Abstract
Objective
The present study aimed to examine the changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia.
Methods
Sixty-two drug naïve, first-episode schizophrenia (SZ group) and 60 healthy individuals (control group) were enrolled in the study. Serum interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) levels, and body weight were measured at baseline for both groups, and repeated for the SZ group at five different time points during 6-month risperidone treatment.
Results
At baseline, serum IL-1β, IL-6, and TNF-α levels in the SZ group (53.28 ± 12.62, 33.98 ± 14.13, 50.08 ± 12.86 pg/mL, respectively) were significantly higher than those in the control group (23.49 ± 15.27, 15.53 ± 7.16, 32.12 ± 15.23 pg/mL, respectively) (p's < 0.001). Within the SZ group, serum IL-1β levels decreased significantly at 2 weeks (48.02 ± 16.00 pg/mL, p < 0.01) and 1 month (44.70 ± 16.63 pg/mL, p < 0.001), but then gradually increased at 2 months (48.49 ± 18.87 pg/mL), 3 months (50.59 ± 18.48 pg/mL) and 6 months (53.64 ± 16.22 pg/mL) to the levels comparable to baseline; serum IL-6 levels changed significantly over the course of treatment (p = 0.001), but reached the levels comparable to baseline at 6 months (37.13 ± 13.23 pg/mL); serum levels of TNF-α increased significantly at 3 months (55.02 ± 16.69 pg/mL, p < 0.01) and 6 months (58.69 ± 13.57 pg/mL, p < 0.001); steady and significant weight gain was observed at each follow-up time point (p's < 0.001), from 56.71 ± 9.25 kg at baseline to 62.72 ± 9.53 kg at 6 months.
Conclusions
Risperidone treatment is associated with changes in serum pro-inflammatory cytokines levels and weight. There is an initial anti-inflammatory effect that reduces with treatment, potentially due to its weight gain side effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past several decades, studies have suggested that activation of the inflammatory response system mediated by cytokines may play a key role in the pathogenesis of schizophrenia (Fan et al. 2007; Song et al. 2009). Abnormal levels of cytokine have been found in both the peripheral blood and the cerebrospinal fluid of schizophrenia patients (Garver et al. 2003; Potvin et al. 2008), and in their relatives (Martinez-Gras et al. 2012; Nunes et al. 2006). Studies in schizophrenia patients have tried to identify specific inflammatory markers relating to the disorder but with conflicting findings (Fan et al. 2007). For example, both Naudin et al. (Naudin et al. 1997) and Lin et al. (Lin et al. 1998) found that, compared with normal controls, patients with chronic schizophrenia had significantly higher serum levels of TNF-α and IL-6. However, in a separate study by Schattner et al. (Schattner et al. 1996), no difference of TNF-α levels was found between schizophrenia patients and normal controls. In a study with 10 neuroleptic-free schizophrenia patients and 10 healthy subjects matched for sex and age, levels of cerebrospinal fluid (CSF) pro-inflammatory interleukin 2 (IL-2) were found to be higher in the patient group (Licinio et al. 1993). But two other research groups were unable to repeat this finding (el-Mallakh et al. 1993; Rapaport et al. 1997). Inconsistent results in the literature might be explained by the heterogeneity of schizophrenia, the differences in illness state (acute versus chronic) (Miller et al. 2011), age and disease duration(Fawzi et al. 2011), the effect of antipsychotic medication (Davey et al. 2012; Myint et al. 2011; Schattner et al. 1996), cigarette smoking (Miller et al. 2011), and comorbid obesity, which is associated with an increased production of pro-inflammatory cytokines and sub-clinical inflammation (Fawzi et al. 2011; Hotamisligil 2006; Odegaard and Chawla 2013; Tateya et al. 2013). Metabolic problems including obesity are commonly seen in patients with schizophrenia; one major contributing factor for these metabolic problems is the use of antipsychotic medications (Amrami-Weizman et al. 2013; Fan et al. 2013; Jassim et al. 2012; Ou et al. 2013).
Addressing possible confounding factors mentioned above, the present study was to characterize the changes in pro-inflammatory cytokines and weight gain during 6-month resperidone treatment in drug naïve, first-episode schizophrenia patients with normal weight.
Methods
Subjects
All subjects provided written informed consent to participate in the study, which was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Inpatients 18 and 45 years old diagnosed with first-episode schizophrenia (disease duration less than 2 years) were recruited. Patients were diagnosed with first-episode schizophrenia according to the criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (Runeson and Rich 1994) and were never previously treated with antipsychotic medications or other psychotropics. The diagnosis of schizophrenia was further determined by a research psychiatrist (X.S.) using the Structured Clinical Interview for DSM-IV Axis I Disorders. Exclusion criteria included ongoing infections or allergies, history of alcohol or other substance use, autoimmune disorders, pregnancy, known medical conditions that might affect metabolism, history of diabetes or lipid disorder, use of anti-diabetic or lipid-lowering agents, or special diets to lower glucose or lipid levels, and use of immunosuppressive agents. Further, those patients who were overweight or obese were excluded from the study (Body Mass Index (BMI) ≥ 24 kg/m2) (Chen et al. 2004). A complete medical history was obtained from all subjects. All subjects were treated in the same hospital and underwent daily physical examination and weekly routine laboratory tests.
After baseline assessment, all patients were treated with risperidone in the dose range from 2 to 6 mg per day based on the clinical judgment of treating psychiatrists. No other medication was allowed during the study except benzodiazepines for insomnia and anticholinergic agents for dystonia reaction.
Healthy control subjects with normal weight were recruited through advertisement. A complete medical history, physical examination, and routine laboratory tests were obtained from all subjects to rule out possible medical conditions. None of them had a history of any psychiatric conditions, or history of alcohol or substance use.
Measures
Symptoms of schizophrenia were assessed for all patient subjects using the Positive and Negative Syndrome Scale (PANSS). The PANSS was administered by the same rater (X.S.) throughout the study. Weight (kg) and height (m) were measured, and BMI was calculated for all subjects.
Venous blood (5 mL) was collected between 7:00 and 8:00 AM to avoid circadian fluctuation of the parameters to be measured. The blood was put into a glass tube and allowed to clot at room temperature. Serum was obtained through centrifugation at 3000 rpm for 10 min, and was then divided into three Eppendorf tubes, and stored at −70 °C for cytokine assay.
Serum levels of IL-1β, IL-6, and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, USA). The assays were performed according to the manufacturer's instructions. The sensitivities of IL-1β, IL-6, and TNF-α were 1.0, 0.7, and 1.6 pg/mL, with inter-assay variation coefficients of 5.6, 4.5, and 5.8 %, respectively, and intra-assay variation coefficients of 4.8, 2.6, and 4.6 %, respectively. Standard curve concentrations were calculated in triplicate for each plate. Absorbencies were measured by a microtiter plate reader (absorbency at 492 nm). All assays were carried out at the same time and were conducted blinded to the subjects' group status.
Statistical analysis
The data were analyzed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL). Group comparison was performed using an independent t test. Chi-square test was used for categorical variables. Measures on cytokines and body weight during 6 months of risperidone treatment within the schizophrenia group were analyzed using repeated measures analysis of variance (ANOVA). Post hoc multiple comparisons (five follow-up time points versus baseline) were performed if the repeated measures ANOVA was significant. A p value <0.05 was considered significant. The Bonferroni correction for the significance threshold (p = 0.05/5 = 0.01) was used in multiple comparisons. Further, stepwise logistic regression model was used to examine the relative predictive value of baseline serum levels of cytokines for the risk of weight gain at 6 months of risperidone treatment.
Results
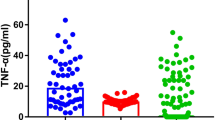
Sixty-two first-episode, drug naïve schizophrenia patients with normal weight (SZ group) and 60 healthy controls with normal weight (control group) were enrolled in the study. There were no significant differences in age, gender, education, smoking status, body weight, and BMI between the two groups (p's > 0.100, Table 1). Serum levels of IL-1β, IL-6, and TNF-α in the SZ group were significantly higher than those in the control group (p's < 0.001, Table 2).
Within the SZ group, a repeated measures ANOVA with a Greehouse-Geisser correction determined that serum levels of IL-1β, IL-6, TNF-α, and body weight changed significantly during 6-month risperidone treatment (p's < 0.001 for IL-1β, TNF-α, and body weight, p = 0.001 for IL-6, Table 3). Post hoc tests revealed that serum levels of IL-1β decreased significantly at 2 weeks (p < 0.01) and 1 month (p < 0.001), but then gradually increased at 2, 3, and 6 months to the levels comparable to baseline. Serum levels of IL-6 decreased at 1 and 2 months compared with baseline (p's < 0.05) but did not reach significance threshold (p < 0.01) after the Bonferroni correction; serum levels of IL-6 then increased at 3 and 6 months to the levels comparable to baseline. Serum levels of TNF-α increased significantly at 3 months (p < 0.01), and 6 months (p < 0.001) compared with the levels at baseline. Steady and significant weight gain was observed at each follow-up time point (p's < 0.001) (Figs. 1 and 2).
SZ patients were divided into weight gain (WG) and non-weight gain (non-WG) groups using 7 % weight gain at 6 months as a cutoff value (Bushe et al. 2013; Kemp et al. 2013). At 6 months, the WG group (N = 49) had significantly higher levels of IL-1β (p = 0.001), IL-6 (p = 0.017), and TNF-α (p = 0.023) compared with the non-WG group (N = 13). The WG group had significantly higher baseline serum levels of IL-1β (p = 0.002), IL-6 (p = 0.024), and non-significant higher baseline serum levels of TNF-α (p = 0.063) compared with the non-WG group; but there was no significant difference in baseline body weight between the two groups (p = 0.673) (Table 4). Further, stepwise logistic regression analysis indicated that, among IL-1β, IL-6, and TNF-α, only higher baseline serum levels of IL-1β predict a greater risk for weight gain at 6 months after controlling for age, gender, education, smoking status, and age of illness onset (odds ratio = 0.91, 95 % Confidence Interval 0.85–0.97, p = 0.005).
Discussion
To our knowledge, the present study was the first to characterize the changes in pro-inflammatory cytokines and weight gain during a relatively long time period (6 months) of risperidone treatment in drug naïve, first-episode schizophrenia patients with normal weight. We found that this group of patients presents an up-regulated inflammatory status at baseline as reflected by elevated serum levels of IL-1β, IL-6, and TNF-α. Our results are consistent with earlier reports from our group (Song et al. 2009) and others (Fineberg and Ellman 2013; Kim et al. 2009; Na and Kim 2007; O'Brien et al. 2008; Potvin et al. 2008).
In our patient study sample, we observed differential change patterns in serum levels of IL-1β, IL-6, and TNF-α during 6 months of risperidone treatment. Serum levels of IL-1β decreased significantly at 2 weeks and 1 month, but eventually increased to the levels comparable to baseline; serum levels of IL-6 decreased initially but went back to the levels comparable to baseline; in contrast, serum levels of TNF-α increased significantly at 3 and 6 months.
Previous studies have examined the anti-inflammatory property of antipsychotic medications with various results (Drzyzga et al. 2006; Kim et al. 2009; Meyer et al. 2009). Inconsistent findings in the literature might be explained by two major considerations. First, antipsychotic medications including risperidone may have direct impact on the inflammatory status (Chen et al. 2012; Leonard et al. 2012; Pollmacher et al. 1996; Rudolf et al. 2002). Second, antipsychotic medications cause various degrees of side effects of weight gain and obesity (Fan et al. 2013), which are closely associated with elevated production of pro-inflammatory cytokine and chronic inflammation (Hotamisligil 2006; Odegaard and Chawla 2013; Ritchie and Connell 2007; Tateya et al. 2013). In our study, steady and significant weight gain was observed during 6 months of risperidone treatment. The changes in serum levels of pro-inflammatory cytokines observed during risperidone treatment might reflect the net effect of direct anti-inflammatory impact of risperidoneand elevated production of pro-inflammatory cytokines associated with steady weight gain over the 6-month follow-up time period.
The present study also found that those drug naïve, first-episode schizophrenia patients with higher baseline serum levels of pro-inflammatory cytokines, especially IL-1β, seem to be at a greater risk for weight gain at 6 months ofrisperidone treatment. It is unclear why baseline levels of various cytokines have differential predictive values for weight gain at 6 months. Our findings align with prior research in the general population. For example, in the Cardiovascular Health Study with individuals ≥65 years old (N = 3,254), those who gained 5 % over a 3-year follow-up time period had higher baseline serum levels of C-reactive protein (CRP), fibrinogen, and factor VIIIc, which are well-established inflammatory markers (Barzilay et al. 2006). In another large study with middle-aged adults (N = 2,792), high serum levels of CRP, fibrinogen, and white blood cell count were more prone to gain weight during a 10-year follow-up time period (Holz et al. 2010). The mechanism that links the baseline inflammatory status and weight gain is not unclear. Some have hypothesized that inflammatory factors chronically stimulate the sympathetic nervous system, which may in turn result in weight increases. An alternate explanation is that inflammation is not directly associated with weight gain, but instead serves as a surrogate indicator of other pathophysiological processes related to weight gain (Vasunilashorn 2013).
The strengths of the present study include the use of a homogeneous group of drug naïve, first-episode schizophrenia patients with normal weight and a relatively long follow-up time period with risperidonemonotherapy. The selection of the study sample in our study minimized potential confounding factors such as previous exposure to antipsychotic medications, variations in disease state (acute versus chronic) and disease duration, and differences in obesity and other metabolic disturbances at baseline. The present study also has a few limitations. (1) Some important factors related to weight control, such as physical activity and food intake, were not measured during the follow-up time period. (2) Adipose tissue is a major source of pro-inflammatory cytokines; waist circumference or waist/hip ratio, which was not measured in our study, might be a closer measurement of adipose tissue. (3) Stress level, which is known to influence inflammatory status, was not measured. (4) Some important metabolic parameters such as fasting glucose and lipids were not measured in the present study.
In summary, our study in a “clean” study sample provided a “clear” dynamic picture on the changes of several important pro-inflammatory cytokines and body weight during 6-month risperidonemonotherapy. As inflammation also plays an important role in the development of schizophrenia and cognitive deficits (Fan et al. 2007), prospective studies are needed to further elucidate the role of inflammation in psychopathology, cognition in addition to metabolism in first-episode schizophrenia. The use of anti-inflammatory agents in combination with antipsychotic agents might be a promising strategy to address multiple clinical challenges in this patient population.
References
Amrami-Weizman A, Maayan R, Gil-Ad I, Pashinian A, Fuchs C, Kotler M, Poyurovsky M (2013) The effect of reboxetine co-administration with olanzapine on metabolic and endocrine profile in schizophrenia patients. Psychopharmacology (Berlin) 230:23–27
Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB (2006) The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (London) 30:1362–1367
Bushe CJ, Slooff CJ, Haddad PM, Karagianis JL (2013) Weight change by baseline BMI from 3-year observational data: findings from the Worldwide Schizophrenia Outpatient Health Outcomes Database. J Psychopharmacol 27:358–365, Oxford, England
Chen C, Lu FC, Department of Disease Control Ministry of Health, P.R.C. (2004) The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci: BES 17:1–36
Chen SL, Lee SY, Chang YH, Chen SH, Chu CH, Tzeng NS, Lee IH, Chen PS, Yeh TL, Huang SY, Yang YK, Lu RB, Hong JS (2012) Inflammation in patients with schizophrenia: the therapeutic benefits of risperidone plus add-on dextromethorphan. J Neuroimmune Pharm 7:656–664
Davey KJ, O'Mahony SM, Schellekens H, O'Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF (2012) Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berlin) 221:155–169
Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS (2006) Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun 20:532–545
el-Mallakh RS, Suddath RL, Wyatt RJ (1993) Interleukin-1 alpha and interleukin-2 in cerebrospinal fluid of schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry 17:383–391
Fan X, Goff DC, Henderson DC (2007) Inflammation and schizophrenia. Expert Rev Neurother 7:789–796
Fan X, Borba CP, Copeland P, Hayden D, Freudenreich O, Goff DC, Henderson DC (2013) Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand 127:217–226
Fawzi MH, Fawzi MM, Fawzi MM, Said NS (2011) C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res 190:91–97
Fineberg AM, Ellman LM (2013) Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry 73:951–966
Garver DL, Tamas RL, Holcomb JA (2003) Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology 28:1515–1520
Holz T, Thorand B, Doring A, Schneider A, Meisinger C, Koenig W (2010) Markers of inflammation and weight change in middle-aged adults: results from the prospective MONICA/KORA S3/F3 study. Obesity 18:2347–2353, Silver Spring, Md
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Jassim G, Skrede S, Vazquez MJ, Wergedal H, Vik-Mo AO, Lunder N, Dieguez C, Vidal-Puig A, Berge RK, Lopez M, Steen VM, Ferno J (2012) Acute effects of orexigenic antipsychotic drugs on lipid and carbohydrate metabolism in rat. Psychopharmacology (Berlin) 219:783–794
Kemp DE, Correll CU, Tohen M, Delbello MP, Ganocy SJ, Findling RL, Chang K (2013) Associations Among Obesity, Acute Weight Gain, and Response to Treatment with Olanzapine in Adolescent Schizophrenia. J Child Adolesc Psychopharmacol
Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2009) Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology 59:123–129
Leonard BE, Schwarz M, Myint AM (2012) The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol 26:33–41, Oxford, England
Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH (1993) Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry 150:1408–1410
Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, Pioli R, Altamura C, Scharpe S, Maes M (1998) The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res 32:9–15
Martinez-Gras I, Garcia-Sanchez F, Guaza C, Rodriguez-Jimenez R, Andres-Esteban E, Palomo T, Rubio G, Borrell J (2012) Altered immune function in unaffected first-degree biological relatives of schizophrenia patients. Psychiatry Res 200:1022–1025
Meyer JM, McEvoy JP, Davis VG, Goff DC, Nasrallah HA, Davis SM, Hsiao JK, Swartz MS, Stroup TS, Lieberman JA (2009) Inflammatory markers in schizophrenia: comparing antipsychotic effects in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Biol Psychiatry 66:1013–1022
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671
Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpe S, Steinbusch HW, Leonard BE, Kim YK (2011) Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain Behav Immun 25:1576–1581
Na KS, Kim YK (2007) Monocytic, Th1 and th2 cytokine alterations in the pathophysiology of schizophrenia. Neuropsychobiology 56:55–63
Naudin J, Capo C, Giusano B, Mege JL, Azorin JM (1997) A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res 26:227–233
Nunes SO, Matsuo T, Kaminami MS, Watanabe MA, Reiche EM, Itano EN (2006) An autoimmune or an inflammatory process in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophr Res 84:180–182
O'Brien SM, Scully P, Dinan TG (2008) Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res 160:256–262
Odegaard JI, Chawla A (2013) Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339:172–177
Ou JJ, Xu Y, Chen HH, Fan X, Gao K, Wang J, Guo XF, Wu RR, Zhao JP (2013) Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berlin) 225:627–635
Pollmacher T, Hinze-Selch D, Mullington J (1996) Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol 16:403–409
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E (2008) Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 63:801–808
Rapaport MH, McAllister CG, Pickar D, Tamarkin L, Kirch DG, Paul SM (1997) CSF IL-1 and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr Res 25:123–129
Ritchie SA, Connell JM (2007) The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis: NMCD 17:319–326
Rudolf S, Peters M, Rothermundt M, Arolt V, Kirchner H (2002) The influence of typical and atypical neuroleptic drugs in the production of interleukin-2 and interferon-gamma in vitro. Neuropsychobiology 46:180–185
Runeson BS, Rich CL (1994) Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. (DSM-III), adaptive functioning in young Swedish suicides. Ann Clin Psychiatry: Off J Am Acad Clin Psychiatr 6:181–183
Schattner A, Cori Y, Hahn T, Sirota P (1996) No evidence for autoimmunity in schizophrenia. J Autoimmun 9:661–666
Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP (2009) The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry 65:481–488
Tateya S, Kim F, Tamori Y (2013) Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne) 4:93
Vasunilashorn S (2013) Retrospective reports of weight change and inflammation in the US National Health and Nutrition Examination Survey. J Obes 2013:601534
Acknowledgments
Funding for this study was provided by the National Natural Science Foundation of China (No.30971058 to X-QS; No.81071090to L-XL), the Natural Science Foundation of Henan (No.102300413208, 112300413226 to L-XL), and the Youth Fund of the First Affiliated Hospital of Zhengzhou University (to X-QS).
Financial disclosures
Dr. Fan has received research support or honoraria from Eli Lilly, AstraZeneca, Bristol-Myer-Squibb, Janssen, and Pfizer. Other authors report no competing interests.
Contributors
Dr. Song and Dr. Fan were responsible for the analysis and interpretation of the data for this paper. All authors contributed to the writing of the paper.
Conflict of interest
None of the authors has any actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence, their work as submitted in the uploaded material.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xueqin Song and Xiaoduo Fan share co-first authorship.
Rights and permissions
About this article
Cite this article
Song, X., Fan, X., Li, X. et al. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology 231, 319–325 (2014). https://doi.org/10.1007/s00213-013-3382-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3382-4