Abstract
Rationale
To improve outcomes for patients undergoing extinction-based therapies (e.g., exposure therapy) for anxiety disorders such as post-traumatic stress disorder (PTSD), there has been interest in identifying pharmaceutical compounds that might facilitate fear extinction learning and recall. Oxytocin (OT) is a mammalian neuropeptide that modulates activation of fear extinction-based neural circuits and fear responses. Little is known, however, about the effects of OT treatment on conditioned fear responding and extinction in humans.
Objectives
The purpose of the present study was to assess the effects of OT in a fear-potentiated startle task of fear conditioning and extinction.
Methods
A double-blind, placebo-controlled study of 44 healthy human participants was conducted. Participants underwent a conditioned fear acquisition procedure, after which they were randomized to treatment group and delivered OT (24 IU) or placebo via intranasal (IN) spray. Forty-five minutes after treatment, participants underwent extinction training. Twenty-four hours later, subjects were tested for extinction recall.
Results
Relative to placebo, the OT group showed increased fear-potentiated startle responding during the earliest stage of extinction training relative to placebo; however, all treatment groups showed the same level of reduced responding by the end of extinction training. Twenty-four hours later, the OT group showed significantly higher recall of extinction relative to placebo.
Conclusions
The current study provides preliminary evidence that OT may facilitate fear extinction recall in humans. These results support further study of OT as a potential adjunctive treatment for extinction-based therapies in fear-related disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-traumatic stress disorder (PTSD) has a prevalence rate of approximately 10 % in the US (Breslau et al. 1998; Kessler et al. 2005) and is associated with significant functional impairment (Druss et al. 2008; Alonso et al. 2004). Evidence for efficacy of exposure-based psychotherapies for PTSD, namely Prolonged Exposure (PE), is much stronger compared to pharmacological treatments (IoMotNA, 2007). PE involves both imaginal exposure to the traumatic memory and in vivo exposure to environmental cues that trigger trauma memories (Foa and Rothbaum 1998). The patient's avoidance of these cues is thought to maintain trauma associations and prevent fear extinction from occurring naturally. Thus, PE reduces PTSD symptoms at least, in part, by supporting extinction of conditioned fear responses to trauma-related cues.
Although evidence for the efficacy of PE is strong, it is not without limitations. A significant number of patients remain symptomatic following a standard course of treatment (Schnurr et al. 2007; Bradley et al. 2005). Several studies support functional and structural abnormalities in important nodes of the extinction learning neurocircuit in PTSD patients, specifically the ventromedial prefrontal cortex (vmPFC) and amygdala, which may be related to the failure of exposure-based treatments in some patients (Etkin and Wager 2007; Meyers and Davis, 2007; Milad et al. 2007; Shin et al. 2006; Acheson et al. 2011). Indeed, PTSD patients have demonstrated stronger fear conditioning, slower rates of extinction, and poor recall of fear extinction memory following extinction training (Norrholm et al. 2011; Milad et al. 2008). Additionally, exposure to trauma cues is an intimidating prospect to some PTSD patients, and thus, discontinuation of treatment may occur unless the provider is skillful in building a therapeutic alliance (Cloitre 2009; Hembree et al. 2003; APA, 2000).
Recent efforts to increase the efficacy of PE have focused on pharmacological augmentation of extinction learning. The most well studied of these agents is d-cycloserine (DCS), a partial NMDA receptor agonist, which has demonstrated efficacy in a range of anxiety disorders (Norberg et al. 2008). A recent randomized, placebo-controlled trial suggested that DCS successfully augments PE for PTSD in severe, treatment-resistant patients (de Kleine et al. 2012). These results are promising and suggest that PE is amenable to pharmacological augmentation. Research is ongoing, with the aim of identifying other compounds that might also have augmentative functions.
One compound identified for its potential to enhance PTSD treatment is oxytocin (OT; Olff et al. 2010). OT is a mammalian neuropeptide with both central and peripheral actions. OT has actions opposite to PTSD on several nodes of the fear extinction neurocircuit thought to mediate PE. Whereas the typical pattern in PTSD is vmPFC hypoactivation paired with amygdala hyperactivation (Etkin and Wager 2007; Shin et al. 2006), acute intranasal (IN) OT has the opposite effect on these structures (Olff et al. 2010; Petrovic et al. 2008; Domes et al. 2007; Kirsch et al. 2005). Further, Sripada et al. (2012) have shown that IN OT increases functional coupling of the medial PFC and the amygdala. Interactions between these structures are known to be important to emotion regulatory processes, specifically fear extinction (Milad and Quirk 2012). Thus, through actions on brain structures implicated in both PTSD and fear extinction/emotion regulation processes, it is possible that OT may be useful as a pharmacological adjunct for exposure-based PTSD treatments. OT also has well-known effects on social behavior (Baumgartner et al. 2008; Heinrichs et al. 2003), which may prove helpful in facilitating development of a therapeutic alliance.
Before OT can be used for extinction therapy, however, more must be known of its effects on fear extinction learning and recall. Indeed, little is known about OT effects on fear extinction learning in humans and there is conflicting data in animals (see “Discussion”). Understanding the nature of OT effects on learned fear vs. extinction is important, as an effect of reducing anxiety completely during exposure may hamper extinction learning and retention (Mueller et al. 2008). Here, we describe a double-blind, placebo-controlled study aimed at testing the effect of an acute dose of IN OT on fear extinction learning in healthy humans using a conditioned fear-potentiated startle paradigm (Norrholm et al. 2011; 2006). We hypothesized that OT treatment prior to extinction training would facilitate extinction and increase extinction recall.
Methods
Participants
Forty-four healthy participants were recruited from the local community via flyers and advertisements on Craigslist.org (see demographics in Table 1). Participants were screened over the phone and upon arrival at the laboratory to assure they did not meet criteria for current or past psychiatric disorders, have a history of cardiac illness, seizure disorder, brain injury, neurologic disorder, or history of head injury with loss of consciousness for more than 1 min. Participants were administered a hearing test to assure they could detect 500, 1,000, 3,000, and 6,000 Hz tones at <45 dB. A urinalysis was administered to rule out use of illegal substances or pregnancy. Since endogenous OT levels can fluctuate across the menstrual cycle, female participants not currently using hormonal contraceptives were scheduled for testing during the follicular phase of the menstrual cycle (e.g., up to 10 days following onset of menstruation).
Assignment to treatment group
Participants were assigned to treatment group in a semirandom fashion, with the only constraints being magnitude of fear learning during acquisition and gender. At the conclusion of the acquisition phase (described below), participants were assigned to active treatment or placebo group (treatment group “A” or “B”), with attention paid to balancing the groups on strength of conditioning and overall startle responsiveness. This procedure allows for evaluation of treatment effects on extinction measures without potential confounds from between-group differences in strength of initial conditioning.
Treatment
OT powder was purchased from Spectrum Laboratories (Gardena, CA, USA) and mixed into IN spray solution (6 IU/spray) by the UCSD Investigational Drug Service Compounding Pharmacy. The OT formulation contained United States Pharmacopeia (USP) grade OT as well as buffers, stabilizers, and preservatives (chlorobutanol, memihydrate, methylparaben, dibasic sodium phosphate, and anhydrous citric acid powder). D.F. holds an Investigational New Drug (IND) for this formulation. Twenty-four international units of OT was administered to the active-drug group prior to extinction training (described below) via two sprays into each nostril. This dose has been shown to produce anxiolytic effects in healthy humans (de Oliveira et al. 2012; Labuschagne et al. 2010). The placebo spray consisted of the same composition of nonactive ingredients and was administered in an identical fashion.
Procedure
Apparatus
Startle pulses (108 dB, 40 ms) were delivered using a San Diego Instruments (SDI, San Diego, CA, USA) SR-HLAB Electromyography (EMG) system. Sound levels were measured using continuous tones calibrated using a Quest Sound Level Meter on the A scale, coupled to the headphones by an artificial ear. EMG responses were recorded using the SDI SR-HLAB EMG system coupled with a Dell desktop computer as previously described (Acheson et al. 2012). Gain was adjusted to 0.5 (0.5 mV electrode input amplified to 2,500 mV signal output) and band-pass filtered (100–1,000 Hz). A 60-Hz notch filter was used to eliminate 60 Hz interference. Sampling rate was 1 kHz. All electrode resistances were <10 kΩ.
The electrical shock stimuli were delivered via a Contact Precision Instruments SHK1 aversive shock stimulator coupled with an IBM ThinkPad notebook computer. Current was passed from the generator to the subject via two Ag/AgCl electrodes filled with electrolyte gel on the subject's nondominant wrist roughly straddling the radial nerve. To maintain important contextual cues, this procedure was continued throughout each testing phase regardless of whether shock was delivered. Before acquisition, shock intensity levels were set manually for each individual by delivering gradually more intense shocks (0–5 mA range) until the subject reported that the shock level was “highly annoying yet not painful.”
Fear conditioning and extinction
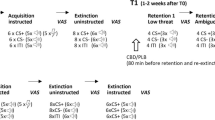
The fear conditioning protocol consisted of three phases: acquisition; extinction, which took place on day 1; and recall, which took place 24 h later. Procedures and timeline for these three phases are illustrated in Fig. 1a and b. Each phase began with six startle pulses presented in the absence of any other stimuli in order for the participants to acclimate/habituate startle responses to baseline level. The acquisition phase consisted of eight 6-s presentations of a blue circle that served as a reinforced conditioned stimulus (CS+) and was followed by a 0.5-s electrical shock unconditioned stimulus (US) in 75 % contingency that coterminated with the CS+, eight 6-s presentations of a yellow circle which served as a nonreinforced conditioned stimulus (CS−) and was never followed by shock, and eight presentations of the startle pulse in the absence of any stimuli (i.e., blank screen; “Noise-Alone trial”; NA) that served as a measure of baseline startle reactivity across the phase. Previous studies have shown that extinction learning is very rapid when 100 % CS-US contingency conditions are used; hence, we elected for a 75 % contingency in order to prolong extinction learning to detect a treatment effect (Phelps et al. 2004; LaBar et al. 1998). Startle pulses were presented 3 s following CS + or CS − onset. Order of stimuli presentation was block randomized with the constraint of two trials of each type (CS+, CS−, and NA) per block. This approach prevents confounds of uneven habituation effects on any one stimulus type and assures accurate temporal match of NA baseline responses to CS + and CS − trials. To measure contingency awareness, participants used a number keypad to report at each CS + or CS − trial whether they expected to receive a shock (“1” key), were unsure (“2” key), or did not expect to receive a shock (“3” key). Following the acquisition phase, contingency awareness was further measured via a questionnaire asking participants which stimuli predicted the shock. Self-reported anxiety was measured by asking how aversive participants found the shock and how anxious they felt in the presence of the blue and yellow circles (CS + and CS−).
After completing the postacquisition questionnaire, participants completed the Profile of Mood States (POMS), Karolinska Sleepiness Scale (KSS), and State Trait Anxiety Inventory (STAI). They were then given either “Drug A” or “Drug B” dependent upon assigned group (see Fig. 1b) and given a 30-min break in which they could read magazines to allow the treatment to take effect. Thirty minutes following treatment administration, participants again completed the POMS and KSS. Forty-five minutes after treatment, participants underwent the extinction phase that consisted of 18 presentations of each stimulus type (CS+, CS−, and NA), in block randomized order as in the acquisition phase. No shocks were presented during this phase. Fewer presentations of each stimulus were used relative to previous studies (e.g., 24 presentations by Norrholm et al. (2011; 2006)) in an attempt to achieve “sub-threshold” extinction. This strategy was employed to avoid a floor effect in control subjects rendering treatment effects uninterpretable. Startle pulses were presented and subjects rated their expectations as in the acquisition phase. Following the extinction phase, participants again rated their subjective anxiety in the presence of both the CS + and CS−. Subjects were then disconnected from the apparatus and left the laboratory.
Participants returned for the recall phase the following day. This phase consisted of eight presentations of each stimulus type (CS+, CS−, and NA), as in the acquisition phase. Startle pulses were presented and expectancy ratings collected as in the previous sessions. Following the recall phase, participants again rated their subjective anxiety to the CS + and CS−. They were then debriefed, compensated, and dismissed.
Data analysis
After analysis, study researchers were unblinded to treatment group.
Startle data
Data collected during the acquisition and extinction phases were analyzed as previously described (Norrholm et al. 2011; 2006) by averaging the responses to each stimulus type within a block. The NA average was then subtracted from both the CS + and CS − responses to create a score representing startle above baseline for that block (e.g., (CS + ) − (NA)). These data were then analyzed using a 2 (CS type) × 4 (Block) × 2 (Treatment group) repeated measures Analysis of Variance (ANOVA), with CS type and Block as within-group factors and Treatment group as a between-group factor. Data collected during the extinction phase were similarly processed, though only the CS + is used to measure extinction learning (Norrholm et al. 2011; 2006). Blocks were then further averaged into early extinction, mid extinction, and late extinction consisting of six CS + presentations each. Previous reports have shown this approach is adequate to detect an extinction learning curve while reducing variability from within-subject startle variation across the session (Norrholm et al. 2011; 2006). These data were analyzed using a 2 (Treatment group) × 3 (Block) repeated measures ANOVA with Block was a within-group factor and Treatment group as a between-group factor. Significant interactions in both phases were followed up with alpha-level adjusted post hoc tests. Startle during the recall phase was analyzed by first computing a CS + score for the first block as described above. An extinction recall index comparing the CS + responses at recall with the maximal CS + responses during conditioning was then computed as described by Milad et al. (2008; 2007) by the following equation: 100 − 100(CS + response during the first block of recall / maximum CS + block across acquisition phase). This index of extinction performance has been shown to be positively and negatively associated with ventral medial prefrontal cortex (PFC) and amygdala activation, respectively, suggesting this measure probes fear extinction circuit function (Milad et al. 2007, 2008). An independent-samples t test was then conducted to test differences between treatment groups on percentage of extinction retained. Calculating the recall index resulted in three individual scores that were outside our a priori range for excluding outliers, ±2.5 standard deviations from the group mean. Removing these data points from the analysis resulted in the loss of one participant from the placebo group and two from the OT group.
Expectancy and self-report
Expectancy responses during EMG testing were coded as: expect a shock = 1, unsure = 0, do not expect a shock = −1. For acquisition, responses were averaged over the last half of the phase (four trials per stimulus type) and analyzed using a 2 (CS type) × 2 (Treatment group) repeated measures ANOVA. For extinction, CS + responses were examined across all trials including the last four trials of acquisition. These data were analyzed with a 2 (Treatment group) × 22 (Trial) repeated measures ANOVA. For the recall phase, expectancy ratings were averaged for the first block CS + responses and analyzed using a 2 (Phase) × 2 (Treatment group) repeated measures ANOVA. Postphase questionnaires were analyzed using 2 (Cue type) × 2 (Treatment group) repeated measures ANOVAs and independent-samples t tests, as appropriate. Change across phases was assessed with a 2 (Phase) × 2 (CS type) × 2 (Treatment group) repeated measures ANOVA. The STAI, delivered before treatment, was analyzed using an independent-samples t test. The KSS and POMS were analyzed using a 2 (Pre–Post-treatment administration) × 2 (Treatment group) repeated measures ANOVA. Significant interactions were followed up with alpha-level adjusted post hoc tests.
Results
Demographics
While there were no significant differences between treatment groups on gender and ethnicity, the placebo group was significantly older than the OT group [Table 1; 7 years difference, t = 2.54, p < 0.02]. In initial analyses, gender was entered as a factor and age as a covariate. However, both were then dropped from the models as neither had a significant effect.
Acquisition
Startle
As expected during fear acquisition, startle responses during the CS + trials were significantly increased over subsequent trials compared to responses to the CS − [Fig. 2a, Cue type × Block: F(3,126) = 5.70, p < 0.001, partial η2 = 0.12]. Post hoc tests showed significant startle potentiation to the CS + relative to the CS − during blocks 2, 3, and 4 (ps < 0.001). There were no significant differences in acquisition across treatment groups.
a Potentiated startle magnitudes for the acquisition phase before treatment. *p < 0.05 vs. CS−. b Potentiated startle magnitude to the CS + during Late acquisition and Early, Mid, and Late extinction phases by Treatment group. *p < 0.05 vs. placebo. #p < 0.05 vs. early extinction phase responding within group. c Potentiated startle magnitudes to the CS + during early extinction depicted in two-trial blocks. *p < 0.05, main effect of oxytocin. Data are depicted as mean ± SEM difference scores in peak startle magnitude during CS + or CS − trials compared to noise-alone trials
Expectancy and self-report
For intrasession expectancy ratings, participants correctly identified the CS + as predictive of the shock [Cue type: F(1,41) = 79.992, p < 0.0001, partial η2 = 0.66]. On a 1 (expect shock) to −1 (do not expect shock) scale, the placebo group averaged 0.55 rating for CS + and −0.57 rating for the CS−. The OT group averaged 0.36 rating for the CS + and −0.73rating for the CS − .
After acquisition, all subjects rated higher levels of anxiety in the presence of the CS + compared to the CS − [Table 3, F(1,36) = 131.17, p < 0.0001, partial η2 = 0.79]. There were no main effects or interactions with treatment group.
Extinction
Psychosocial questionnaires
OT treatment had no effect on STAI or KSS scores (Table 2). With POMS scores analyzed by subscale, all participants showed a significant reduction in ratings of tension/anxiety [F(1,42) = 11.348, p < 0.002, partial η2 = 0.21] and fatigue [F(1,42) = 6.362, p < .016, partial η2 = 0.13] from the first to the second test.
Startle
Subjects receiving OT showed significantly higher potentiated startle to the CS + during early extinction relative to subjects receiving placebo (p < 0.05) [Fig. 2b, Group × Block: F(2,84) = 5.89, p < 0.004, partial η2 = 0.12; Block: F(2,84) = 11.39, p < 0.0001, partial η2 = 0.21]. This difference disappeared by the mid and late extinction blocks. To further clarify the nature of the interaction, separate repeated measures ANOVAs were run to assess the main effect of block for each treatment group. The group receiving OT showed a significant decrease in response magnitude across the extinction phase [F(2,42) = 16.38, p < 0.0001, partial η2 = 0.44], though this effect was not significant in the placebo group. To further investigate the initial treatment group difference at early extinction, a 2 (Treatment group) × 3 (Block) repeated measures ANOVA was conducted on the responses during early extinction broken down into blocks of two trials each, revealing a main effect of treatment group [Fig. 2c; F(1,42) = 4.07, p < 0.05]. Though the interaction was not significant, inspection of the figure shows that both groups appear to have equal levels of responding during the first two trials, with the placebo group rapidly attenuating potentiation to the CS + relative to the OT group. To confirm extinction learning across groups, we compared the last block of extinction to the last block of acquisition trials for both groups, with both groups showing a significant reduction in CS + responses (Fig. 2b Block: F(1,41) = 23.96, p < 0.0001).
Expectancy and self-report
Expectancy ratings to the CS + decreased across the late acquisition and extinction phases equally across treatment groups [Fig. 3; main effect of trial: F(21,714) = 25.45, p < 0.0001, partial η2 = 0.43]. From acquisition to extinction phases, anxiety in response to the CS + decreased (p < 0.0001), while anxiety in response to the CS − remained unchanged [Table 3; Cue type × Phase interaction [F(1,36) = 39.24, p < 0.0001, partial η2 = 0.52]. OT treatment had no significant effects on anxiety ratings.
Recall
Startle
When the data was normalized for individual rates of conditioned responding, the OT group exhibited significantly higher percent extinction recall scores relative to placebo [Fig. 4; t = −2.34, p < 0.03]. The OT group also trended to exhibit a greater change in raw CS + scores between acquisition and recall phases compared to placebo [Fig. 4 inset; F(1,42) = 3.39, p < .08].
Extinction recall index during the Recall phase by Treatment group (see text for equation). *p < 0.05. Data are depicted as mean index scores ± SEM. Inset maximum potentiated startle magnitudes during the Acquisition phase by Treatment group and potentiated startle magnitudes for the Recall phase by Treatment group. Data are depicted as mean ± SEM difference scores in peak startle magnitude during CS + trials compared to noise-alone trials
Expectancy and self-report
Compared to acquisition, both groups showed significant reductions in shock expectancy ratings to the CS + during recall [phase: F(1,40) = 22.66, p < 0.0001, partial η2 =0.36]. On a 1 (expect shock) to −1 (do not expect shock) scale, placebo group responses to the CS + changed from 0.55 (0.33) to 0.05 (0.62) and OT group responses changed from 0.36 (0.54) to −0.25 (0.61). Compared to acquisition, anxiety in response to the CS + was significantly reduced after recall (p < 0.001), while anxiety in response to the CS − remained minimal across acquisition and recall phases [Table 3; Cue type × Phase: F(1,36) = 49.55, p < 0.0001].
Discussion
The current study represents the first test of OT effects on extinction of conditioned fear in healthy subjects. OT did not facilitate extinction, but our prediction that it would increase extinction recall was supported. Before treatment, both groups showed similar levels of startle potentiation to the CS+, suggesting equal conditioned fear levels (Fig. 2a, b, and c). During the first block of extinction training (45 min after treatment), fear extinction was transiently inhibited in the OT treatment group, but by the end of training, OT and placebo groups displayed equal levels of reduced CS + responding (Fig. 2b and c). On day 2, participants given OT on day 1 displayed significantly greater extinction recall relative to placebo (Fig. 3). Extinction recall (extinction index; Milad et al. 2008; 2007) is anchored to pretreatment levels of CS + responding, thus is not confounded by increased CS + responding in the OT group during early extinction (Fig. 2b). There were no treatment effects on expectancy ratings or self-reported anxiety, suggesting that the effects of OT on fear-potentiated startle were independent of explicit contingency awareness. These findings are the first to support a facilitative effect of OT on extinction recall, supporting further research into OT as an adjunctive pharmacological treatment in combination with PE.
Few studies have looked at the effect of OT on conditioned fear and extinction learning in humans. In a previous study in healthy subjects, Petrovic et al. (2008) used an evaluative conditioning procedure to investigate the effect of OT on aversively conditioned social cues (faces). They found that OT given shortly following acquisition abolished conditioned responding and attenuated amygdala activation relative to placebo. This finding is similar to effects of intraventricular (ICV) OT reversing conditioned social avoidance in rodents (Lukas et al. 2011). Findings of reduced conditioned aversive responses are at odds with the present study, which found that OT treatment transiently inhibited extinction of conditioned fear. Inhibition during initial extinction training in this study is somewhat surprising given the evidence for acute reductions in amygdala activation and anxiolytic effects after IN OT treatment in humans (Petrovic et al. 2008; de Oliveira et al. 2012; Labuschagne et al. 2010; Domes et al. 2007; Kirsch et al. 2005). However, as recently described by Bartels (2012), the “amygdala-dampening” interpretation of IN OT effects on limbic circuitry may be overly simplistic based on the recent finding, suggesting that OT modulation of circuit activation as well as behavior are stimulus-/context-specific (e.g., presence of social cues) with increased amygdala activation and anxiety/mistrust behaviors reported after IN OT in some studies (for review, see Meyer-Lidenberg et al. (2011). Indeed, using similar measures and treatment parameters as the present study, Grillon et al. (2012) found that OT increases potentiated startle responses to unpredictable threat (i.e., “context fear”) but not specific cued threat. In the current study, the beginning of a new Fear Potentiated Startle (FPS) session (extinction training) may have elicited a sense of uncertainty regarding the CS-US contingency, resulting in increased potentiated startle responses in the OT group as shown by Grillon et al. (2012). However, examination of expectancy ratings at the beginning of the extinction phase would suggest that explicit uncertainty was not responsible for the observed effect. Thus, the acute OT modulation of conditioned fear depends on the conditioned cue, social or nonsocial, likely via the type of circuits recruited by these specific stimuli.
Timing of OT administration during the fear learning and extinction processes may also be a factor in OT inhibition of early extinction. In our study, the end of acquisition training and the beginning of extinction training were separated by approximately 1.5 h. Thus, the present observation of OT-induced inhibition of early extinction may be due to acute effects of OT administration immediately after acquisition testing and right before the onset of extinction training. When OT was administered, fear memories were likely still consolidating that may have been facilitated by OT treatment, resulting in the observed transient resistance to extinction. Similarly, in rats, ICV OT prior to extinction training inhibits extinction, whereas OT increased extinction when administered prior to fear conditioning (Toth et al. 2012). Future studies separating acquisition and extinction training by a longer period are required to determine if, like in animals, OT administration before fear conditioning also facilitates subsequent extinction in humans.
The effect of OT treatment to increase recall of extinction 24 h later suggests that OT treatment enhanced consolidation of fear extinction training. While computational models of fear conditioning (i.e., Rescorla and Wagner, 1972) might suggest that enhancement of recall could be due to enhanced startle in the OT group during early extinction training, early extinction responses did not significantly correlate with recall scores, arguing against this explanation. Procognitive effects of OT on other forms of memory are emerging, including facilitation of social memory (Striepens et al. 2011; Herzmann et al. 2012) and verbal learning in some cases (Feifel et al. 2012; but see Heinrichs et al. 2004).
Neuroimaging and animal research have demonstrated that the PFC mediates extinction recall via an inhibitory effect on the amygdala that mediates conditioned fear responding (for review, see Milad and Quirk (2012)). OT treatment increases coupling between the rostral medial frontal cortex and amygdala (Sripada et al. 2012). Thus, OT treatment may act to facilitate extinction learning via increased coupling of this circuit during extinction training. OT treatment also increases long-term glutamatergic neurotransmission in the mPFC (Ninan 2011), which may enhance neuroplasticity in this structure and, consequently, extinction learning. Only one other study that we are aware of has assessed OT effects on extinction learning in humans. Guastella et al. (2009) tested the effect of OT as an adjunctive treatment to a short exposure therapy trial (four sessions of public speaking) for social anxiety disorder. OT facilitated extinction of negative self-assessments during public speaking; however, this effect did not generalize to overall symptom reduction. Thus, the translation of OT effects on extinction to its use as an adjunctive therapy is still uncertain, requiring more study with alternate exposure techniques and patient populations.
In summary, the current findings support the hypothesis that OT treatment facilitates recall of fear extinction; however, it may facilitate learned fear responses acutely under certain circumstances (present findings and Grillon et al. (2012)). A potential limitation of the current findings is that OT affected potentiated startle measures, but not self-reported anxiety. However, response desynchrony between physiological and self-report measures of anxiety has long been documented in the literature and is not uncommon (e.g., Hodgson and Rachman (1974)). This desynchrony may be due to self-reported anxiety being measured only once at the end of each phase, reducing its sensitivity. Overall, the current findings support further research of IN OT as an adjunctive treatment for exposure-based therapy for conditioned fear disorders such as PTSD. OT may not only support the extinction-mediated effects of exposure therapy but also have utility in increasing feelings of trust and facilitating therapeutic alliance, which could theoretically result in a more robust response to treatment in some patients along with a reduction in rates of treatment discontinuation.
References
Acheson DT, Stein MB, Paulus MP, Geyer MA, Risbrough VB (2012) The effect of pregabalin on sensory motor gating in ‘low’ gating humans and mice. Neuropharmacology 63:480–485
Acheson DT, Gresack J, Risbrough VB (2011) Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62:674–685
Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, Girolamo G, Graaf R, Demyttenaere K et al (2004) Disability and quality of life impact of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl 109:21–27
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (fourth edition, text revision). Washington, DC
Bartels A (2012) Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology 37:1795–1796
Baumgartner T, Heinrichs M, Vonlathen A, Fischbacher U, Fehr E (2008) Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58:639–650
Bradley R, Greene J, Russ E, Dutra L, Westen D (2005) A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 162:214–227
Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis CG, Andreski P (1998) Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 55:626–632
Cloitre M (2009) Effective psychotherapies for posttraumatic stress disorder: a review and critique. CNS Spectrum 14:32–43
Committee on Treatment of Posttraumatic Stress Disorder IoMotNA (2007) Treatment of posttraumatic stress disorder: an assessment of the evidence. The National Academies Press, Washington, DC
de Kleine RA, Hendriks G, Kusters WJC, Broekman TG, van Minnen A (2012) A randomized placebo-controlled trial of d-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry
de Oliveira DC, Chagas MH, Garcia LV, Crippa JA, Zuardi AW (2012) Oxytocin interference in the effects induced by inhalation of 7.5 % CO(2) in healthy volunteers. Hum Psychopharmacol 27(4):378–385
Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007) Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 62:1187–1190
Druss BG, Hwang I, Petukhova M, Sampson NA, Wang PS, Kessler RC (2008) Impairment in role functioning in mental and chronic medical disorders in the United States: results from the National Comorbidity Survey Replication. Mol Psychiatry 14:728–737
Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488
Feifel D (2012) Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology 37:304–305
Foa EB, Rothbaum BO (1998) Treating the trauma of rape: cognitive–behavioral therapy for PTSD. Guilford, New York
Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS (2009) A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 34:917–923
Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B (2012) Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. doi:10.1038/mp.2012.156
Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54:1389–1398
Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH (2004) Selective amnesic effects of oxytocin on human memory. Physiol Behav 83(1):31–38
Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X (2003) Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress 16:555–562
Herzmann G, Young B, Bird CW, Curran T (2012) Oxytocin can impair memory for social and non-social visual objects: a within-subject investigation of oxytocin's effects on human memory. Brain Res 1451:65–73
Hodgson R, Rachman S (1974) II. Desynchrony in measures of fear. Behav Res Ther 12:319–326
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627
Kirsch P, Esslinger C, Chen Q et al (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25:11489–11493
LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998) Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20:937–945
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ (2010) Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35(12):2403–2413
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011) The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36(11):2159–2168
Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63:129–151
Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008) Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42:515–520
Milad MR, Wright CL, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007) Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62:446–454
Mueller D, Porter JT, Quirk GJ (2008) Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28:369–375
Meyer-Lindengergy A, Domes G, Kirsch P, Heinrichs M (2011) Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–538
Myers KM, Davis M (2007) Mechanisms of fear extinction. Molecular Psychiatry 12:120–150
Ninan I (2011) Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem 119(2):324–331
Norberg MM, Krystal JH, Tolin D (2008) A meta-analysis of D-cycloserine and facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126
Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ (2011) Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 15:556–563
Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ (2006) Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn Mem 13:681–685
Olff M, Langeland W, Witteveen A, Denys D (2010) A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectrum 15:522–530
Petrovic P, Kalisch R, Singer T, Dolan RJ (2008) Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci 28:6607–6615
Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004) Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43(6):897–905
Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF (eds) Classical Conditioning II. Appleton-Century-Crofts, p 64–99
Schnurr PP, Friendman MJ, Engel CC, Foa EB, Shea M, Chow BK et al (2007) Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 297:820–830
Shin LM, Rauch SL, Pitman RK (2006) Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071:67–79
Sripada CS, Phan KL, Labuschagne 2012 I, Welsh R, Nathan PJ, Wood AG (2012) Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol 30:1–6
Striepens N, Kendrick KM, Maier W, Hurlemann R (2011) Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 32(4):426–450
Toth I, Neumann ID, Slattery DA (2012) Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology
Acknowledgments
These studies were supported by the Veterans Affairs Center for Excellence in Stress and Mental Health (VB, JL), Defense Medical Research and Development Program award #DM102425 (VB, DA), R34 MH091285 (DF), and a Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (DA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acheson, D., Feifel, D., de Wilde, S. et al. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology 229, 199–208 (2013). https://doi.org/10.1007/s00213-013-3099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3099-4