Abstract
Rationale
Numerous studies indicate the potential antidepressant actions of several mGlu5 receptor antagonists, including 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP). The explanation for the mechanism of these effects might be a key step in finding new antidepressant drugs (AD).
Objectives
The aim of the present study was to investigate the possible role of the serotonergic system in the antidepressant-like activity of MTEP in the tail suspension test (TST) in C57BL/6J mice, using selected antagonists of serotonergic receptors and by applying two different methods of serotonin (5-HT) depletion.
Results
The results of our studies showed that the mGlu5 receptor antagonist, MTEP, similar to the fluoxetine used as reference AD, did not induce antidepressant-like effects in mice pretreated with tryptophan hydroxylase inhibitor, para-chlorophenylalanine. On the other hand, MTEP worked as a potential AD in the TST in mice fed on a tryptophan-free (TRP-free) diet for 3 weeks. However, fluoxetine, which was used as a reference control was also active in this experiment, suggesting that a TRP-free diet was not sufficiently effective in reducing the 5-HT level. Furthermore, we showed that the 5HT2A/2C antagonist, ritanserin, yet not the 5-HT1A antagonist, WAY100635, 5HT1B antagonist, SB224289 or 5HT4 antagonist, GR125487, reversed the antidepressant-like effects of MTEP in the TST. Finally, a sub-effective dose of MTEP co-administered with a sub-effective dose of citalopram induced an antidepressant-like effect in the TST in mice.
Conclusion
The results of our studies suggest the involvement of serotonergic system activation in the antidepressant-like effects of the mGlu5 antagonist, MTEP, in the TST in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral studies and some clinical trials have shown that modulation of the glutamatergic system might be an efficient way to achieve effective and rapid antidepressant activity. Antidepressant-like effects of several functional NMDA receptor antagonists, AMPA receptor potentiators and mGlu receptor ligands in animal models of depression and some clinical trials have been described (for review see: Chaki et al. 2012; Pałucha and Pilc 2007; Szewczyk et al. 2012). The most promising data came from the set of clinical studies describing the strong and rapid therapeutic effect of an uncompetitive NMDA receptor trapping blocker, ketamine, in the treatment-resistant depression (Berman et al. 2000; Diazgranados et al. 2010; Ibrahim et al. 2011; Zarate et al. 2006). However, ketamine has its significant limitations, including undesirable effects and the route of administration. In all of the clinical studies investigating the potential antidepressant activity of the drug, ketamine was given intravenously by an anesthesiologist, and hospitalization was required for at least 24 h post-infusion (Aan Het Rot et al. 2012). Thus, ketamine cannot be considered as a new, safe AD for outpatients. On the other hand, these studies have provided a great hope that modulation of the glutamatergic system, including NMDA receptor activity, might provide a new generation of rapid and efficient ADs.
Since mGlu5 receptors are functionally involved in the mild modulation of the activity of NMDA receptors (see review: Szewczyk et al. 2012), they might become a target for new and safer ADs. Several studies have confirmed this hypothesis. First of all, increasing number of behavioral data have shown the potential antidepressant-like effect of several mGlu5 receptor negative allosteric modulators (NAMs), such as MPEP, MTEP, and GRN-529 (Belozertseva et al. 2007; Hughes et al. 2012; Li et al. 2006; Pałucha et al. 2005; Tatarczyńska et al. 2001). Furthermore, the positron emission tomography study, which showed lower levels of mGlu5 binding in several brain regions of unmedicated patients with major depression and postmortem analyses, illustrating lower levels of mGlu5 protein expression in the prefrontal cortices of depressed patients, when compared to healthy control subjects, also indicated the possible involvement of the mGlu5 receptor in depression (Deschwanden et al. 2011).
Although the activity of mGlu5 NAMs in several animal models of depression have been described, the mechanism of their antidepressant-like action is still not clear. Among many possible explanations, there is an argument for the possible involvement of the serotonergic system in the antidepressant-like action of mGlu5 receptor antagonists. Firstly, acute treatment with MPEP has been shown to increase plasma corticosterone concentrations and this effect was blocked with a 5-HT1A antagonist, whereas the repeated 5-day treatment with MPEP induced desensitization of the HPA axis to stimulation with a 5-HT1A agonist (Bradbury et al. 2003). These neuroendocrine effects of MPEP are typical of conventional monoamine-based ADs, and suggest the involvement of the serotonergic system in the action of MPEP. Another argument comes from the behavioral study by Belozertseva et al. (2007), who investigated the antidepressant-like effects of MTEP in the forced swim test (FST) in rats. The authors have shown that the profile of behavioral changes induced by MTEP is typical of ADs acting via modulation of the serotonergic system, e.g., selective serotonin reuptake inhibitors (SSRIs) (Detke et al. 1995), such as in a dose-dependent reduction in the immobility time and an increase in the swimming behavior, without changes in the climbing behavior of rats were observed (Belozertseva et al. 2007).
In the present study, we decided to investigate the possible role of the serotonergic system in the antidepressant-like activity of MTEP in the TST in mice, applying two alternative methods of serotonin depletion (i.e., para-chlorophenylalanine (PCPA) administration and TRP-free diet) and using specific antagonists of 5HT receptors, which are believed to be involved in antidepressant effects of drugs (i.e., 5-HT1A, 5HT1B, 5HT2A/2C, and 5HT4 antagonists). Furthermore, the effect of MTEP administration on the antidepressant-like effect of the SSRI, citalopram, has been investigated in the TST in mice.
Material and methods
Animals and housing
Male C57BL/6J mice (Charles River, Germany), weighing 23–25 g at the beginning of the experiment, were used in the study. The animals were kept under standard laboratory conditions of lighting (light phase 0700–1900) and temperature (19–21 °C). Food and water were freely available. Each experimental group consisted of seven to nine animals. All the subjects were experimentally naive and used only once in each test. The experiments were performed during the light period (0900–1400 hours) by an observer unaware of the treatment. All procedures were conducted according to the guidelines of the National Institutes of Health Animal Care and Use Committee, and were approved by the ethics Committee of the Institute of Pharmacology, Polish Academy of Sciences in Kraków.
Drug administration
MTEP, SB 224289 (Tocris Cookson Ltd., Bristol, UK), GR125487 (Tocris Cookson Ltd., Bristol, UK), ritanserin (Tocris Cookson Ltd., Bristol, UK), and PCPA (Sigma-Aldrich, St. Louis, USA) were dispersed in a suspension of 0.5 % methylcellulose, which was used as a vehicle. WAY100635 (N-{2-{4-(2-methoxyphenyl)-1-piperazinyl}ethyl}-N-(2-pyridinyl) cyclo-hexanecarbox-amide fumarate) (Sigma-Aldrich, St. Louis, USA), fluoxetine hydrochloride (Ascent Scientific Ltd., Bristol, UK) and citalopram (Ascent Scientific Ltd., Bristol, UK) were dissolved in sterile water. Ritanserin, SB 224289 and GR 125487 were administered intraperitoneally (IP), 60 min before the experiment. WAY100635 was given subcutaneously (SC), 45 min before the test. MTEP, citalopram and fluoxetine, were administered IP, 30 min before the experiment. The drugs were injected at a constant volume of 10 ml/kg.
Pharmacological depletion of 5-HT
To pharmacologically deplete the 5-HT level, the mice were pretreated with the tryptophan hydroxylase inhibitor, PCPA (300 mg/kg, IP), at a dose of 300 mg/kg. Injections were given twice daily (at 0900 and 1800 hours), for three consecutive days. The experiments were made on the fourth day, and started at 1000 hours. Vehicle-treated mice were injected with 0.5 % methylcellulose according to the same schedule as PCPA-treated mice.
Tryptophan-free diet
To naturally reduce the 5-HT level, a TRP-free synthetic amino acid diet (MP Biomedicals, Illkirch Cedex, France) was used. The animals were grouped ten per cage and fed a TRP-free diet or control diet (mouse diet, purified, MP Biomedicals, Illkirch Cedex, France) for 3 weeks. The animals were weighed daily. After 3 weeks, the mice were used in the TST or in the locomotor activity test.
Tail suspension test
The tail suspension test was used to investigate potential antidepressant-like effects. The immobility of mice was induced by tail suspension according to the procedure of Steru et al. (1985). C57BL/6J mice were individually suspended by their tails by a plastic string positioned horizontally 75 cm above the tabletop using adhesive tape placed about 1 cm from the tip of the tail. The immobility duration was recorded for 6 min. The mice were considered immobile only when they hung down passively and were completely motionless.
Locomotor activity test
The spontaneous locomotor activity of mice was measured in Plexiglas locomotor activity chambers (40 × 20 × 15 cm) in a 20-station photobeam activity system (Opto-M3 Activity Meter, Columbus Instruments, USA) where the animals were placed individually 30 min after the injection of MTEP. Other drugs were given according to the injection schedule presented in the “Drug administration” section. The total number of ambulations during 5 min was recorded.
Statistical analysis
The data was presented as the means ± SEM and evaluated by the one-way ANOVA followed by Dunnett's post hoc test or the two-way ANOVA followed by Bonferroni's post hoc test (see figure legends). GraphPad Prism version 4.00 for Windows 2000 (GraphPad Software, San Diego CA, USA) was used to analyze the data.
Results
The effect of PCPA pretreatment on the antidepressant-like activity of MTEP and fluoxetine in the TST
MTEP, given at a dose of 3 mg/kg (IP), significantly decreased the immobility time of vehicle-treated mice in the TST [F(1,26) = 6.961, p = 0.0139]. Two-way ANOVA also revealed a significant interaction between PCPA and MTEP [F(1,26) = 10.23, p = 0.0036], showing that MTEP was not active in the TST in 5-HT-depleted mice (Fig. 1a). Fluoxetine, used as a reference drug at a dose of 20 mg/kg (IP), significantly decreased the immobility time of vehicle-treated mice in the TST [F(1,25) = 39.52, p < 0.0001]. Moreover, two-way ANOVA revealed significant interaction between PCPA and fluoxetine [F(1,25) = 10.18, p = 0.0038] (Fig. 1b) indicating that fluoxetine was not significantly active in PCPA-pretreated mice in the TST.
The effect of PCPA pretreatment on the antidepressant-like activity of MTEP (a) or fluoxetine (b) in the TST in C57BL/6J mice. Values are expressed as the means ± SEM and analyzed by two-way ANOVA followed by Bonferroni's post hoc test, ***p < 0.001, *p < 0.05 vs. control group, ## p < 0.01 interaction between PCPA and MTEP or fluoxetine
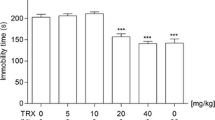
The effect of a TRP-free diet on the antidepressant-like activity of MTEP and fluoxetine in the TST
The TRP-free diet did not change immobility of vehicle-treated mice [F(1,31) = 2.399, p > 0.05]. MTEP, administered at a dose of 3 mg/kg induced an antidepressant-like effect in mice fed on a control diet [F(1,31) = 13.06, p = 0.0011]. The TRP-free diet (3 weeks) did not influence this effect, i.e., no interaction between the MTEP and the TRP-free diet was observed [F(1,31) = 0.166, p > 0.05] (Fig. 2a). Similar effects were induced by fluoxetine (20 mg/kg, IP), used in the study as a positive control. Fluoxetine significantly decreased the immobility time of mice fed on the control diet [F(1,32) = 20.41, p < 0.0001], whilst TRP-free diet did not influence immobility of vehicle-treated mice [F(1,32) = 0.671, p > 0.05]. Furthermore, the action of fluoxetine was not changed by the TRP-free diet, i.e., no interaction between the fluoxetine and the TRP-free diet was observed [F(1,32) = 2.302, p > 0.05] (Fig. 2b), suggesting that the kind of diet had no influence on the antidepressant-like effects of fluoxetine or MTEP in the TST. On the other hand, we observed the relationship between the type of diet and the weight of animals, i.e., the mice fed on the TRP-free diet gradually lost weight [F(4,144) = 125, p < 0.0001] (Fig. 3), contrary to mice fed on the control diet which gained weight during the 3 weeks' experiment [F(4,149) = 162, p < 0.0001] (Fig. 3).
The effect of sub-effective doses of citalopram and MTEP on the antidepressant-like activity in the TST
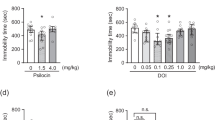
Citalopram, used in the TST at a non-active dose of 1 mg/kg, co-administered with a non-active dose of MTEP (0.1 mg/kg), did not change the behavior of the mice in the TST [F(1,27) = 0.04839; p > 0.05] (Fig. 4c). However, citalopram, used at the same dose of 1 mg/kg, co-administered with a higher, but still non-active dose of MTEP (1 mg/kg), induced a significant decrease in the immobility time of mice [F(1,28) = 9.971; p = 0.0038] in the TST, i.e., a significant interaction between MTEP (1 mg/kg) and citalopram (1 mg/kg) was observed (Fig. 4c). Sub-effective doses of citalopram and MTEP were defined on the basis of dose–response curves, performed in the preceding set of experiments (Fig. 4a, b).
The effect of MTEP (a) or citalopram (b) on the antidepressant-like effects in the TST in C57BL/6J mice. Values expressed as means ± SEM were analyzed by one-way ANOVA. ***p < 0.001 vs. respective control group. c The effects of co-administration of sub-active doses of MTEP (0.1 or 1 mg/kg) and citalopram (1 mg/kg) on the immobility time of mice in the TST. Values are expressed as the means ± SEM and analyzed by two-way ANOVA followed by Bonferroni's post hoc test, **p < 0.01 interaction between MTEP (1 mg/kg) and citalopram (1 mg/kg)
The effect of 5HT antagonists, on the antidepressant-like activity of MTEP in the TST
MTEP, given at a dose of 3 mg/kg, significantly decreased the immobility time of vehicle-treated mice in the TST [F(1,30) = 24.89, p < 0.0001] (Fig 5a). 5HT1A receptor antagonist, WAY100635 (0.1 mg/kg, SC), was not active in the TST when given by itself and it did not influence the MTEP-induced attenuation of the antidepressant-like activity of mice in the TST [interaction: F(1,30) = 0.3184, p = 0.05768] (Fig. 5a). Similar effects were obtained when the 5HT1B receptor antagonist, SB224289 (0.1 mg/kg, IP), and 5HT4 receptor antagonist GR125487 (10 mg/kg, IP) were applied, i.e., two-way ANOVA showed that a non-active dose of SB224289 did not change the MTEP (3 mg/kg)-induced decrease in the immobility of mice [interaction: F(1,31) = 1,707; p = 0.201] (Fig. 5b) and a non-active dose of GR125487 did not influence the antidepressant-like effect of MTEP in the TST [interaction: F(1,24) = 1,111; p = 0.302] (Fig. 5d). Conversely, the 5HT2A/2C receptor antagonist, ritanserin (0.5 mg/kg, IP), when given by itself, did not change the behavior of the animals in the TST [F(1,24) = 3.035; p = 0.093], whilst it significantly antagonized the MTEP (3 mg/kg)-induced decrease in the immobility time of mice [interaction: F(1,26) = 8.189, p = 0.008] (Fig. 5c). However, the lower tested dose of ritanserin, (0.1 mg/kg, IP), did not influence the MTEP (3 mg/kg)-produced antidepressant-like effects in the TST in mice, i.e., two-way ANOVA revealed no interaction between MTEP (3 mg/kg) and ritanserin (0.1 mg/kg) [interaction: F(1,26) = 0.874, p = 0.358].
Effects of 5HT1A receptor antagonist, WAY100635 (0.1 mg/kg; a), 5HT1B receptor antagonist, SB224289 (0.1 mg/kg; b), 5HT2A/2C receptor antagonist, ritanserin (0.1 or 0.5 mg/kg; c) or 5HT4 antagonist, GR125487 (10 mg/kg; d), on the antidepressant-like activity of MTEP (3 mg/kg) in the TST in C57BL/6J mice. Values are expressed as the means ± SEM and analyzed by two-way ANOVA followed by Bonferroni's post hoc test, ***p < 0.001, **p < 0.01 vs. control group, # p < 0.05 interaction between ritanserin and MTEP
The effect of MTEP on the locomotor activity of PCPA-pretreated mice
MTEP (3 mg/kg, IP) did not influence the locomotor activity of mice in a 5-min experimental session [F(1,28) = 1.798, p = 0.19]. PCPA, given by itself, did not change locomotor activity and it did not influence the locomotor activity of mice injected with MTEP [F(1,28) = 0.0093, p = 0.92] (Fig. 6a).
Effects of PCPA pretreatment (a), TRP-free diet (b), WAY100635 (0.1 mg/kg) (d), ritanserin (0.5 mg/kg) (d), SB224289 (0.1 mg/kg) (e) or GR125487 (10 mg/kg) (e) on the locomotor activity of MTEP (3 mg/kg)-treated C57BL/6J mice and the effect of co-administration of MTEP (1 mg/kg) and citalopram (1 mg/kg) on the locomotor activity of C57BL/6J mice (c). Values expressed as the means ± SEM were evaluated by two-way ANOVA followed by Bonferroni's post hoc test, **p < 0.01 vs. control group
The effect of MTEP on the locomotor activity of mice fed a TRP-free diet
MTEP (3 mg/kg, IP) did not change the locomotor activity of mice fed on a control diet [F(1,28) = 0.644, p = 0.42]. The TRP-free diet (3 weeks) did not influence the locomotor activity of control mice and did not change the locomotor activity of mice administered with MTEP [F(1,28) = 0.665, p = 0.42] (Fig. 6b).
The effect of citalopram and MTEP co-administration on the locomotor activity of mice
MTEP, used at a dose of 1 mg/kg, did not change the basal locomotor activity of mice [F(1,26) = 0.096, p = 0.759]. Similarly, citalopram, used at a dose of 1 mg/kg, did not influence this parameter [F(1,26) = 0.104, p = 0.749] and did not change the mobility of MTEP (1 mg/kg)-treated mice [F(1,26) = 1.877, p = 0.182].
The effect of 5-HT antagonists on the locomotor activity of MTEP-treated mice
The 5HT1A antagonist, WAY100635, at a dose of 0.1 mg/kg (SC), did not change the basal locomotor activity of mice [F(1,28) = 0.0006, p = 0.97] and did not influence the locomotor activity of mice injected with MTEP [F(1,28) = 2.494, p = 0.1255] (Fig. 6d). 5HT2A/2C antagonist, ritanserin, at a dose of 0.5 mg/kg (IP) decreased the locomotor activity of mice in a 5-min session [F(1,28) = 23.26, p < 0.0001] and decreased the locomotor activity of mice injected with mGlu5 antagonist, MTEP, since no significant interaction between the “ritanserin” and “ritanserin + MTEP” groups was observed [F(1,28) = 0.205, p = 0.6542] (Fig. 6d). Both 5HT1B receptor antagonist, SB 224289 (0.1 mg/kg) and 5HT4 receptor antagonist, GR 125487 (10 mg/kg), did not significantly influence the exploratory locomotor activity of mice [F(1,27) = 0.009, p = 0.92], [F(1,24) = 0.627, p = 0.44], respectively (Fig. 6e). Furthermore, two-way ANOVA showed no interactions between MTEP and SB 224289 [F(1,27) = 0.023, p = 0.879] or GR 125487 [F(1,24) = 1.005, p = 0.326] in the locomotor activity test in mice (Fig. 6e).
Discussion
In the present study, we found that the antidepressant-like effect of the potent and systemically active mGlu5 receptor antagonist, MTEP, in the TST in C57BL/6J mice, depends on the activation of the serotonergic system. The TST was used in the study, since it has been shown to detect the antidepressant-like activity of not only typical ADs but also atypical and new potential ones, including the mGlu receptor ligands (Chaki et al. 2004; Cryan et al. 2005; Pałucha et al. 2005; 2007). The active dose of MTEP (3 mg/kg), has been used based on in vivo receptor occupancy studies, showing that 1 h after peripheral MTEP administration, the dose required to occupy 50 % of mGlu5 receptors (ED50) was 1.2 mg/kg and full receptor occupancy was achieved with the 10 mg/kg dose of MTEP (Busse et al. 2004). Furthermore, MTEP used at a dose of 3 mg/kg has been previously shown to induce antidepressant-like effects in C57BL/6J mice in the TST (Pałucha et al. 2005). The dose–response curve of the MTEP activity in the TST in mice, presented in the present study, confirms the previous results.
To investigate the role of the serotonergic system in the antidepressant-like effects of MTEP, two different methods of 5-HT depletion were introduced; namely pharmacological depletion and a TRP-free diet. The pharmacological depletion of 5-HT was achieved by tryptophan hydroxylase inhibitor administration. PCPA, which was used in the study, was given for 3 days, twice daily at a dose of 300 mg/kg. The same schedule of treatment has been used in our previous studies, which showed the reduced brain 5-HT level by 77 % (Pałucha-Poniewiera et al. 2010a) and the lack of effect by a selective serotonin reuptake inhibitor (SSRI), citalopram, in PCPA-pretreated mice in the TST (Pałucha-Poniewiera et al. 2010b). In the present study, we show that another SSRI, fluoxetine, which was used as a positive control, did not cause an antidepressant-like effect in PCPA-pretreated mice in the TST, while it was effective in this test in vehicle-treated mice. It means that the attenuation of the 5-HT level after PCPA treatment was enough to prevent the action of fluoxetine. An antagonist of the mGlu5 receptor, MTEP, similar to fluoxetine, did not decrease the immobility time of mice in the TST in 5-HT-depleted mice, while it was significantly effective in vehicle-treated mice. These results suggest that a regular level of 5-HT in the brain is essential to the antidepressant-like action of MTEP in the TST.
Another way to diminish the 5-HT level in the brain was via sub-chronic dietary tryptophan depletion, which has been proposed as an animal model of depression (Franklin et al. 2012). Behavioral experiments on rats have shown that 2 weeks of a low-TRP diet induced changes in rat behavior in the FST, i.e., the floating time increased and the swimming time decreased, suggesting pro-depressive behavior (Franklin et al. 2012). In our study, we did not observe such changes after 3 weeks of a TRP-free diet. However, different species of animals, diet and behavioral procedures were used in the studies, which may account for the different results. Our experiments did not only indicate the lack of pro-depressive effects produced by a TRP-free diet. We also illustrated the lack of effect of a TRP-free diet on the antidepressant-like effect of fluoxetine in the TST in mice. Fluoxetine was active both in mice fed on a control diet and the TRP-free diet. The similar effects were induced by MTEP, which produced an antidepressant-like effect both in mice fed on a control and the TRP-free diet. However, using these results as our basis, we cannot conclude that 5-HT is not involved in the action of MTEP in the TST, since a reference drug, fluoxetine, which unquestionably works via the 5-HT-dependent mechanism, is also active in mice fed on the TRP-free diet. It seems that the 5-HT level in the brains of mice fed on the TRP-free diet was high enough to let fluoxetine (or MTEP) normally work as an antidepressant in the TST. Altogether, in our hands, the TRP-free diet turned out not to be a good model of depression in C57BL/6J mice and, as far as we are concerned, no binding conclusion on the possible role of 5-HT in the action of MTEP, should be drawn from “TRP-free diet” studies. One of the reasons of the failure in our attempt to use a TRP-free diet as an efficient method of 5-HT depletion might be the strain of mice used in the studies. It has been shown that acute removal of the tryptophan from the diet resulted in a 74 % reduction of the plasma ratio of tryptophan to the sum of other large neutral amino acids in Swiss mice, although there was only a 40 % reduction in C57BL/6 mice, and no changes in the TST or FST were observed (van Donkelaar et al. 2010). Furthermore, some data suggest that a long term (3 weeks) TRP-free diet is much less effective in reducing 5-HT level in the brain then short-term (3 days) PCPA treatment. It has been shown that 3-weeks of a TRP-free diet induced a decrease in the 5-HT level in several brain structures in rats by about 50 % (Kot et al. 2012), while 3 days of PCPA treatment decreased brain 5-HT level by almost 80 % (O'Leary et al. 2007; Pałucha-Poniewiera et al. 2010a).
It is worth mentioning that in our study, the TRP-free diet produced some changes in mice, e.g., statistically significant loss of body weight and hair loss on the back were observed. These effects did not result from possible differences in food consumption, since the amount of food consumed was calculated daily and no significant differences between mice fed on the TRP-free diet and the control diet were observed.
We also measured the locomotor activity of mice, since changes in exploratory locomotor activity of animals induced by tested compounds can often provide false positive or false negative results in the TST. In our study, we did not observe any significant changes in the locomotor activity of mice induced by PCPA or the TRP-free diet, although a non-significant tendency to decrease locomotor activity was observed after PCPA treatment. In our previous studies, we observed a decrease in the locomotor activity of mice induced by PCPA (Pałucha-Poniewiera et al. 2010a; b). Furthermore, MTEP, used at a dose of 3 mg/kg, did not influence any spontaneous locomotor activity of animals, which is in line with the previous results (Belozertseva et al. 2007; Halberstadt et al. 2011; Pałucha et al. 2005). Thus, it seems that the behavioral effects in the TST were associated with antidepressant-like action, but not with changes in the mobility of animals.
To further investigate the role of the serotonergic system in the antidepressant-like effect of MTEP, we combined sub-effective doses of MTEP and the SSRI, citalopram, in the TST in mice. The dose–response curves of the antidepressant-like activity of both compounds had been performed before the experiment in order to choose sub-effective doses of the drugs. Co-administration of non-active doses of MTEP (0.1 mg/kg) and citalopram (1 mg/kg) did not influence the immobility time of mice in the TST. However, MTEP used at a higher, yet still sub-effective dose of 1 mg/kg, co-administered with citalopram (1 mg/kg), produced significant antidepressant-like activity in this test. Such a dose-dependent effect suggests the synergistic action of the SSRI and the mGlu5 receptor antagonist, indicating that both compounds act at different targets and the stimulation of which leads to the same final results.
The locomotor activity study showed that citalopram and MTEP, given alone or combined at doses used in the TST, did not change the behavior of mice, ensuring the specificity of the antidepressant-like effect of combination citalopram and MTEP.
We also investigated the possible role of selected serotonin receptors in the antidepressant-like action of MTEP. 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4 receptors were chosen in the studies, since they play a crucial role in the regulation of serotonergic neurotransmission and in the mechanism of action of several ADs, including SSRIs, monoamine oxidase (MAO) inhibitors, and tricyclics (O'Neill et al. 1996; Middlemiss et al. 2002; Hensler 2006; Lucas et al. 2010). Furthermore, their blockade has been shown to antagonize the action of the SSRIs in the TST in mice (O'Neill et al. 1996; Miyata et al. 2004).
We showed that the pretreatment of mice with the 5-HT1A receptor antagonist WAY100635, 5-HT1B receptor antagonist SB224289 or 5-HT4 receptor antagonist GR125487, did not block the antidepressant-like activity of MTEP in the TST, while the 5-HT2A/2C receptor antagonist ritanserin, dose-dependently reversed the MTEP-induced decrease in the immobility time of mice in this test, suggesting the possible role of 5HT2A/2C receptors in this action. On the other hand, two-way ANOVA showed that ritanserin, used at a dose of 0.5 mg/kg, produced a decrease in exploratory locomotor activity. Additionally, mice co-administered with ritanserin (0.5 mg/kg) and MTEP (3 mg/kg) were also less active then the control animals. It might suggest the possibility of a false positive result in the TST. However, we suppose that this small decrease in locomotor activity did not influence the behavior of mice in the TST, since ritanserin, when given alone, did not induce any changes in the immobility time of mice in the TST, when compared to the control animals, although it decreased exploratory activity when used at the same dose.
The mechanism involving activation of the serotonergic system in the antidepressant-like effect of MTEP in the TST is unknown. Direct effects of MTEP on the serotonergic receptors, transporters or metabolism are highly unlikely. MTEP has been described as a selective mGlu5 receptor NAM (Cosford et al. 2003). Wide-range counterscreening in a large number of in vitro assays showed no activity of MTEP in functional assays at iGlu receptors (including NMDA NR2B receptors (at >300 μM), AMPA and kainate) and mGlu receptors (mGlu1, mGlu2, mGlu3, and mGlu7) (10 μM)). However, as far as we know, MTEP and its metabolites have been not tested against all glutamate and monoaminergic transporters and receptors (Busse et al. 2004; Cosford et al. 2003) and thus, the direct effects of MTEP on the serotonergic system cannot be definitely excluded.
An indication that MTEP may influence the action of the serotonin transporter is illustrated in a study by Stachowicz et al. (2007), who showed that the rise of the 5-HT level in rat's prefrontal cortex after MTEP administration was not accompanied by the rise in 5-HIAA, indicating a possible dysfunction of the membrane transporter. It is worth mentioning that the similar action of another mGlu5 antagonist, MPEP, on the dopamine transporter was observed by Page et al. (2001) in striatal synaptosomes. However, there are no data concerning any influence of MTEP on a direct blockade of the serotonergic transporters.
Furthermore, binding assays revealed the affinity of MTEP on MAOA. However, it rather did not play a role in the action of MTEP in our experiments, since MTEP was shown to displace [125I]RTI-55 from MAOA with IC50 = 30 μM, while the brain concentration of MTEP after a dose of 3 mg/kg, IP, 1 h post-administration was only 1.4 ± 0.2 μM (Cosford et al. 2003).
Another possible explanation for the role of the serotonergic system in the antidepressant-like effects of MTEP comes from the study by Stachowicz et al. (2007), which showed a dose-dependent increase in serotonin release in the prefrontal cortex in rats after acute, peripheral MTEP administration and the involvement of serotonergic receptors in the anxiolytic-like effects of this drug. Interestingly, the anxiolytic-like activity of MTEP in the conflict drinking Vogel test in rats was also dependent on 5HT2A/2C, but not on the activation of 5HT1A receptors (Stachowicz et al. 2007). This might suggest similar mechanisms of antidepressant and anxiolytic actions of MTEP.
A functional interaction between the serotonergic 5HT2A receptor and mGlu5 receptor has also been demonstrated by Halberstadt et al. (2011), who showed that an increase in locomotor activity, induced by a high dose of mGlu5 antagonist, MPEP (20 mg/kg) was blocked by the selective 5HT2A antagonist, M100907 (0.1 mg/kg) in C57BL/6J mice. Furthermore, it has been demonstrated that gene deletion of mGlu5 receptors increased the behavioral response to the 5-HT2A agonist, DOM (2,5-dimethoxy-4-methylamphetamine) (Halberstadt et al. 2011).
Biochemical studies applying the technique of in vivo measurements of receptor-activated phosphoinositide hydrolysis, indicate the possible interaction between the 5HT2A and mGlu5 receptors at the level of the second messenger systems' activation. Both receptors are positively coupled to phospholipase C, through Gq/11 proteins, and induce phosphoinositide hydrolysis (Barnes and Sharp 1999; Conn and Pin 1997). The study by Molinaro et al. (2009) showed that the stimulation of [3H] inositol monophosphate formation by 5HT2A agonist, DOI, was partially reduced by MPEP (3 mg/kg), suggesting that a component of the action of DOI was mediated by the endogenously released glutamate acting at mGlu5 receptors.
It is worth noting that there is strong evidence for the functional interaction between other glutamatergic receptors, namely mGlu2/3 receptors, and serotonergic 5HT2A receptors, based on electrophysiological, behavioral and biochemical studies (Marek et al. 2000; 2006; Molinaro et al. 2009; Winter et al. 2004; Zhai et al. 2003). The study by González-Maeso et al. (2008) also sheds light onto the molecular nature of this interaction, which refers to the heterodimeric complex formed by 5-HT2A and mGlu2 receptors. The similar complex of mGlu5 and 5HT2A receptors cannot be excluded, since both receptors are not only functionally linked, but they are also localized in the similar brain areas. Immunocytochemical, in situ hybridization and receptor autoradiography studies suggest that 5HT2A receptor is located on neurons and local (GABAergic) interneurons in various brain areas including the cerebral cortex, i.e., it has been found in cortical pyramidal (projection) neurons, which are known to be glutamatergic (for review, see Barnes and Sharp 1999). mGlu5 receptors are also highly expressed in several limbic structures, including the cerebral cortex (Romano et al. 1995; Shigemoto et al. 1993).
Altogether, our study shows the involvement of the serotonergic system in the antidepressant-like activity of mGlu5 NAM, MTEP, in the TST in mice. 5HT2A receptor may be involved in this process. However, more studies are needed to clarify the mechanism of this interaction.
References
Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152
Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY (2007) Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol 17:172–179
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JA (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Schaffhauser H, Rao SP, Varney MA, Anderson JJ (2003) Metabotropic glutamate receptor 5 antagonist-induced stimulation of hypothalamic-pituitary-adrenal axis activity: interaction with serotonergic systems. Neuropharmacology 44:562–572
Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND (2004) The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29:1971–1979
Chaki S, Ago Y, Pałucha-Poniewiera A, Matrisciano F, Pilc A (2012) mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology. doi:10.1016/j.neuropharm.2012.05.022
Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A (2004) MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46:457–467
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Ann Rev Pharmacol Toxicol 37:205–237
Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA (2003) 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem 46:204–206
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 168:727–734
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802
Franklin M, Bermudez I, Murck H, Singewald N, Gaburro S (2012) Sub-chronic dietary tryptophan depletion—an animal model of depression with improved face and good construct validity. J Psychiatr Res 46:239–247
González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97
Halberstadt AL, Lehmann-Masten VD, Geyer MA, Powell SB (2011) Interactive effects of mGlu5 and 5-HT2A receptors on locomotor activity in mice. Psychopharmacology 215:81–92
Hensler JG (2006) Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 30:203–214
Hughes ZA, Neal SJ, Smith DL, Sukoff Rizzo SJ, Pulicicchio CM, Lotarski S, Lu S, Dwyer JM, Brennan J, Olsen M, Bender CN, Kouranova E, Andree TH, Harrison JE, Whiteside GT, Springer D, O'Neil SV, Leonard SK, Schechter LE, Dunlop J, Rosenzweig-Lipson S, Ring RH (2012) Negative allosteric modulation of metabolic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology. doi:10.1016/j.neuropharm.2012.04.007
Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA Jr (2011) Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1155–1159
Kot M, Pilc A, Daniel WA (2012) Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacol Res 66:292–299
Li X, Need AB, Baez M, Witkin JM (2006) Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther 319:254–259
Lucas G, Du J, Romeas T, Mnie-Filali O, Haddjeri N, Pineyro G, Debonnel G (2010) Selective serotonin reuptake inhibitors potentiate the rapid antidepressant-like effects of serotonin4 receptor agonists in the rat. PLoS One 5:e9253
Marek GJ, Wright RA, Schoepp DD (2006) 5-Hydroxytryptamine2A (5-HT2A) receptor regulation in rat prefrontal cortex: interaction of a phenethylamine hallucinogen and the metabotropic glutamate2/3 receptor agonist LY354740. Neurosci Lett 403:256–260
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000) Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292:76–87
Middlemiss DN, Price GW, Watson JM (2002) Serotonergic targets in depression. Curr Opin Pharmacol 2:18–22
Miyata S, Hirano S, Kamei J (2004) Diabetes attenuates the antidepressant-like effect mediated by the activation of 5-HT1A receptor in the mouse tail suspension test. Neuropsychopharmacology 29:461–469
Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G (2009) Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol 76:379–387
O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I (2007) Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology 192:357–371
O'Neill MF, Fernandez AG, Palacios JM (1996) GR127935 blocks the locomotor and antidepressant-like effects of RU24969 and the action of antidepressants in the mouse tail suspension test. Pharmacol Biochem Behav 53:535–539
Page G, Peeters M, Najimi M, Maloteaux JM, Hermans E (2001) Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem 76:1282–1290
Pałucha A, Brański P, Szewczyk B, Wierońska JM, Kłak K, Pilc A (2005) Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 81:901–906
Pałucha A, Kłak K, Brański P, van der Putten H, Flor PJ, Pilc A (2007) Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology 194:555–562
Pałucha A, Pilc A (2007) Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther 115:116–147
Pałucha-Poniewiera A, Brański P, Lenda T, Pilc A (2010a) The antidepressant-like action of metabotropic glutamate 7 receptor agonist N,N′-bis(diphenylmethyl)-1,2-ethanediamine (AMN082) is serotonin-dependent. J Pharmacol Exp Ther 334:1066–1074
Pałucha-Poniewiera A, Wierońska JM, Brański P, Stachowicz K, Chaki S, Pilc A (2010b) On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology 212:523–535
Romano C, Sesma MA, Mcdonald CT, Omalley K, Vandenpol AN, Olney JW (1995) Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355:455–469
Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57
Stachowicz K, Gołembiowska K, Sowa M, Nowak G, Chojnacka-Wójcik E, Pilc A (2007) Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent. Neuropharmacology 53:741–748
Steru L, Chermat R, Thierry B, Simon P (1985) Tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Szewczyk B, Pałucha-Poniewiera A, Poleszak E, Pilc A, Nowak G (2012) Investigational NMDA receptor modulators for depression. Expert Opin Investig Drugs 21:91–102
Tatarczyńska E, Kłodzińska A, Chojnacka-Wójcik E, Pałucha A, Gasparini F, Kuhn R, Pilc A (2001) Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol 132:1423–1430
van Donkelaar EL, Blokland A, Lieben CK, Kenis G, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J (2010) Acute tryptophan depletion in C57BL/6 mice does not induce central serotonin reduction or affective behavioural changes. Neurochem Int 56:21–34
Winter JC, Eckler JR, Rabin RA (2004) Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology 172:233–240
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-d aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK (2003) Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28:45–52
Acknowledgments
This work was supported by Funds for Statutory Activity of the Institute of Pharmacology Polish Academy of Sciences and by the grant POIG.01.01.02-12-004/09 “Depression-Methods-Therapy”, task. 3.7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pałucha-Poniewiera, A., Brański, P., Wierońska, J.M. et al. The antidepressant-like action of mGlu5 receptor antagonist, MTEP, in the tail suspension test in mice is serotonin dependent. Psychopharmacology 231, 97–107 (2014). https://doi.org/10.1007/s00213-013-3206-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3206-6