve RL, Colombo PJ (2007) Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn Mem 14:195–199
Burgers PM, Eckstein F, Hunneman DH (1979) Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem 254:7476–7478
Cone J, Wang S, Tandon N, Fong M, Sun B, Sakurai K, Yoshitake M, Kambayashi J, Liu Y (1999) Comparison of the effects of cilostazol and milrinone on intracellular cAMP levels and cellular function in platelets and cardiac cells. J Cardiovasc Pharmacol 34:497–504
Croom KF, Dhillon S (2011) Bevacizumab: a review of its use in combination with paclitaxel or capecitabine as first-line therapy for HER2-negative metastatic breast cancer. Drugs 71:2213–2229
Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Pérez-Roldán JM, García-Barroso C, Franco R, Aguirre N, García-Osta A (2011) Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br J Pharmacol 164:2029–2041
Davis RL (1996) Physiology and biochemistry of Drosophila learning mutants. Physiol Rev 76:299–317
Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr (1998) Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation 98:678–686
Dooley M, Lamb HM (2000) Donepezil: a review of its use in Alzheimer’s disease. Drugs Aging 16:199–226
Florian C, Mons N, Roullet P (2006) CREB antisense oligodeoxynucleotide administration into the dorsal hippocampal CA3 region impairs long- but not short-term spatial memory in mice. Learn Mem 13:465–472
Fujioka T, Fujioka A, Duman RS (2004) Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci 24:319–328
Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K (1999) Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron 24:701–713
Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107:618–626
Goelet P, Castellucci VF, Schacher S, Kandel ER (1986) The long and the short of long-term memory—a molecular framework. Nature 322:419–422
Goldberg ND, Walseth TF, Stephenson JH, Krick TP, Graff G (1980) 18O-Labeling of guanosine monophosphate upon hydrolysis of cyclic guanosine 3′:5′-monophosphate by phosphodiesterase. J Biol Chem 255:10344–10347
Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O (2004) Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest 114:1624–1634
Hiramatsu M, Takiguchi O, Nishiyama A, Mori H (2010) Cilostazol prevents amyloid β peptide(25–35)-induced memory impairment and oxidative stress in mice. Br J Pharmacol 161:1899–1912
Jung MW, McNaughton BL (1993) Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3:165–182
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038
Kida S (2012) A functional role for CREB as a positive regulator of memory formation and LTP. Exp Neurobiol 21:136–140
Kojima N, Borlikova G, Sakamoto T, Yamada K, Ikeda T, Itohara S, Niki H, Endo S (2008) Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J Neurosci 28:6459–6472
Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS (2005) Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 36:782–786
Lakics V, Karran EH, Boess FG (2010) Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59:367–374
LeDoux JE (1995) Emotion: clues from the brain. Annu Rev Psychol 46:209–235
Lee JH, Park SY, Shin YW, Kim CD, Lee WS, Hong KW (2007) Concurrent administration of cilostazol with donepezil effectively improves cognitive dysfunction with increased neuroprotection after chronic cerebral hypoperfusion in rats. Brain Res 1185:246–255
Leutgeb JK, Leutgeb S, Moser MB, Moser EI (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315:961–966
Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang HT (2009) Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34:2404–2419
Lu YF, Hawkins RD (2002) Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol 88:1270–1278
Lucki I, Dalvi A, Mayorga AJ (2001) Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 155:315–322
Lugnier C (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109:366–398
Maren S, Aharonov G, Fanselow MS (1997) Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res 88:261–274
Mayford M, Kandel ER (1999) Genetic approaches to memory storage. Trends Genet 15:463–470
Monti B, Berteotti C, Contestabile A (2006) Subchronic rolipram delivery activates hippocampal CREB and arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology 31:278–286
Mori K, Yamashita H, Nagao M, Horiguchi J, Yamawaki S (2002) Effects of anticholinergic drug withdrawal on memory, regional cerebral blood flow and extrapyramidal side effects in schizophrenic patients. Pharmacopsychiatry 35:6–11
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Moser DJ, Boles Ponto LL, Miller IN, Schultz SK, Menda Y, Arndt S, Nopoulos PC (2012) Cerebral blood flow and neuropsychological functioning in elderly vascular disease patients. J Clin Exp Neuropsychol 34:220–225
Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ (2004) Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol 14:1907–1915
Nagakura A, Niimura M, Takeo S (2002) Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol 135:1783–1793
Neunuebel JP, Knierim JJ (2012) Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci 32:3848–3858
O’Donnell ME, Badger SA, Sharif MA, Young IS, Lee B, Soong CV (2009) The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. J Vasc Surg 49:1226–1234
Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE (2008) The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem 15:792–805
Park SH, Kim JH, Bae SS, Hong KW, Lee DS, Leem JY, Choi BT, Shin HK (2011) Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid β-induced cognitive deficits associated with decreased amyloid β accumulation. Biochem Biophys Res Commun 408:602–608
Patel DS, Anand IS, Bhatt PA (2012) Evaluation of antidepressant and anxiolytic activity of phosphodiesterase 3 inhibitor—cilostazol. Indian J Psychol Med 34:124–128
Pepeu G, Giovannini MG (2009) Cholinesterase inhibitors and beyond. Curr Alzheimers Res 6:86–96
Perez-Gonzalez R, Pascual C, Antequera D, Bolos M, Redondo M, Perez DI, Pérez-Grijalba V, Krzyzanowska A, Sarasa M, Gil C, Ferrer I, Martinez A, Carro E (2013) Phosphodiesterase 7 inhibitor reduced cognitive impairment and pathological hallmarks in a mouse model of Alzheimer’s disease. Neurobiol Aging 34:2133–2145
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285
Pratt CM (2001) Analysis of the cilostazol safety database. Am J Cardiol 87:28D–33D
Prickaerts J, Sik A, van Staveren WC, Koopmans G, Steinbusch HW, van der Staay FJ, de Vente J, Blokland A (2004) Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int 45:915–928
Prickaerts J, Sik A, van der Staay FJ, de Vente J, Blokland A (2005) Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology (Berl) 177:381–390
Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O (2009) Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci 29:8075–8086
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S (2013) Creating a false memory in the hippocampus. Science 341:387–391
Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J (2009) Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 202:419–443
Rutten K, Lieben C, Smits L, Blokland A (2007) The PDE4 inhibitor rolipram reverses object memory impairment induced by acute tryptophan depletion in the rat. Psychopharmacology (Berl) 192:275–282
Steele RJ, Morris RG (1999) Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9:118–136
Takase H, Hashimoto A, Okutsu R, Hirose Y, Ito H, Imaizumi T, Miyakoda G, Mori T (2007) Anti-atherosclerotic effect of cilostazol in apolipoprotein-E knockout mice. Arzneimittelforschung 57:185–191
Tota S, Hanif K, Kamat PK, Najmi AK, Nath C (2012) Role of central angiotensin receptors in scopolamine-induced impairment in memory, cerebral blood flow, and cholinergic function. Psychopharmacology (Berl) 222:185–202
van der Staay FJ, Rutten K, Bärfacker L, Devry J, Erb C, Heckroth H, Karthaus D, Tersteegen A, van Kampen M, Blokland A, Prickaerts J, Reymann KG, Schröder UH, Hendrix M (2008) The novel selective PDE9 inhibitor BAY 73–6691 improves learning and memory in rodents. Neuropharmacology 55:908–918
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Warburton EC, Brown MW (2010) Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia 48:2262–2272
Xu Y, Zhang HT, O’Donnell JM (2011) Phosphodiesterases in the central nervous system: implications in mood and cognitive disorders. Handb Exp Pharmacol 204:447–485
Yanai S, Okaichi Y, Okaichi H (2004) Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging 25:325–332
Yanai S, Semba Y, Endo S (2012) Remarkable changes in behavior and physiology of laboratory mice after the massive 2011 Tohoku earthquake in Japan. PLoS One 7:e44475
Yin JC, Del Vecchio M, Zhou H, Tully T (1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81:107–115
Yoo HD, Cho HY, Lee YB (2010) Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol 69:27–37
Yuede CM, Dong H, Csernansky JG (2007) Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol 18:347–363
Acknowledgments
Authors thank Ms. Masako Suzuki, Tomoko Arasaki, and Kazuko Nakanishi for their technical help. This work is supported in part by JSPS KAKENHI (24730642, 25293331, and 25560382), the Naito Foundation, Japan Foundation for Aging and Health, and Otsuka Pharmaceutical Co., Ltd.
Conflict of interest
Authors declare that there are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 75 kb)
Rights and permissions
About this article
Cite this article
Yanai, S., Semba, Y., Ito, H. et al. Cilostazol improves hippocampus-dependent long-term memory in mice. Psychopharmacology 231, 2681–2693 (2014). https://doi.org/10.1007/s00213-014-3442-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3442-4