Abstract
Rational
Although it has been recognized that inhibition of calcineurin induced depressive-like behavior, the underlying neural mediators have not yet been identified. Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase that regulates protein synthesis in synapses, has been demonstrated to be involved in the rapid antidepressant effects of ketamine.
Objective
To investigate a potential role of mTOR signaling pathway which interferes with depressive-like behavior induced by calcineurin blockade and to determine the neurobiological mechanisms underlying mood-related disorders.
Methods
Calcineurin inhibitor cyclosporine A (CsA) and tacrolimus (FK506) were microinjected into the medial prefrontal cortex (mPFC) in rats, and the depressive-like behavior was measured in sucrose preference test and forced swim test. Additionally, mTOR activity was tested by the levels of phosphorylation of p70s6 kinase (p70s6k) and 40S ribosomal protein S6 (rps6).
Results
Chronic microinjection of CsA or FK506 into mPFC increased depressive-like behaviors and decreased mTOR activity, but acute CsA or FK506 had no effects on both behavioral phenotype and mTOR activity. Furthermore, activation of mTOR by NMDA reversed the depressive-like behavior induced by chronic CsA or FK506 administration. Moreover, inhibition of mTOR by rapamycin reversed the antidepressant effects of ketamine. Finally, traditional antidepressant venlafaxine prevented the depressive-like performance induced by chronic CsA or FK506 treatment.
Conclusion
These findings indicate that calcineurin-inhibition-induced depressive-like behavior is mediated by blockade of the mTOR signaling pathway and raise the possibility that stimulation of specific brain mTOR may be sufficient to decrease risk of affective disorders in patients treated with calcineurin inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammalian target of rapamycin (mTOR) is a member of serine/threonine protein kinase and plays an essential role in cell growth and survival through its regulation on initiation of translation (Guertin and Sabatini 2005). mTOR function is regulated by the activation of glutamate N-methyl-d-aspartate (NMDA) receptors (Gong et al. 2006). Activation of mTOR has been functionally linked with protein synthesis in synapses, resulting in the formation, maturation, and function of new spine synapses (Hoeffer and Klann 2010). p70S6 kinase (p70s6k) is the major protein kinase of mTOR phosphorylation (Chiang and Abraham 2005). mTOR phosphorylates and activates 40S ribosomal protein S6 (rps6). Therefore, phosphorylation of p70s6k and rps6 can be used to measure mTOR activity. Rapamycin can block mTOR signaling activation via disruption of mTOR–protein complex formation (Beretta et al. 1996; Kim et al. 2002; Proud 2007). It has been reported recently that inhibition of mTOR by rapamycin reversed the treatment effect of ketamine, a non-selective NMDA receptor antagonist, in several animal models of depression (Li et al. 2010, 2011), suggesting that activation of mTOR signaling is required for the antidepressant actions of ketamine.
The Ca2+-dependent protein phosphatase, calcineurin (also known as protein phosphatase type 2B, PP2B), has been reported to participate in neurotransmission, neuronal structure, and neuronal excitability (Buttini et al. 1993; Mansuy 2003; Polli et al. 1991). The association of calcineurin activity with psychiatric disorders derives from the clinical observations that the rate of anxiety and depression increased in patients that are treated chronically with calcineurin inhibitor cyclosporine-A (CsA) to prevent rejection after organ transplantation (Bahi et al. 2009; de Groen et al. 1987). Increasing evidence proposed that calcineurin signaling is involved in the pathophysiology of depression and the antidepressant treatment. Preclinical studies showed that inhibition of amygdala calcineurin induced depressive-like behavior in mice (Bahi et al. 2009). A recent study also showed that the process that rats were exposed to chronic stress, a well-established procedure that has been widely used to investigate neurobiology of depression, decreased calcineurin activity in CA3 of the hippocampus (Zhu et al. 2011). These findings suggest that calcineurin might play a critical role in the pathophysiology of affective disorders. However, the neurobiological mechanisms underlying mood-related alterations induced by calcineurin blockade is still unknown.
The medial prefrontal cortex (mPFC) is a critical brain region in the network that is implicated in mood-related disorders. Decreased activity of mPFC and reduced serotonergic neurotransmission is considered as a salient feature of depression (Juckel et al. 1999). Postmortem studies found that the reduction of neuronal activity and cortical volume in mPFC is associated with impaired cellular proliferation and plasticity in depressed patients (Drevets 2001; Mayberg 1997). Based on these previous studies, we assessed the role of the mTOR signaling pathway in the mPFC in calcineurin-inhibitor-induced depressive-like behavior by directly modifying the function of mTOR. In the present study, we used two inhibitors of calcineurin, CsA and tacrolimus (FK506), to investigate the potential role of mTOR signaling in rat models of depression and to assess the role of this pathway in mediating the development of depressive-like behavior and the behavioral response to antidepressant treatment.
Materials and methods
Animals
Male Sprague-Dawley rats (weighing 200–220 g upon arrival) were individually housed under a constant temperature (23 ± 2 °C) and maintained on a 12/12-h light/dark cycle (light was on at 8:00 p.m. and off at 8:00 a.m.) with free access to food and water. All of the behavioral tests and drug administrations were performed during the animals’ dark phase. All of the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Local Animal Use Committee.
Drugs
Cyclosporine A (CsA) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in saline containing 2 % dimethylsulfoxide (DMSO) at a dose of 4 μg/μl, which has been reported previously that can inhibit calcineurin activity (Zhu et al. 2011). FK506 was purchased from Tocris Bioscience (Bristol, UK) and was dissolved in 20 % DMSO at a dose of 2 μg/μl according to preliminary experiments. NMDA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in sterile saline at a dose of 500 ng/μl. Rapamycin was purchased from Xinmei Technology Corporation (Tianjin, China) and was dissolved in 20 % DMSO at a dose of 100 μg/μl. The effective dose of NMDA and rapamycin was selected based on the investigation reported previously (Wang et al. 2010). All of the aforementioned drugs or vehicle were bilaterally infused into mPFC at a volume of 0.5 μl per side per day. Ketamine was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was administered intraperitoneally (i.p.) at a dose of 10 mg/kg based on previous reports that it produces antidepressant actions in behavioral models of depression (Li et al. 2010, 2011; Maeng et al. 2008). Venlafaxine was purchased from Chengdu Daxi’nan Pharmaceutical Co., Ltd (Chengdu, Sichuan Province, China) and was dissolved in saline at a dose of 40 mg/kg. All of the drug administrations were performed in the dark phase of rat.
Experimental procedures
Intracerebral cannula implantation and intracranial injections
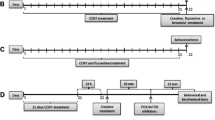
Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and guide cannulae (23 gauge, Plastics One, Roanoke, VA) were placed at a 23° angle toward the midline and were implanted bilaterally 1 mm above the medial prefrontal cortex with the stereotaxic coordinates anterior/posterior [A/P], −3.2 mm; lateral [L], ± 2.5 mm; dorsal/ventral [D/V], −3.3 mm) (Aguiar et al. 2009; Paxinos et al. 1980) (Fig. 1). Vehicle, CsA (4 μg/μl), or FK506 (2 μg/μl) was intracranially microinjected using 10-μl Hamilton syringes (Hamilton, Reno, NV) that were connected via polyethylene-50 tubing to 30-gauge injectors (Plastics One). A total volume of 0.5 μl was infused into each side over 1 min, and the injection syringe was left in the place for an additional 1 min to allow for diffusion. Vehicle, CsA, or FK506 was administered either acutely (one injection) or chronically (14 days) to observe the effects on p70s6k/rps6 activity and depressive-like behavior. NMDA (500 ng/μl) was infused 0.5 μl per side bilaterally with an injection cannula into the mPFC 30 min before each CsA or FK506 microinjection. Rapamycin (100 μg/μl, 0.5 μl per side) was infused bilaterally into the mPFC 30 min before ketamine injection. At the end of the experiments, the rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused. Cannula placements were assessed using Nissl staining with a thickness of 30 μm under light microscopy. Subjects with misplaced cannulae were excluded from the statistical analysis. Schematic representations of the injection sites and photomicrographs of representative cannula placements in the mPFC are shown in Fig. 1.
Sucrose preference test
The sucrose preference test was used to assess anhedonia induced by the blockade of calcineurin. The measurement of sucrose preference was performed as previously described (Willner et al. 1987). Rats were trained to adapt to a 1 % sucrose solution (w/v) for 48 h at the beginning of the experiment, in which two bottles of 1 % sucrose solution were placed in each cage. After adaptation, rats were deprived of water and food for 24 h, followed by the sucrose preference test, in which rats were housed in individual cages for 4 h and had free access to two bottles containing 1 % sucrose or tap water. The bottles were counterbalanced across the left and right sides of the cages throughout the experiment. The position of the two bottles was varied every 2 h during the test. At the end of 4 h, sucrose and water consumption (ml) were measured, and sucrose preference (%) was calculated as the ratio of sucrose consumption to sucrose plus water consumption.
Forced swim test
The forced swim test was similar to the protocol used previously (Porsolt et al. 1978). Briefly, rats were placed into a 25 cm diameter × 65 cm height plastic cylinder filled to a 30 cm depth with 23–25 °C water for 15 min. After 15 min, the rats were removed, dried, and returned to their home cages. They were placed again in the cylinders 24 h later, and the 5-min swim test was conducted and was videotaped. Immobility was defined as the minimum movement required to passively keep the animal’s head above water without other motions. The results are expressed as the time (in seconds) that the animals spent immobile during the 5-min test.
Open field test
To exclude the possibility that calcineurin inhibition induces locomotor alterations in the FST, we measured the effects of CsA or FK506 on locomotor activity. The open field test was used to measure the locomotor activity as previously described (Lin et al. 2005). Briefly, the apparatus consisted of a 75 cm × 75 cm × 40 cm square arena divided into 25 equal squares (15 cm × 15 cm) on the floor of the arena. Each rat was placed in the center of the cage, and the number of crossings (entering the adjacent square line) was counted for 5 min.
Tissue sample preparation
Rats were decapitated, and brains were extracted based on our previous study (Lu et al. 2005). Subsequently, bilateral tissue punches of the mPFC (16 gauge) were obtained from approximately 1-mm-thick coronal sections cut in a Reichert-Jung 2800 Frigocut E cryostat at −20 °C. The rostral faces of the coronal sections were approximately 3.2 mm from bregma. Tissue punches were homogenized (10–15 s × 3, 5 s interval) with an electrical disperser after being lysed with RIPA lysis buffer (Beyotime Biotechnology, China) for 30 min. Afterward, the homogenate was subjected to 10,000 × g centrifugation at 4 °C for 20 min. All of the aforementioned procedures were performed under low temperature (0–4 °C). The protein concentrations of all samples were determined using the BCA assay kit (Beyotime Biotechnology). The protein concentration was normalized by diluting samples with RIPA lysis buffer.
Calcineurin activity assay
The methods used for assay calcineurin activity were essentially the same as previously described (Foster et al. 2001; Lian et al. 2001; Mitsuhashi et al. 2000). Briefly, 10-μg total protein and 80-μl buffer (1.7-mM calmodulin, 1.7-mM EGTA, 50-nM microcystin LR, 8.3-mM ascorbic acid, and 0.17 % β-mercaptoethanol) were added to each well of a standard 96-well plate and preincubated for 10 min at 30 °C. To block the activity of other phosphatases, okadaic acid (5 μM) was added to the buffer for assays of calcineurin. Reactions were started by addition of 20-mM para-nitrophenyl phosphate (p-NPP, Sigma-Aldrich), which is a substrate for purified calcineurin. After a 15-min incubation time, absorbance was read at 620 nm using a Shimadzu UV spectrophotometer.
Western blot assays
Samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (12.5 % acrylamide/0.27 % N,N′-methylenebisacryalamide resolving gel) for approximately 30 min at 80 V in stacking gel and approximately 1 h at 120 V in resolving gel. Proteins were transferred electrophoretically to Immobilon-P transfer membranes (Millipore, Bedford, MA, USA) at 0.25 A for 3 h. Membranes were washed with TBST (Tris-buffered saline plus 0.05 % Tween-20, pH 7.4) before dipping in blocking buffer (5 % skimmed dry milk in TBST) overnight at 4 °C. Membranes were then incubated for 1 h at room temperature with anti-phospho-p70s6k antibody (1:500; Cell Signaling Technology), anti-p70s6k antibody (1:500; Cell Signaling Technology), anti-phospho-rps6 antibody (1:500; Cell Signaling Technology), or anti-rps6 antibody (1:500; Cell Signaling Technology) in TBST plus 5 % bovine serum albumin. After the membrane was shaken in 4 × 6 min washes in TBST buffer, the blots were incubated for 45 min at room temperature with horseradish peroxidase-conjugated secondary antibody (goat antirabbit or mouse IgG; Santa Cruz Biotechnology and Vector Labs, respectively) diluted 1:5,000 in blocking buffer. The blots were then shaken in 4 × 6 min washes in TBST. Afterward, the blots were incubated with a layer of Super Signal enhanced chemiluminescence substrate mixture (Pierce Biotechnology, Rockford, IL, USA) for 1 min at room temperature. Finally, the blots were exposed against X-ray film (Eastman Kodak Company). Band intensities were quantified using Quantity One software (version 4.0.3) from Bio-Rad Corporation (Hercules, CA, USA).
Experimental design
Experiment 1: effects of mPFC calcineurin inhibition on the depressive-like behavior
In this experiment, we first assessed the effects of CsA and FK506 administration on calcineurin activity. We used eight group of rats that were infused with CsA (2 μg/side), FK506 (1 μg/side), or vehicle into mPFC acutely (one single infusion) or chronically (once daily, for 14 days). One hour after the last infusion, rats were decapitated, and brains were collected for protein concentration assay and the subsequent calcineurin activity measurement. Another eight groups of rats were used to determine the effects of acute and chronic calcineurin blockades in mPFC on the depressive-like behavior. One hour after the last drug injections (on day 1 in acute group; on day 14 in chronic group), the rats underwent the sucrose preference test. Subsequently, the rats were placed into the cylinder filled with water and were adapted to swim for 15 min. Twenty four hours later, the rats were exposed to the FST, and the immobility time was recorded. Additionally, separate eight groups of rats were infused with CsA or FK506 for 1 or 14 days; 1 h after the last infusion, the rats were exposed to the open field test to measure the locomotor activity.
Experiment 2: effects of calcineurin inhibitor on mPFC p70s6k/rps6 activity
To assess the effects of acute and chronic calcineurin inhibitions on p70s6k/rps6 activity, four groups of rats were microinjected with acute (1 day) or chronic (14 day) CsA/FK506 or vehicle into mPFC. One hour after the last drug injections, the rats were decapitated, and their brains were extracted for subsequent determination of p70s6k and rps6 in mPFC using Western blotting.
Experiment 3: effects of activation of mPFC p70s6k/rps6 activity on the depressive-like behavior induced by calcineurin inhibitor
The aim of this experiment was to investigate the effect of activation of p70s6k/rps6 activity in the mPFC on the depressive-like behavior induced by calcineurin inhibitor CsA or FK506 in sucrose preference test and forced swim test. Eight groups of rats were used according to the experimental assignment (Fig. 3a). Rats were administered with the corresponding drugs for 14 days. The sucrose preference test and forced swim test were conducted on days 14 and 15, respectively. On day 16, the rats were killed, and the brains were collected for the subsequent measurement of p70s6k/rps6 expression using Western blotting.
Experiment 4: effects of inhibition of mPFC p70s6k/rps6 activity on the antidepressant action of ketamine in rats treated with calcineurin inhibitor
We then determined whether the antidepressant effect of ketamine in the depressive-like behavior induced by calcineurin inhibitor CsA or FK506 could be altered by mTOR inhibitor rapamycin. Based on the experimental procedure, eight groups of rats were used (Figs. 4a and 5a). Rats were treated with CsA or FK506 for 16 days. Rapamycin and ketamine were injected on day 14. The sucrose preference test and forced swim test were conducted on days 15 and 16, respectively.
Experiment 5: effects of traditional antidepressant venlafaxine on the depressive-like behavior induced by calcineurin inhibitors
This experiment is to test whether traditional antidepressant venlafaxine could prevent the depressive-like behavior induced by calcineurin inhibitors. Four groups of rats were used, as described in Fig. 6a. Rats were infused with CsA/FK506 into mPFC every other day for 35 days and were treated with venlafaxine from day 14 to day 35. Sucrose preference test was performed on day 36. The next day (day 37), the forced swim test was conducted.
Data analysis
Data are expressed as mean ± SEM. The statistical analysis of depressive-like behavior and p70s6k/rps6 activity in CsA/FK506- and vehicle-treated rats was performed using unpaired Student’s t-test. Data from the acute and chronic CsA or FK506 administration were analyzed separately. For the other experiments, data were analyzed using analysis of variance (ANOVA) with appropriate one-way or two-way analysis followed by Tukey’s post hoc test (for details, see the “Results” section). Values of p < 0.05 were considered statistically significant.
Results
Chronic but not acute inhibition of mPFC calcineurin by CsA or FK506 induced depressive-like behavior
Calcineurin activity in mPFC was significantly inhibited by both acute and chronic CsA or FK506 administrations (Table 1). To determine whether chronic microinjections of CsA or FK506 would cause neuronal lesion within the injection brain region, we measured the neuronal activity by the expression level of immediate early gene c-fos in mPFC. The results revealed that rats infused with CsA or FK506 repeatedly, compared with vehicle controls, did not display altered levels of c-fos mRNA (data not shown), suggesting that chronic CsA or FK506 microinjections did not induce the change of functional activity of mPFC.
In the sucrose preference test, rats treated chronically with CsA or FK506 in mPFC consumed less sucrose solution (p < 0.001, Table 1). In the forced swim test, chronic infusions of CsA or FK506 in mPFC significantly increased the immobility time (p < 0.01, Table 1), while the acute infusions of CsA or FK506 did not change the depressive-like symptoms. Furthermore, there were no differences in both chronic and acute CsA/FK506 injections in locomotor activity, suggesting that mPFC CsA or FK506-infusion-induced depression-like behavior was not due to the alterations on the normal locomotion (Table 1).
Chronic but not acute inhibition of calcineurin by CsA or FK506 reduced mTOR activity
To determine whether calcineurin inhibition affects mTOR activity in mPFC, we used eight groups of rats: chronic and acute treatment with CsA or FK506. The results showed that the rats that were chronically microinjected with CsA or FK506 decreased phosphorylated, but not total, p70s6k and rps6 in the mPFC (Fig. 2a–d). Data analysis from the Western blot revealed that phosphorylated p70s6k levels in rats in the chronic CsA (p < 0.05) and FK506 (p < 0.01) treatment group were lower than those of the vehicle groups, but with no alterations on the total p70s6k (Fig. 2a, b). Similarly, chronic CsA (p < 0.05) and FK506 (p < 0.05) administration significantly reduced the phosphorylated rps6 levels without changes on the total rps6 (Fig. 2c, d). However, no significant differences were observed in phosphorylated p70s6k and rps6 levels between acute infusions of CsA/FK506 and vehicle groups.
Chronic but not acute treatment with calcineurin inhibitor CsA or FK506 decreased p70s6k and rps6 phosphorylation in the mPFC. a, b Percentage of phosphorylated p70s6k in the mPFC. c, d Percentage of phosphorylated rps6 in the mPFC. The representative band intensity of the Western blot is shown at the bottom. Data are expressed as a percentage of the values obtained for rats infused with vehicle. * p < 0.05; ** p < 0.01, compared with rats infused with corresponding vehicle in chronic group (n = 6 per group). p phosphorylated, t total
Activation of the mTOR pathway blocked the depressive-like behavior induced by chronic calcineurin inhibition
We further determined whether activation of mTOR activity in the mPFC could reverse the depressive-like behavior induced by chronic CsA or FK506 treatment. It has been reported that NMDA, an agonist of the NMDA receptor, can activate the mTOR pathway through its action on the NMDA receptor (Lenz and Avruch 2005; Wang et al 2010). Data analysis from the Western blot assay revealed that NMDA increased mTOR activity as measured by phosphorylated p70s6k and rps6 levels in naïve rats and in CsA or FK506 treatment group (Fig. 3b, c). In the sucrose preference test, microinjection of CsA or FK506 into mPFC reduced sucrose preference (Fig. 3d, e), whereas mPFC preinfusions of NMDA increased sucrose preference compared with vehicle–CsA and vehicle–FK506 rats. The statistical analysis revealed a significant effect of CsA (F 1,32 = 60.48, p < 0.001) and NMDA (F 1,32 = 8.99, p < 0.01) and a significant CsA × NMDA interaction (F 1,32 = 21.75, p < 0.001) on sucrose preference. The statistical analysis also revealed a significant effect of FK506 (F 1,32 = 42.19, p < 0.001) and NMDA (F 1,32 = 4.99, p < 0.05) and a significant FK506 × NMDA interaction (F 1,32 = 7.76, p < 0.01) on sucrose preference. In the forced swim test, microinjection of the calcineurin inhibitor CsA or FK506 into the mPFC increased immobility compared with the vehicle group (Fig. 3f, g). Notably, this increase in immobility was blocked by pretreatment with NMDA. Two-way ANOVA with two between-subjects factors, CsA (0 and 2 μg) and NMDA (0 and 250 ng), revealed significant effects of CsA (F 1,32 = 18.64, p < 0.001) and NMDA (F 1,32 = 8.76, p < 0.01) and a significant CsA × NMDA interaction (F 1,32 = 4.61, p < 0.05) on immobility. The ANOVA statistical analysis also revealed a significant effect of FK506 (F 1,32 = 7.20, p < 0.05) and NMDA (F 1,32 = 4.76, p < 0.05) and a significant FK506 × NMDA interaction (F 1,32 = 7.29, p < 0.05) on immobility in forced swim test. Altogether, these results suggest that activation of mTOR is effective in preventing the depressive-like behavior induced by chronic calcineurin inhibition.
Activation of p70s6k–rps6 phosphorylation by NMDA reversed the depressive-like behavior induced by chronic CsA or FK506 in sucrose preference and forced swim test. a Experimental design for drug treatment and behavioral tests. SPT sucrose preference test, FST forced swim test. b, c Percentage of phosphorylated p70s6k and rps6 in the mPFC. The representative band intensity of the Western blot is shown at the bottom. Data are expressed as a percentage of the values obtained for rats infused with vehicle. ** p < 0.01, compared with rats infused with corresponding vehicle in chronic group; # p < 0.05, compared with rats infused with CsA-NMDA or FK506-NMDA group (n = 6 per group). p phosphorylated. d, e Sucrose preference after infusions of NMDA or its vehicle into the mPFC. Bilateral infusions of NMDA into the mPFC increased the sucrose preference in chronic CsA- or FK506-treated rats. f, g Immobility time after infusions of NMDA or its vehicle into the mPFC. Bilateral infusions of NMDA into the mPFC decreased the immobility time in the forced swim test measured for 5 min. Data are expressed as mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001, different from CsA-NMDA or FK506-NMDA group (n = 8 per group)
Inhibition of mPFC mTOR activity abolished the behavioral effects of antidepressant-like actions of ketamine in chronic CsA- or FK506-treated rats
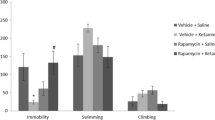
The findings that chronic CsA or FK506 treatment induced a decrease of mTOR activity and activation of mTOR by NMDA reversed the depressive-like behavior suggest that mTOR might play a key role in the depressive-like behavior induced by calcineurin inhibition. We further determined the role of mTOR signaling in the actions of ketamine in CsA- or FK506- induced depressive-like phenotype. Acute injection of ketamine rapidly reversed CsA-induced depressive-like behavior in the sucrose preference test (F 1,40 = 58.33, p < 0.001, Fig. 4b), whereas mPFC pretreatment with rapamycin, a selective inhibitor of mTOR, blocked this antidepressant effect of ketamine (Fig. 4b). These results showed that activation of mTOR is required for the antidepressant action of ketamine. The statistical analysis revealed significant effects of ketamine (F 1,40 = 12.72, p < 0.01) and rapamycin (F 1,40 = 8.32, p < 0.01) and a significant ketamine × rapamycin interaction (F 1,40 = 35.23, p < 0.001) for sucrose preference. In addition, chronic CsA infusion increased the immobility time in forced swim test (F 1,40 =12.40, p < 0.01), which was reduced by a single administration of ketamine (Fig. 4c). Preinfusion with rapamycin increased the immobility time compared with the vehicle–ketamine group in forced swim test, having no effects on ketamine-free groups. These findings suggested that inhibition of mPFC p70s6k–rps6 phosphorylation by rapamycin abolished the antidepressant effect of ketamine in CsA-administrated rats.
Inhibition of mPFC p70s6k–rps6 phosphorylation by rapamycin reversed the antidepressant effect of ketamine in CsA-administrated rats. a Timeline of the experimental procedure. Rats were treated with CsA for 16 days. On day 14, rapamycin was administrated 30 min after CsA injection; 30 min later, ketamine was used. The sucrose preference and forced swim tests were conducted on days 15 and day 16, respectively. b Sucrose preference after infusions of rapamycin or its vehicle into the mPFC. Bilateral pretreatment with rapamycin 30 min before ketamine into the mPFC reversed the antidepressant response of ketamine in the sucrose preference. c Immobility time after infusions of rapamycin or its vehicle into the mPFC. Bilateral infusions of rapamycin into the mPFC blocked the behavioral effects of ketamine in the forced swim test. Data are expressed as mean ± SEM. * p < 0.05; *** p < 0.001, different from vehicle group (n = 8 per group). SPT sucrose preference test, FST forced swim test
Additionally, we determined whether inhibition of mTOR could reverse the antidepressant effect of ketamine in FK506-administrated rats. The results showed that ketamine completely reversed the behavioral deficits in the sucrose preference and forced swim test induced by chronic administration of FK506 (Fig. 5b, c). However, the reversal effects of ketamine were completely blocked by rapamycin pretreatment in these two tests. The statistical analysis revealed significant effects of ketamine (F 1,40 = 28.53, p < 0.001) and rapamycin (F 1,40 = 5.31, p < 0.05) and a significant ketamine × rapamycin interaction (F 1,40 = 20.67, p < 0.001) for sucrose preference. The statistical analysis also revealed significant effects of ketamine (F 1,40 = 10.18, p < 0.01) and rapamycin (F 1,40 = 4.99, p < 0.01) and a significant ketamine × rapamycin interaction (F 1,40 = 4.60, p < 0.05) for immobility.
Inhibition of mPFC p70s6k–rps6 phosphorylation by rapamycin reversed the antidepressant effect of ketamine in FK506-administrated rats. a Timeline of the experimental procedure. Rats were treated with FK506 for 16 days. On day 14, rapamycin was infused 30 min after FK506 injection; 30 min later, ketamine was administrated. The sucrose preference and forced swim tests were conducted on days 15 and day 16, respectively. b Sucrose preference after infusions of rapamycin or its vehicle into the mPFC. Bilateral pretreatment with rapamycin 30 min before ketamine into the mPFC reversed the antidepressant response of ketamine in the sucrose preference. c Immobility time after infusions of rapamycin or its vehicle into the mPFC. Bilateral infusions of rapamycin into the mPFC blocked the behavioral effects of ketamine in the forced swim test. Data are expressed as mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001, different from vehicle group (n = 8 per group). SPT sucrose preference test, FST forced swim test
Traditional antidepressant venlafaxine prevented the depressive-like behavior induced by calcineurin inhibition
Chronic treatment with both CsA (p < 0.001) and FK506 (p < 0.001) for 35 days significantly decreased the sucrose preference (Fig. 6b, c), while administration of antidepressant venlafaxine reversed this behavioral deficits. Chronic CsA (p < 0.001) and FK506 (p < 0.001) treatment also increased the immobility in FST (Fig. 6d, e), whereas the immobility was reduced in rats that received venlafaxine chronically. These results suggest that traditional antidepressant venlafaxine could prevent the depressive-like performance induced by chronic treatment with calcineurin inhibitors.
Traditional antidepressant venlafaxine prevented the depressive-like performance induced by chronic calcineurin inhibition. a Experimental procedure for CsA/FK506 and venlafaxine administration and behavioral tests. b, c Chronic administration of CsA or FK506 induced a decrease in sucrose preference, while antidepressant venlafaxine reversed this behavioral deficit. d, e The increased immobility time caused by chronic CsA or FK506 was blocked by venlafaxine treatment in the forced swim test. Data are expressed as mean ± SEM. *** p < 0.001, different from vehicle–saline group (n = 8 per group). SPT sucrose preference test, FST forced swim test
Discussion
The present results demonstrate that chronic but not acute mPFC inhibition of calcineurin activity increased depressive-like behaviors including anhedonia and desperate symptoms in rats. Our results are consistent with earlier reports and confirm the findings that chronic administration of calcineurin inhibitors lead to an increase in anxiety- and depression-like behaviors (Bahi et al. 2009; Zhu et al. 2011). Additionally, mTOR activity was significantly decreased by microinjection of calcineurin inhibitor CsA or FK506 in the mPFC. Activation of mTOR by NMDA blocked the depressive-like behavior induced by chronic CsA or FK506. Inhibition of mTOR by rapamycin prevented the rapid antidepressant effect of ketamine in rats administrated chronically with calcineurin inhibitors. Finally, the depressive-like behavior induced by chronic calcineurin inhibitors can be prevented by traditional antidepressant venlafaxine. These findings suggest that mTOR signaling plays a key role in calcineurin inhibition that induced depressive-like behavior and that activation of mTOR activity in the mPFC may be sufficient to decrease the risk of affective disorders in patients treated with calcineurin inhibitor.
mTOR, a member of the phosphatidylinositol 3 kinase (PI3K) family, is a downstream target of brain-derived neurotrophic factor (BDNF), which has been implicated in the pathophysiology and the treatment of several psychiatric disorders (Chen et al. 2001; Shirayama et al. 2002). mTOR is a ubiquitously expressed serine/threonine kinase involved in cell growth, cellular nutrient, and protein stability. A major role of mTOR on synaptic plasticity is the regulation of translation initiation through regulating two key components of the translation initiation, p70s6 kinase and the eukaryotic initiation factors 4E (eIF4E) binding proteins (4E-BPs) (Dufner and Thomas 1999; Klann and Dever 2004). Activation of the mTOR pathway specifically increased phosphorylation of p70s6k, a downstream target of mTOR (Chiang and Abraham 2005; Hoeffer and Klann 2010; Sekulic et al. 2000). Phosphorylated p70s6k further activates rps6, a component of the 40 S ribosomal subunit (Hoeffer and Klann 2010). Therefore, the protein levels of phosphorylated p70s6k and its substrate rps6 are reasonable indicators to reflect mTOR signaling function (Martin et al. 2007; Nathan et al. 2004). Previous postmortem studies proposed that there was a significant reduction in mTOR and the downstream components p70S6K, eukaryotic initiation factors 4B (eIF4B) protein level in PFC in depressed subjects compared to healthy controls (Jernigan et al. 2011), suggesting that deficits in mTOR signaling in the PFC is linked to the pathophysiology of depression.
CsA and FK506 are two potent calcineurin inhibitors used as immunosuppressive agent to prevent transplant rejection. It was recently reported that chronic inhibition of hippocampal CA3 calcineurin induced depressive-like behavior in normal rats and blocked the behavioral effects of antidepressant venlafaxine via the downregulation of mGluR2/3 function (Zhu et al. 2011). Similarly, we found here that chronic blockade of mPFC calcineurin also induced depressive-like phenotype. We also found that chronic mPFC infusion of calcineurin inhibitors CsA or FK506 induced reductions of both phosphorylated p70s6k and rps6, suggesting that calcineurin blockade produced dysregulation of mTOR signaling function. Moreover, the findings that acute CsA or FK506 neither decreased mTOR activity nor altered the depressive-like behavior confirmed our hypothesis that the impairment of mTOR function contributed to the depressive-like behavior induced by calcineurin inhibition. Therefore, we hypothesized that activation of mTOR in the mPFC might affect the depressive-like behavior induced by chronic treatment of calcineurin inhibitors. NMDA can increase mTOR activity through the PI3K–Akt signaling pathway (Hou and Klann 2004). In this study, we infused NMDA before each CsA or FK506 injection into mPFC and found that these behavioral deficits were reversed by NMDA, suggesting that mTOR pathway is involved in the depressive-like symptoms induced by calcineurin inhibition.
In the current study, we also detected the mTOR activity directly after injection of NMDA into mPFC, and we found that administration of NMDA (250 ng per side) significantly increased mTOR activity, which is consistent with an earlier experiment that reported that the same dose of NMDA infused into nucleus accumbens core increased phosphorylated p70s6k and rps6 (Wang et al. 2010). Therefore, it is possible that the result that NMDA abolished the depressive-like performance is due to the activation of mTOR pathway, suggesting that dysfunction of mTOR participated in the process of depressive-like behavior induced by calcineurin inhibition. Activation of mTOR signaling resulting in rapid and sustained elevation of synapse associated proteins and spine number in the mPFC represents a mechanism for the rapid antidepressant actions of ketamine. Additionally, it has been indicated that NMDA receptor antagonist ketamine, a fast-acting antidepressant, increased synapse-associated protein synthesis and spine formation via activation of mTOR pathway in the mPFC in rats (Li et al. 2010), suggesting that mTOR signaling in the mPFC is linked to the antidepressant treatment of ketamine. Our findings demonstrated that pretreatment with rapamycin, a specific inhibitor of mTOR (Hoeffer and Klann 2010), completely prevented the antidepressant behavioral response of ketamine in CsA- or FK506- treated rats. Nonetheless, the molecular mechanism of calcineurin through which to regulate mTOR signaling and downstream targets that was involved in the development of depression remains elusive.
Glutamate system has become the focus of investigation into affective disorders. Increasing evidence suggests that abnormality of the glutamatergic system may contribute to the impairments in synaptic and neural plasticity (Swanson et al. 2005). NMDA receptor function was altered in the frontal cortex of suicide victims compared to controls (Nowak et al. 1995). In addition to ketamine, another NMDA receptor antagonist MK-801 also increased proteins in the mTOR/p70S6K pathway in the rat frontal cortex (Yoon et al. 2008). In the present study, we found that a single injection of ketamine reversed both the anhedonic-like state and desperate behavior induced by mPFC calcineurin inhibition. Chronic calcineurin inhibitor injection into mPFC might induce cell loss and underlie the mechanism of the deficits of behavior. Since chronic calcineurin inhibitor administration decreased mTOR activity, it is interesting to determine whether calcineurin inhibition could affect the impairments of mTOR-mediated plasticity and glia renewal in the mPFC. Our data raise the possibility that disruptions to mTOR function in the mPFC might contribute to the modulation of calcineurin on glial cell, as glial reduction in the mPFC has been associated with depressive-like symptoms in rodents (Banasr and Duman 2008). Both the mTOR inhibitors and calcineurin inhibitor are increasingly used in organ transplantations as immunosuppressive drugs (Budde et al. 2011; Gungor et al. 2011). mTOR regulates the activity of protein phosphatase 2A (PP2A), and these phosphatases can regulate mTOR substrates (Hoeffer and Klann 2010). More evidence is needed to further determine the regulatory role of mTOR kinase on calcineurin activity.
In conclusion, chronic inhibition of mPFC calcineurin activity decreased mTOR activity and resulted in depressive-like behavior. Activation of mTOR normalized the depressive-like behavior induced by chronic treatment of two potent calcineurin inhibitors. Inhibition of mTOR reversed the antidepressant-like effect of ketamine in calcineurin-inhibitor-treated rats. Our findings provide the evidence that mTOR pathway and function have been linked with the expression of depressive-like symptoms and the production of antidepressant response. Critical regulation of calcineurin via the mTOR pathway might achieve promising therapeutic effects in depression and other mood disorders.
References
Aguiar DC, Terzian AL, Guimaraes FS, Moreira FA (2009) Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl) 205:217–225
Bahi A, Mineur YS, Picciotto MR (2009) Blockade of protein phosphatase 2B activity in the amygdala increases anxiety- and depression-like behaviors in mice. Biol Psychiatry 66:1139–1146
Banasr M, Duman RS (2008) Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64:863–870
Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N (1996) Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J 15:658–664
Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377:837–847
Buttini M, Limonta S, Luyten M, Boddeke H (1993) Differential distribution of calcineurin A alpha isoenzyme mRNA’s in rat brain. Naunyn Schmiedebergs Arch Pharmacol 348:679–683
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT (2001) Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50:260–265
Chiang GG, Abraham RT (2005) Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280:25485–25490
de Groen PC, Aksamit AJ, Rakela J, Forbes GS, Krom RA (1987) Central nervous system toxicity after liver transplantation. The role of cyclosporine and cholesterol. N Engl J Med 317:861–866
Drevets WC (2001) Neuroimaging and neuropathological studies of depression: implications for the cognitive–emotional features of mood disorders. Curr Opin Neurobiol 11:240–249
Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109
Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A (2001) Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci 21:4066–4073
Gong R, Park CS, Abbassi NR, Tang SJ (2006) Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem 281:18802–18815
Guertin DA, Sabatini DM (2005) An expanding role for mTOR in cancer. Trends Mol Med 11:353–361
Gungor O, Kircelli F, Carrero JJ, Hur E, Demirci MS, Asci G, Toz H (2011) The effect of immunosuppressive treatment on arterial stiffness and matrix Gla protein levels in renal transplant recipients. Clin Nephrol 75:491–496
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75
Hou L, Klann E (2004) Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24:6352–6361
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779
Juckel G, Mendlin A, Jacobs BL (1999) Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology 21:391–398
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Klann E, Dever TE (2004) Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci 5:931–942
Lenz G, Avruch J (2005) Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem 280:38121-38124
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761
Lian Q, Ladner CJ, Magnuson D, Lee JM (2001) Selective changes of calcineurin (protein phosphatase 2B) activity in Alzheimer’s disease cerebral cortex. Exp Neurol 167:158–165
Lin YH, Liu AH, Xu Y, Tie L, Yu HM, Li XJ (2005) Effect of chronic unpredictable mild stress on brain–pancreas relative protein in rat brain and pancreas. Behav Brain Res 165:63–71
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352
Mansuy IM (2003) Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun 311:1195–1208
Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ (2007) Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem 282:36112–36120
Mayberg HS (1997) Limbic–cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9:471–481
Mitsuhashi S, Shima H, Kikuchi K, Igarashi K, Hatsuse R, Maeda K, Yazawa M, Murayama T, Okuma Y, Nomura Y (2000) Development of an assay method for activities of serine/threonine protein phosphatase type 2B (calcineurin) in crude extracts. Anal Biochem 278:192–197
Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, Zhou H, Smith M, Kimberly D, Glass J (2004) Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res 10:5820–5827
Nowak G, Ordway GA, Paul IA (1995) Alterations in the N-methyl-d-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res 675:157–164
Paxinos G, Watson CR, Emson PC (1980) AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3:129–149
Polli JW, Billingsley ML, Kincaid RL (1991) Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res 63:105–119
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Proud CG (2007) Cell signaling. mTOR, unleashed. Science 318:926–927
Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT (2000) A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 60:3504–3513
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4:131–144
Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L (2010) Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30:12632–12641
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93:358–364
Yoon SC, Seo MS, Kim SH, Jeon WJ, Ahn YM, Kang UG, Kim YS (2008) The effect of MK-801 on mTOR/p70S6K and translation-related proteins in rat frontal cortex. Neurosci Lett 434:23–28
Zhu WL, Shi HS, Wang SJ, Wu P, Ding ZB, Lu L (2011) Hippocampal CA3 calcineurin activity participates in depressive-like behavior in rats. J Neurochem 117:1075–1086
Acknowledgments
This work was supported in part by a grant from the National Natural Science Foundation of China (No. 30870895 to J.L.Y).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, JJ., Zhang, Y., Wang, Y. et al. Inhibition of calcineurin in the prefrontal cortex induced depressive-like behavior through mTOR signaling pathway. Psychopharmacology 225, 361–372 (2013). https://doi.org/10.1007/s00213-012-2823-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2823-9