Abstract
Rationale
Rats develop preferences for places associated with the immediate rewarding effects of cocaine and aversions for places paired with the drug’s delayed negative effects. The motivation to seek cocaine should therefore depend upon the relative magnitude of these two opposing effects of the drug.
Objective
The current study tested this notion by assessing the relative persistence of the positive and negative associations formed between environmental cues and the immediate or delayed effects of cocaine.
Methods
Rats were administered 1.0 mg/kg intravenous cocaine and placed into a distinctive environment either immediately or 15-min after injection, alternating daily with pairings of a second environment with saline. After four drug-place and four saline-place pairings, rats were returned to their home cages for 1, 7, or 21 days after which a 15-min place preference test was conducted. In a second experiment, the effectiveness of a single reconditioning session (one drug-place and one saline-place pairing) to reactivate learned cocaine-place associations was assessed after 1 or 3 weeks of drug abstinence.
Results
Places associated with the immediate effects of cocaine were preferred (CPP), while places associated with the delayed effects of cocaine were avoided (CPA). The persistence of these effects differed with CPP remaining viable at 3 weeks of withdrawal, while CPA was no longer present after 1 week. Reconditioning with an additional cocaine-place pairing failed to reinstate the CPA.
Conclusions
Cue-induced “relapse” of cocaine-seeking behavior may be fueled in part by an increased persistence of positive relative to negative associations with drug-paired stimuli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “Opponent Process” theory of motivation postulates that any stimulus capable of producing a positive affective response will also activate an opposing response whose onset is delayed and whose function is to return the organism to emotional homeostasis (Solomon and Corbit 1974). For example, human cocaine addicts report that the initial drug-induced state of euphoria (the “high”) is temporally displaced by profound feelings of anxiety, agitation, depression, and fatigue (the “crash”) (Anthony et al. 1989; Hamner 1993; Resnick and Resnick 1984; Roselli and Ardila 1996; Smith 1986; Washton et al. 1983; Williamson et al. 1997). The delayed onset of these negative effects occur even while plasma levels of cocaine remain high, suggesting that such effects are not a result of drug withdrawal, but more directly related to the drug’s activation of biologically independent opponent processes (Van Dyke and Byck 1982).
Laboratory animals similarly show both positive and negative effects of cocaine. The drug is readily self-administered (Hill and Powell 1976; Roberts et al. 1977; Ross et al. 1978; see reviews by Gawin 1988; Johanson 1988), produces preferences for places associated with its administration (Mucha et al. 1982; Mueller and Stewart 2000; Spyraki et al. 1982b; for review, see Tzschentke 2007), and reduces brain stimulation thresholds (Ahmed et al. 2002; Markou and Koob 1991, 1992). Müller et al. (2008) have suggested that these initial positive effects of cocaine might be attributable, at least in part, to short-lived anxiolytic effects of drugs. Others, meanwhile, have shown that cocaine enhances the anxiogenic-like response of subjects in the elevated plus maze (Rogerio and Takahashi 1992; Schank et al. 2008), reduces center entries in the open field (Simon et al. 1994; Yang et al. 1992), increases suppression of drinking in the presence of a conditioned aversive stimulus (Fontana and Commissaris 1989), and produces greater avoidance of an aversive brightly illuminated white area of a two compartment white/black test box (Costall et al. 1989; Erb et al. 2006). The dual positive and negative effects of cocaine can also be observed in the same animal on the same trial. Rats running a straight alley for i.v. cocaine develop a unique pattern of approach-avoidance behavior (quickly approaching the goal, then stopping and retreating back to the start box) that reflects the presence of mixed positive and negative associations that subjects form with the cocaine-paired goal box (Ettenberg and Geist 1991; see reviews by Ettenberg 2004, 2009).
If one accepts that cocaine produces mixed rewarding and anxiogenic effects, then it seems reasonable to suggest that the motivation to seek the drug must be influenced by the relative magnitude of these two opposing actions. Given that drug-paired cues have long been thought to play a critical role in the reinstatement (relapse) of drug-seeking behavior after a period of abstinence (Alleweireldt et al. 2001; Childress et al. 1987, 1988; Meil and See 1996; O’Brien et al. 1992; Tran-Nguyen et al. 1998; Weiss et al. 2001), it was of interest to determine the relative magnitude and persistence of the effects of environmental cues associated with the either the positive or negative effects of cocaine. To accomplish this, we made use of the fact that rats develop conditioned preferences for distinct places paired with the immediate effects of i.v. cocaine and aversions for places associated with the effects of the drug present 15-min postinjection (Ettenberg et al. 1999; Ettenberg and Bernardi 2007; Knackstedt et al. 2002). While others have demonstrated that a cocaine-induced conditioned place preference (CPP) can persist for up to 6 weeks of withdrawal (Mueller and Stewart 2000) and that a single reconditioning session can reinstate a CPP (Sticht et al. 2010), almost nothing is known about the persistence and relative strength of the negative effects of cocaine-paired cues. The current study therefore examined the persistence of these conditioned place preferences and aversions after varying periods of drug abstinence to determine whether the relative behavioral impact of positive versus negative drug-paired cues might differentially shift during drug withdrawal.
Materials and methods
Subjects
Adult male Sprague–Dawley rats (n = 128), weighing 330–360 g at the time of surgery (Charles River Laboratories, Wilmington, MA, USA) served as subjects. Rats were individually housed in plastic cages within a temperature-controlled (23 °C) vivarium maintained on a reverse 12-h light–dark cycle (lights off at 0800 hours). Free access to food (Purina Rat Chow) and water was provided throughout the duration of the study. All animal handling and experimental procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California at Santa Barbara’s Institutional Animal Care and Use Committee.
Surgery
Rats were acclimated to human handling for 1 week prior to intravenous (i.v.) catheterization. Catheter construction consisted of thin polyethylene tubing (13 mm long, 0.3 mm inner, and 0.64 mm outer diameters; Dow Corning Corp, Midland, MI, USA) that was preassembled to fit a stainless steel guide cannula (Item 313G; Plastics One, Roanoke, VA, USA). The guide cannula was in turn affixed to a 2-cm square of Mersilene surgical mesh using dental cement (Bard; Warwick, RI, USA). Catheterization was accomplished during isoflurane-induced deep anesthesia (4 % for induction and 1.5–2.5 % for maintenance). To prevent respiratory congestion and reduce postsurgical pain, rats were treated with atropine (0.04 mg/kg, intramuscularly) and the nonopiate analgesic flunixin meglumine (Phoenix Pharmaceuticals, Belmont, CA, USA; 2 mg/kg, subcutaneously). The open end of the catheter was inserted into the right jugular vein and secured in place by silk sutures. The other end of the catheter was passed subcutaneously to the midline of the animal’s back where the attached guide cannula protruded through a 2-mm hole and the Mersiline mesh was laid flat against the subdermal tissue. All incisions (in the neck and back) were tightly closed by suture. Following surgery, rats received the antibiotic, icarcillin disodium/clavulanate potassium (Timetin; 50 mg/kg, i.v.), and 0.1 ml of heparin (6.0 IU/0.1 ml prepared in 0.9 % physiological saline, i.v.) to maintain catheter patency.
Subjects were given 1 week to recover from surgery during which the catheters were flushed daily with 0.1 ml of Timetin antibiotic (20 mg/kg, i.v.) and 0.1 ml of heparinized 0.9 % physiological saline. Catheter patency was tested on the day prior to behavioral testing and 2 h after the final conditioning trial by observing the behavioral impact of an i.v. injection of the fast-acting barbiturate, methohexital sodium (Brevital; 2.0 mg/kg/0.1 ml). A single animal did not lose its righting reflex in response to Brevital prior to the start of the experiment and was recatheterized using the left jugular vein and given additional days for recovery. Animals that failed the final Brevital test (n = 7) were removed from the data analyses.
Drugs
Cocaine hydrochloride was generously provided by the National Institute of Drug Abuse. The drug was dissolved in a vehicle of 0.9 % physiological saline and noncontingently delivered in a volume of 0.1 ml over a period of 4.3 s via a 10-ml syringe that was seated in a motorized syringe pump (Razel Scientific Instruments, St Albans, VT, USA). Vehicle “control” injections entailed the same injection parameters as the cocaine but with no drug added to the solution. The dose for the current study (1.0 mg/kg) was identified as the optimal dose in prior runway studies (i.e., it produced the fastest start times and runtimes, and fewest approach-avoidance retreats; Raven et al. 2000) and was found to produce robust conditioned place preferences and aversions (Ettenberg and Bernardi 2007; Ettenberg et al. 1999).
Conditioned place preference apparatus
Two identical CPP boxes consisted of wood-constructed rectangular enclosures (156 cm long × 34 cm wide × 30 cm high) that could be subdivided into three separate compartments: two larger chambers (61 × 30 cm) at opposite ends separated by a smaller intermediate chamber (34 × 30 cm). One of the larger compartments was painted black with an acrylic (Plexiglas ®) flooring and scented with acetic acid (2 % solution, swabbed 5 cm above the compartment floor). The other larger chamber was painted white and covered with soft gray bedding (Carefresh; Absorption Corp, Ferndale, WA, USA), without any additional odor cues. The smaller intermediate chamber had a wood floor that was painted gray. Each compartment, therefore, had unique visual, tactile, and olfactory properties and yielded no reliable inherent preferences prior to conditioning. Situated above each apparatus was a digital camera that detected and recorded the precise location of the animal in real time via a desktop computer running Any-Maze software (Stoelting Co, Wood Dale, IL, USA).
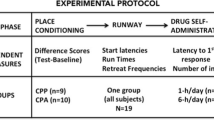
Experiment 1: persistence of CPP and CPA
Place conditioning involved three stages: a preconditioning baseline preference test, eight place conditioning trials, and a final preference test conducted after varying periods of drug abstinence. During the baseline trial, the interior walls separating each compartment were removed, and subjects were placed into the middle gray section of the apparatus. The time spent in each of the three compartments was then recorded over a 15-min preconditioning trial. Each rat was then exposed to eight alternating conditioning trials on which an injection of either vehicle or cocaine (1.0 mg/kg, i.v.) was administered followed by placement into either the white or black compartment for 5 min. On the following day, each rat received the alternate treatment and was placed in the alternate colored environment. This continued in an alternating manner until each subject had experienced four cocaine and saline pairings. Animals were placed into the conditioning chamber either immediately after injection (0 min “reward” group) or 15-min postinjection (15 min “aversion” group). The order of testing (receiving vehicle or cocaine on the first conditioning trial) and the drug-paired compartment (either black or white) were counterbalanced within each group (i.e., an unbiased CPP/CPA design was employed; see Carr et al. 1988). Following the completion of place conditioning, animals were returned to their home cages for either 1, 7, or 21 days. A final 15-min place preference test was then conducted exactly as described for the baseline trial.
By way of summary, the experimental protocol described above produced six groups of animals: three tested for place preferences 1 day (n = 11), 1-week (n = 10), or 3 weeks (n = 11) after conditioning to the immediate effects of cocaine, and three groups tested for place aversions to the delayed negative effects of cocaine 1 day (n = 12), 1 week (n = 15), or 3 weeks (n = 14) after conditioning. For each group, the comparison of interest was performance on test day (postconditioning) relative to that observed prior to conditioning (i.e., on baseline). For that reason, no vehicle control groups (i.e., saline pairings with both compartments) were employed (e.g., see also Manzanedo et al. 2012; Mueller and Stewart 2000; Paolone et al. 2009; Spyraki et al. 1982a,b; Zakharova et al. 2009).
Experiment 2: effects of reconditioning on CPP and CPA expression
A second experiment was conducted to further investigate the relative strength of the associations formed between the drug-paired cues (i.e., the conditioning environments) and the positive or negative effects of cocaine. This involved the administration of a single reconditioning trial conducted on the day prior to preference testing either 1 or 3 weeks after completion of the initial place conditioning protocol. In this experiment, 10 days of CPP/CPA place conditioning were employed as described for experiment 1—i.e., a baseline trial, 8 days of place conditioning (with immediate or delayed cocaine), and a final preference test. All animals then experienced a second preference test either 1 or 3 weeks after the first preference test. On the day preceding this second test, animals were exposed to a reconditioning session in which they received a saline-place pairing in the morning and a cocaine-place pairing 4–5 h later. The order of injections was not counterbalanced to ensure that vehicle animals were tested at a time when no residual cocaine was present. This procedure generated four groups: two tested for “reinstated” place preferences either 1 week (n = 8) or 3-weeks (n = 13) after the initial preference test, and two additional groups tested at the same time points for cocaine-induced place aversions (n = 10 and 16, respectively).
Upon completion of the experiments described above, a one-pairing control group was added to ensure that a single reconditioning session was insufficient in and of itself to produce place conditioning. These subjects underwent a baseline trial, a single saline and cocaine pairing on the same day (i.e., as in the reconditioning protocol described above), and a preference test conducted on the next day. The drug dose, temporal manipulations of cocaine (i.e., immediate or delayed conditioning), and the procedures for the baseline and preferences tests were as previously described. These animals were assigned to either a 0-min (immediate cocaine) group (n = 8) or a 15-min delayed cocaine group (n = 10).
Statistical analyses
Of the 128 rats that completed the two experiments, 11 were removed from the data analyses due to either cannula patency failure or excessive deviation from group mean performance (e.g., strong inherent place biases during preconditioning baseline). Differences in group sample sizes stemmed from these factors and from planned larger n’s in the 3-week groups where a greater frequency of cannula failure was expected (but not always realized).
In experiment 1, a mixed four-factor analysis of variance (ANOVA) (conditioning × compartment × trial × withdrawal period) was employed to analyze the times spent by each group in the cocaine- and saline-paired sides of the apparatus on baseline versus test day and across the different withdrawal time points. Thus, the four main factors in the analysis were as follows: “conditioning” compared the performance of animals in the immediate versus delayed conditioning groups; “compartment” refers to the time spent in the cocaine- versus saline-paired sides of the apparatus; “trial” compared baseline to test-day performance across all animals; and “withdrawal period” compared group performance across 1, 7, or 21 days of withdrawal.
In experiment 2, two separate ANOVAs were employed: one analyzed the performance of all subjects after the initial preference test (i.e., a mixed design trial × compartment × conditioning analysis)—this served as a replication of the 1-day group in experiment 1; a second ANOVA (another three-factor mixed design: compartment × conditioning × withdrawal) examined the impact of “reconditioning” on subsequent preference behavior tested either 1 or 3 weeks after the initial preference test. Data from the one-pairing control group (that received a single immediate or delayed pairing of cocaine with one compartment and saline with the other) were analyzed using the same three-factor mixed design ANOVA. In all instances, statistically reliable results revealed by ANOVA were further examined post-hoc using Bonferonni-protected t tests.
Results
Experiment 1: persistence of CPP and CPA
Figure 1 depicts the mean time (seconds) + SEM spent in the cocaine- and saline-paired environments on test day for the three groups of animals conditioned to the immediate positive effects of cocaine (left panels) and the three groups conditioned to the delayed negative effects of the drug (right panels). The top, middle, and bottom panels of the figure reflect the results from groups tested 1 day, 1 week, or 3 weeks after conditioning. The four-factor mixed-design ANOVA computed on the data depicted in the figure yielded several statistically significant results. There was a reliable difference between baseline and test day scores [main effect of “trial”; F(1,72) = 33.002, p < .01], between the time spent in the cocaine-paired side of the apparatus versus the saline-paired side [main effect of “compartment”; F(1,72) = 4.329, p < .05], and between preference scores across the three withdrawal periods [main effect of “withdrawal”; F(1,72) = 3.719, p < .05]. In addition to these reliable main effects, significant interactions were identified for compartment × conditioning [F(1,72) = 5.840, p < .05], compartment × trial [F(1,72) = 10.884, p < .01], and compartment × trial × conditioning [F(1,72) = 12.497, p < .01]. To identify the sources of these interactions, repeated measures Bonferonni-corrected t tests compared the times each group spent in each compartment on baseline and test days. These results confirmed that there were no differences in the times spent in one versus the other compartment on baseline for any group (p > .05)—hence, prior to conditioning, the animals exhibited no inherent compartment/place biases. In contrast, times spent in the cocaine paired-compartment versus the saline-paired compartment differed both as a function of the temporal timing (immediate or delayed) of the cocaine injection, and the length of the abstinence period between conditioning and testing. Figure 1 clearly shows that animals in the three immediate-cocaine (0 min) groups spent more time on the cocaine-paired side of the apparatus at all three time points [1 day, t(10) = 3.343 p < .01; 1 week, t(9) = 4.386, p < .01; 3 weeks, t(11) = 3.312 p < .01], while delayed-cocaine 15-min group exhibited an aversion for the cocaine-paired side of the apparatus only at the 1-day test point [t(11) = −1.796, p < .05].
Mean time (seconds) + SEM spent in the saline-paired (white bars) and cocaine-paired (dark bars) compartments for six groups of rats during baseline and on test day: a 0-min delay group (top left, n = 11) and 15-min delay group (top right, n = 12) tested 1 day after place conditioning; two groups tested 1 week postconditioning, a 0-min delay group (middle left, n = 10) and a 15-min delay group (middle right, n = 15); two groups tested 3 weeks postconditioning, a 0-min delay group (bottom left, n = 11), and a 15-min delay group (bottom right, n = 14). *p < .05, significant differences in the time spent in the cocaine- versus saline-paired compartment on test day
Experiment 2: effects of reconditioning on CPP and CPA expression
Figure 2 shows the mean time (+SEM) that each group spent in the cocaine- and saline-paired compartments on baseline and test days. The top two panels reflect a replication of the 1-day group in experiment 1. A three-factor repeated measures ANOVA computed on the data depicted in the top two panels revealed a significant main effect of trial [baseline versus test; F(1, 46) = 8.306, p < .01] and more importantly a significant trial × conditioning × compartment interaction [F(1, 46) = 20.115, p < .01]. Post hoc Bonferonni-protected repeated measures t tests confirmed that the two groups (immediate versus delayed cocaine) exhibited no inherent place biases prior to conditioning (i.e., times spent in the two compartments on baseline were not different from one another for either group; p > .05). However, subjects spent more time in the cocaine-paired side if they were exposed to the immediate positive effects of cocaine [t(20) = 2.469, p < .01] and less time in the drug-paired compartment of the apparatus if conditioned to the delayed negative effects [t(26) = 1.916, p < .01]. This confirms the results observed in experiment 1 as well as those reported previously by our laboratory (e.g., see Ettenberg et al. 1999; Ettenberg and Bernardi 2007; Knackstedt et al. 2002).
Mean time (seconds) + SEM spent in either the saline-paired (gray bars) or cocaine-paired (black bars) compartment during baseline and/or test day. The top portion of the figure (a) depicts the data from two groups of rats tested 1 day after place conditioning: a 0-min delay group (top left panel, N = 21) and a 15-min delay group (top right panel, n = 26). The bottom portion of the figure (b) depicts the test day data from the four subgroups that experienced reconditioning. A 0-min delay group reconditioned and retested after 1 week (n = 8) or 3 weeks (n = 13), and 15-min delay group reconditioned and retested after 1 week (n = 10) or 3 weeks (n = 16). * p < .05, significant differences in the time spent in the cocaine-paired and vehicle-paired compartments on test day
The bottom two panels of Fig. 2 depict the performance of animals that were reconditioned 1 day prior to a second preference test conducted either 1 or 3 weeks after the initial 1-day preference test. The three-factor mixed-design ANOVA (conditioning × withdrawal × compartment) computed on these data revealed a significant main effect of compartment [time spent in the cocaine-paired compartment differed from that spent in the saline-paired compartment; F(1, 46) = 4.723, p < .05] and a significant compartment × conditioning interaction [F(1, 46) = 7.107, p < .05]. Post hoc Bonferonni-protected t tests clearly suggest that both the main effect and interaction stem from the fact that reconditioning served to maintain a strong CPP 1 week [t(7) = 2.187, p < .05] and 3 weeks [t(12) = 2.494, p < .05] after conditioning, while reconditioning had no impact on the expression of CPA at either time point (p > .05).
The single-pairing control group (data not shown) produced no statistically reliable conditioning, compartment, trial, or interaction effects (p > .05). Thus, the reconditioning procedure itself was insufficient to produce reliable cocaine-induced place preferences or aversions.
Discussion
Human cocaine users report that after the initial euphoria subsides, a negative affective state ensues that is characterized by feelings of anxiety, stress, and agitation (e.g., see Van Dyke and Byck 1982). Clearly, a thorough understanding of the factors that contribute to cocaine use must include knowledge about how both the positive and negative aspects of the drug experience interact to motivate drug-seeking behavior. In the current study, place conditioning was employed to assess the dual and opposing actions of cocaine as both reward and aversion can be measured using the same behavioral model. Rats placed into a distinctive environment immediately after a 1.0 mg/kg i.v. cocaine injection, exhibited a CPP for that cocaine-paired environment when tested drug-free on the day after the final conditioning trial. This reaffirms a well-established finding in the cocaine literature (e.g., see reviews by Aguilar et al. 2009; Calcagnetti et al. 1995) and is consistent with numerous other studies in which a CPP has been observed by pairing a novel and distinctive environment with any one of a variety of positive incentive (rewarding) stimuli including drugs of abuse (Aguilar et al. 2009; Carr et al. 1989; Tzschentke 2007), intracranial stimulation (Kling-Petersen et al. 1995), and natural rewards such as sucrose (White and Carr 1985), food (Spyraki et al. 1982a), sex (Miller and Baum 1987), or social interaction (Calcagnetti and Schechter 1992).
In contrast to cocaine’s initial rewarding effects, and consistent with Solomon and Corbit’s (1974) classic “Opponent Process” theory of motivation, the affective response to cocaine after the initial “high” had subsided was found to be aversive in nature. Animals placed into a distinctive environment beginning 15-min postinjection subsequently exhibited aversions for the drug-paired environment when tested drug-free 1 day after the completion of place conditioning trials. Given that the half-life of a 1.0 mg/kg i.v. injection of cocaine has been reported to exceed 18-min in rat (Barbieri et al. 1992), the aversive effects of cocaine present 15-min postinjection in the current study are unlikely to have stemmed from the absence of the drug in brain and/or plasma. Indeed, as discussed in “Introduction,” human cocaine users similarly report negative effects even while plasma levels of cocaine remain high, suggesting that such effects are not a result of drug withdrawal, but more directly related to the drug’s activation of biologically independent opponent processes (Van Dyke and Byck 1982).
The sensitivity of the place conditioning method to detect negative or aversive effects of stimuli has of course been well documented in the literature. For example, subjects have been shown to produce learned aversions (CPA) for places associated with painful stimuli (Johansen et al. 2001; Lei et al. 2004; Kung et al. 2003), fearful stimuli (Gao et al. 2004; Selden et al. 1991), and drugs known to induce anxiogenic or aversive states (Swerdlow et al. 1983; Wagner and Katz 1984; Watanabe et al. 2002). Thus, the present results replicate our own previous findings that animals exhibit preferences for places associated with the initial rewarding effects of i.v. cocaine and aversions for places associated with the delayed effects of the drug (Ettenberg et al. 1999; Ettenberg and Bernardi 2007; Knackstedt et al. 2002).
Of particular relevance and importance in the current study are the data describing the relative persistence of the positive and negative associations that animals form with cocaine-paired contextual cues. While studies on the persistence of CPP have been reported in the literature, the current study is novel in its comparison of the relative strength of CPP and CPA over the course of drug abstinence. Place preferences remain intact without priming injections for up to 6 weeks of withdrawal (Li et al. 2010; Meuller and Stewart 2000) and can be reinstated with a cocaine-prime injection immediately prior to the CPP test (Itzhak and Martin 2002; Mcgeehan and Olive 2006; Szumlinski et al. 2002; Zavala et al. 2003). In the current study, animals conditioned to the immediate effects of cocaine continued to spend more time in the cocaine-paired compartment than the saline-paired compartment for up to three weeks of withdrawal (left panels in Fig 1). This was not the case for cocaine-induced CPA. The current results demonstrate that the expression of learned aversions for places paired with the delayed negative effects of cocaine weaken over the course of withdrawal. More specifically, while animals exhibited an initial aversion of the cocaine-paired side (relative to the saline-paired side) at the 1-day test point, that effect was no longer statistically detectable after 1 or 3 weeks of withdrawal (Fig 1; right panels).
The data from experiment 1 demonstrate that environments associated with the immediate rewarding effects of cocaine retain their conditioned incentive properties longer than environments paired with the negative/aversive effects of the drug. It would seem then that the strength of cocaine-place associations differs as a function of the affective properties of the drug that are being paired with the conditioning environment. This conclusion is supported by the results of experiment 2. When animals were provided an additional cocaine- and saline-place exposure on the reconditioning trial, they exhibited CPP but not CPA during the next day’s preference test. This was not an artifact of the reconditioning method itself since single cocaine-place pairings were insufficient to produce either CPP or CPA in the single-pairing control group (see also Nomikos and Spyraki 1988). Therefore, the most parsimonious conclusion to be drawn from these data is that the reconditioning session was insufficient to reinstate and/or adequately strengthen the previously formed association between the environmental cues and the negative effects of cocaine. It is, of course, possible that had different doses of cocaine been used different levels of positive and negative affect would have been produced. However, the selection of the current 1.0 mg/kg dose of cocaine was based upon the fact that this dose produced the strongest drug-seeking behavior in experienced but nondrugged animals running a straight alley for cocaine (e.g., Raven et al. 2000). More specifically, the dose employed in the current study has been shown to be highly motivating (animals leave the start box quickly and quickly approach the goal) despite having significant negative anxiogenic effects (subjects exhibit a growing hesitancy—retreat behaviours—about goal box entry). A goal of our research program, therefore, has been to assess the relative impact of these positive and negative effects of cocaine on cue-induced drug-seeking behavior. In that context, experiment 2 demonstrates that the behavioral impact of a single re-exposure to cocaine, after a period of drug withdrawal, is more effective in activating prior positive than negative drug-place associations.
Collectively, these results are consistent with those we have observed in the drug self-administration runway. As indicated above, rats running for i.v. cocaine exhibit approach-avoidance retreat behaviors that develop as a consequence of the mixed positive and negative associations that subjects form with the cocaine-paired goal box (for reviews by Ettenberg 2004, 2009). However, when cocaine-paired olfactory cues were presented after varying periods of nonreinforced extinction trials, approach behavior was maintained while retreats weaken over the course of drug abstinence (Su et al. 2011, 2012). Thus, in both the runway and place conditioning models, the associations of environmental stimuli with the positive effects of cocaine are more persistent than those with the negative effects of the drug. While drug-paired environmental cues have long been thought to play a critical role in the reinstatement of drug-seeking behavior after a period of drug abstinence (for review, see Crombag et al. 2008; Shaham et al. 2003), our data suggest that “relapse” in drug-seeking behavior may be fueled, at least in part, by an increase in the salience of positive relative to negative associations with drug-paired stimuli. Furthermore, the persistence of positive drug-cue associations coupled with the weakening of negative drug-cue associations over time, may also contribute to the increasing risk of relapse in response to presentation of drug-paired cues as abstinence continues—i.e., the “incubation of craving” effect (e.g., Bedi et al. 2011; Grimm et al. 2001; Lu et al. 2004; Pickens et al. 2011).
References
Aguilar MA, Rodriguez-Arias M, Minarro J (2009) Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev 59:253–277
Ahmed SH, Kenny PJ, Koob GF, Markou A (2002) Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5:625–626
Alleweireldt AT, Weber SM, Neisewander JL (2001) Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav 69:555–560
Anthony JC, Tien AY, Petronis KR (1989) Epidemiological evidence on cocaine use and panic attacks. Am J Epidemiol 129:543–549
Barbieri EJ, Ferko AP, DiGregorio GJ, Ruch EK (1992) The presence of cocaine and benzoylecgonine in rat cerebrospinal fluid after the intravenous administration of cocaine. Life Sci 51:1739–1746
Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H (2011) Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69:708–711
Calcagnetti DL, Schechter MD (1992) Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav 51:667–672
Calcagnetti DJ, Keck BJ, Quatrella LA, Schechter MD (1995) Blockade of cocaine-induced conditioned place preference: relevance to cocaine abuse therapeutics. Life Sci 56:475–483
Carr GD, Phillips AG, Fibiger HC (1988) Independence of amphetamine reward for locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology 94:221–226
Carr GD, Fibiger HC, Phillips AG (1989) Place preference as a measure of drug reward. In: Liebman JM, Cooper SJ (eds) The neuropharmacological basis of reward. Clarendon, Oxford, pp 264–319
Childress AR, McLellan AT, Ehrman R, O’Brien CP (1987) Extinction of conditioned responses in abstinent cocaine or opioid users. NIDA Res Monogr 76:189–195
Childress AR, McLellan AT, Ehrman R, O’Brien CP (1988) Classically conditioned responses in opioid and cocaine dependence: a role for relapse. NIDA Res Monogr 84:25–40
Costall B, Kelly ME, Naylor RJ, Onaivi ES (1989) The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav 33:197–203
Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363:3233–3243
Erb S, Hanan K, Kristoffer R (2006) A study of the lasting effects of cocaine pre-exposure on anxiety-like behaviors under baseline conditionings and in response to central injections of corticotropin-releasing factor. Pharmacol Biochem Behav 85:206–213
Ettenberg A, Geist TD (1991) An animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology 103:455–461
Ettenberg A, Raven MA, Danluck DA, Necessary BD (1999) Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav 64:507–512
Ettenberg A (2004) Opponent processes properties of self-administered cocaine. Neurosci Biobehav Rev 27:721–728
Ettenberg A, Bernardi RE (2007) Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav 87:171–178
Ettenberg A (2009) The runway model of drug self-administration. Pharmacol Biochem Behav 91:271–277
Fontana DJ, Commissaris RL (1989) Effects of cocaine on conflict behavior in the rat. Life Sci 45:819–827
Gao YL, Ren WH, Zhang YQ, Zhao ZA (2004) Contribution of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110:343–353
Gawin FH (1988) Chronic neuropharmacology of cocaine: progress in pharmacotherapy. J Clin Psychiatry 49(Suppl):11–16
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Hamner MB (1993) PTSD and cocaine abuse. Hosp Comm Psychiatry 44:591–592
Hill SY, Powell BJ (1976) Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav 5:701–704
Itzhak Y, Martin JL (2002) Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharm 26:130–134
Johansen JP, Fields HL, Manning BH (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 98:8077–8082
Johanson CE (1988) Behavioral studies of the reinforcing properties of cocaine. NIDA Res Monogr 88:107–124
Kling-Peterson T, Ljung E, Wolter L, Svensson K (1995) Effects of dopamine D2 preferring compounds on conditioned place preference and intracranial self-stimulation in the rat. J Neural Transm Gen Sect 101:27–39
Knackstedt LA, Samimi MM, Ettenberg A (2002) Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav 72:931–936
Kung JC, Su NM, Ran RJ, Chai SC, Shu BC (2003) Contribution of the anterior cingulate cortex to laser-pain conditioning. Brain Res 970:58–72
Lei LG, Sun S, Gao YJ, Zhao ZQ, Zhang YQ (2004) NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp Neurol 189:413–421
Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue FL, Shaham Y, Lu L (2010) Basolateral amygdala cd5k activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci 30:10351–10359
Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47(Suppl 1):214–226
Manzanedo C, Garcia-Pardo MP, Rodrigues-Arias M, Miñarro J, Aguilar MA (2012) Pre-treatment with high doses of cocaine decreases the reinforcing effects of cocaine in the conditioned place preference paradigm. Neurosci Lett 516:29–33
Markou A, Koob GF (1991) Post-cocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26
Markou A, Koob GF (1992) Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology 7:213–224
McGeehan AJ, Olive MF (2006) Attenuation of cocaine-induced reinstatement of cocaine conditioned place preference by acamprosate. Behav Pharmacol 17:363–367
Meil WM, See RE (1996) Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7:754–763
Miller RL, Baum MJ (1987) Naloxone inhibits matings and conditioned place preferences for an estrous female in male rats soon after castration. Pharmacol Biochem Behav 26:781–789
Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105
Mueller D, Stewart J (2000) Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:37–47
Müller CP, Carey RJ, Wilkisz M, Schwenzner S, Jocham G, Huston JP, De Souza Silva MA (2008) Acute anxiolytic effects of cocaine: the role of test latency and activity phase. Pharmacol Biochem Behav 89:218–226
Nomikos GG, Spyraki C (1988) Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology (Berl) 94:119–125
O’Brien CP, Childress AR, McLellan AT, Ehrman R (1992) Classical conditioning in drug dependent humans. Ann NY Acad Sci 654:401–414
Paolone G, Botreau F, Stewart J (2009) The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology 202:403–409
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420
Raven MA, Necessary BD, Danluck DA, Ettenberg A (2000) Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol 8:117–124
Resnick RB, Resnick EB (1984) Cocaine abuse and its treatment. Psychiatr Clin N Am 7:713–728
Roberts DC, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620
Rogerio R, Takahashi RN (1992) Anxiogenic properties of cocaine in the rat elevated plus-maze. Pharmacol Biochem Behav 43:631–633
Roselli M, Ardila A (1996) Cognitive effects of cocaine and polydrug abuse. J Clin Exp Neuropsychol 18:122–135
Ross JW, Laska FJ, Fennessy MR (1978) Brain biogenic amines and intravenous self-administration of cocaine in rats. Clin Exp Pharmacol Physiol 5:351–359
Schank JR, Liles LC, Weinshenker D (2008) Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63:1007–1012
Selden NR, Everitt BJ, Robbins TW (1991) Telencephalic but not diencephalic noradrenaline depletion enhances behavioral but not endocrine measures of fear conditioning to contextual stimuli. Behav Brain Res 43:139–154
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse, history, methodology and major findings. Psychopharmacology 168:3–20
Simon P, Dupris J, Costentin J (1994) Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 61:59–64
Smith DE (1986) Cocaine-alcohol abuse: epidemiological, diagnostic and treatment considerations. J Psychoactive Drugs 18:117–129
Solomon RL, Corbit JD (1974) An opponent-process theory of motivation. I. Temporal dynamics of affect. Psych Review 81:119–145
Spyraki C, Fibiger HC, Phillips AG (1982a) Attenuation of haloperidol of place preference conditioning using food reinforcement. Psychopharmacology 77:379–382
Spyraki C, Fibiger HC, Phillips AG (1982b) Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res 253:195–203
Sticht M, Mitsubata J, Tucci M, Leri F (2010) Reacquisition of heroin and cocaine place preference involves a memory consolidation process sensitive to systemic and intra- ventral tegmental area naloxone. Neurobiol Learn Mem 93:248–260
Su Z-I, Wenzel J, Baird R, Ettenberg A (2011) Comparison of self-administration behavior and responsiveness to drug-paired cues in rats running an alley for intravenous heroin and cocaine. Psychopharmacology (Berl) 214:769–778
Su Z-I, Kichaev G, Wenzel J, Ben-Shahar O, Ettenberg A (2012) Weakening of negative relative to positive associations with cocaine-paired cues contributes to cue-induced responding after drug removal. Pharmacol Biochem Behav 100:458–463
Swerdlow NR, van der Kooy D, Koob GF, Wenger JR (1983) Cholecystokinin produces conditioned place-aversions, not place-preferences, in food-deprived rats: evidence against involvement in satiety. Life Sci 32:2087–2093
Szumlinski KK, Price KL, Frys KA, Middaugh LD (2002) Unconditioned and conditioned factors contributing to the “reinstatement” of cocaine place conditioning following extinction in C57BL/6 mice. Behav Brain Res 136:151–160
Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL (1998) Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19:48–59
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Van Dyke C, Byck R (1982) Cocaine. Sci Am 246:128–141
Wagner JA, Katz RJ (1984) Anxiogenic action of benzodiazepine antagonists RO 15–1788 and CGS 8216 in the rat. Neurosci Lett 48:317–320
Washton AM, Gold MS, Pottash AC (1983) Intranasal cocaine addiction. Lancet 10:1374
Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M (2002) Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol 88:399–406
Weiss F, Martin-Fardon R, Cioccocioppo R, Kerr TM, Smith DL, Ben-Shahar O (2001) Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharm 25:361–372
White NM, Carr GD (1985) The conditioned place preference is affected by two independent reinforcement processes. Pharmacol Biochem Behav 23:37–42
Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J (1997) Adverse effects of stimulant drugs in a community of drug uses. Drug Alcohol Depend 44:87–94
Yang XM, Gormon AL, Dunn AJ, Goeders NE (1992) Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav 41:643–650
Zavala AR, Weber SM, Rice JH, Allweireldt AT, Neisewander JL (2003) Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction and reinstatement of cocaine-conditioned place preference. Brain Res 990:157–164
Zakharova E, Leoni G, Kichko I, Izenwasser S (2009) Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res 198:45–50
Acknowledgments
The authors acknowledge, with gratitude, Dr. Osnat Ben-Shahar, Stephanie Waldroup, and Hilary Hammond for their assistance in various aspects of the project. This research was funded by NIDA grants DA05041 and DA033370 awarded to A.E.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, ZI., Santoostaroam, A., Wenzel, J. et al. On the persistence of cocaine-induced place preferences and aversions in rats. Psychopharmacology 229, 115–123 (2013). https://doi.org/10.1007/s00213-013-3086-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3086-9