Abstract
Rationale
Decision-making deficits, measured using the Iowa Gambling Task (IGT), are observed in many psychiatric populations. Additionally, evidence suggests that the environment also influences the development of these same disorders.
Objective
To determine the direct influence of the environment on decision-making by utilizing the rat gambling task (rGT), a risky decision-making test modeled after the IGT.
Methods
Male rats reared in isolation, in pairs, or in an enriched environment were trained on the rGT as adults. During the rGT, animals chose from four different options. The optimal strategy on the rGT and IGT is the same: to favor options associated with smaller immediate rewards and less punishment/loss. Impulsive action is also measured during rGT performance by recording the number of premature responses made, similar to the five-choice serial reaction time task.
Results
Compared to pair-housed rats, isolated and environmentally enriched rats were slower at learning the optimal strategy. However, following training, only isolation-reared rats chose the disadvantageous options more often. Amphetamine altered decision-making on the rGT in socially housed animals, yet isolates were unaffected. Conversely, amphetamine increased premature responding similarly in all groups. This increase was attenuated by prior administration of a dopamine D1 or D2 antagonist; however, the ability of amphetamine to alter decision-making was not blocked by either drug.
Conclusions
Housing environment affects animals’ ability to learn and perform a decision-making task. Additionally, amphetamine’s effect on impulsive action appears to be mediated by the dopaminergic system, whereas its effect on risky decision-making may be mediated by other neurotransmitters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental factors are known to significantly impact the etiology of many psychiatric disorders, including addiction, depression, schizophrenia, pathological gambling, and attention-deficit hyperactivity disorder (Brown 2011; Halperin and Healey 2011; Laviola et al. 2008; Nithianantharajah and Hannan 2006; Solinas et al. 2010; Welte et al. 2004). In particular, it has been suggested that there is an increased prevalence of psychiatric disorders among individuals exposed to a greater number of negative factors (e.g., low socioeconomic status, increased stress levels, poor relationships with peers and family, etc.), whereas a positive environment (e.g., average to high socioeconomic status, lower levels of stress, positive relationships, etc.) may be protective (see Solinas et al. 2010 for a review).
Decision-making deficits, measured using the Iowa Gambling Task (IGT), have also been observed in such disorders (Bechara et al. 2001; Cella et al. 2010; Garon et al. 2006; Goudriaan et al. 2005; Shurman et al. 2005; Smoski et al. 2008). During the IGT, participants choose between decks of cards. With each card drawn, the subject either wins or loses points (Bechara et al. 1994). The optimal strategy is to pick from decks associated with smaller gains, but also smaller losses; however, many patients with these psychiatric disorders choose more frequently from the tempting high-reward decks associated with greater long-term loss. Therefore, although environmental factors may affect psychiatric prognosis, the direct influence of positive/negative environments on decision-making itself remains unclear. Given that decision-making deficits may precede the onset of the full psychiatric syndrome (for e.g., Vitaro et al. 1999), understanding the nature of any such environmental influence may be of great utility.
One obstacle to empirical study of the influence of the environment on cognitive functioning concerns the large amount of variation present in the human population, which is difficult to categorize and control. Animal models incorporating a variety of standardized environmental conditions are therefore crucial to understanding the link between environment and decision-making. Two environmental manipulations that can be employed in a laboratory setting are post-weaning isolation housing and environmental enrichment, both of which have long-term effects on behavior in addition to changes in brain chemistry and morphology (Simpson and Kelly 2011; Solinas et al. 2010).
Environmentally enriched rats are often group-housed in large cages containing objects and toys that promote natural behaviors, such as exploring and burrowing, and provide animals with greater sensory, cognitive, and motor stimulation compared to standard housing conditions (Nithianantharajah and Hannan 2006). This form of enrichment is generally considered beneficial: enriched rats exhibit an anti-depressant-like phenotype on the forced-swim test and outperform controls on several cognitive measures (Simpson and Kelly 2011). In contrast, rats reared in isolated conditions show a depressive phenotype in addition to numerous other maladaptive behaviors such as hyperactivity and an increased startle-response (Brenes et al. 2008; Fone and Porkess 2008; Heidbreder et al. 2000).
The condition in which animals are reared influences their response to psychostimulants such as amphetamine. Amphetamine exerts its actions by increasing the concentration of monoamines in the extracellular space. Specifically, amphetamine drastically increases dopamine levels by inhibiting the dopamine transporter and promoting presynaptic release (Floor and Meng 1996; Jones et al. 1998). Compared to rats reared in isolated conditions, animals reared in an enriched environment exhibited an enhanced locomotor response to an acute dose of amphetamine, yet demonstrated a reduced rate of amphetamine self-administration and a decreased sensitization response to repeated amphetamine injections (Bardo et al. 1995, 2001; Bowling et al. 1993). Furthermore, animals reared in an enriched environment have a greater locomotor response to an acute dose of a dopamine agonist (apomorphine) (Hoffmann et al. 2009). These results suggest that enriched animals may be differentially sensitive to manipulations of the dopaminergic system compared to rats reared in isolation.
We have recently developed a rat gambling task (rGT), based in part on the IGT, to assess similar decision-making processes in laboratory rodents (Zeeb et al. 2009; Zeeb and Winstanley 2011). The optimal strategy on the rGT is the same as the IGT—to select options associated with smaller, immediate gains and less long-term loss while avoiding disadvantageous options associated with greater gains but larger penalties. Previous experiments have shown that acute administration of amphetamine may alter decision-making preferences on the rGT by causing animals to become hypersensitive to loss (Zeeb et al. 2009). Furthermore, amphetamine also increased levels of motor impulsivity during rGT performance (Zeeb et al. 2009). The ability of amphetamine to elevate levels of motor impulsivity has been attributed to increased dopamine activity (Robbins 2002). Therefore, amphetamine’s effect on decision-making preferences may also be due to alterations in dopamine release. Furthermore, given the data reviewed above, different rearing conditions may therefore affect choice behavior on this task.
In the current study, rats were reared from postnatal day (PND) 22 in three different conditions—in an enriched environment, in isolation (single-housed), or pair-housed—then trained on the rGT as adults to determine the effects of different rearing environments on decision-making. We hypothesized that animals raised in isolation may perform worse on the rGT compared to pair-housed rats whereas environmental enrichment may enhance performance and facilitate training on the rGT, as rearing rats in an enriched environment tends to have a beneficial effect compared to rearing rats in isolation. Animals were then challenged with an acute dose of amphetamine and both dopamine D1 and D2 receptor antagonists (SCH 23390 and eticlopride, respectively). We predicted that animals raised in an enriched environment may be more sensitive to the effects of amphetamine on motor control, compared to pair-housed rats, as enrichment tends to enhance the acute effects of amphetamine on locomotion (Bardo et al. 1995, 2001; Bowling et al. 1993). Additionally, in an attempt to block the effects of amphetamine on rGT performance, animals received an injection of either the dopamine D1 or D2 receptor antagonists prior to amphetamine administration.
Methods
Subjects and housing conditions
Subjects
Subjects were 32 male Long-Evans rats. Animals arrived from Charles River Laboratories (St. Constant, QC, Canada) on PND 22. Upon arrival, rats were housed in either an enriched environment (n = 16), pair-housed (n = 8), or isolation-housed (n = 8), for the duration of the experiment. Animals were housed under a 12-h reverse light cycle (lights off at 8:00 am) in a temperature-controlled colony room. All rats were located in the same room but only directly interacted with the animal(s) they were housed with. Rats were handled 5 to 6 days per week in addition to bi-weekly cage changing. Water was available ad libitum. Rats were given free access to food until 1 week prior to training on the rGT (PND 64, 300–375 g), at which point animals were restricted to 14 g of standard laboratory rat chow per day (approximately 85 % of their free-feeding weight), available immediately after rGT testing. Pair- and isolation-housed rats were fed in their home cages, whereas animals housed in the enriched environment were fed individually in standard cages before being placed back into the enrichment cage. Behavioral testing took place between 12:00 pm and 5:00 pm, 5 to 6 days per week. Following completion of all behavioral testing, animals were sacrificed by increasing exposure to carbon dioxide. Experimental protocols were approved by the Animal Care Committee of the University of British Columbia and were in accordance with the Canadian Council of Animal Care.
Rearing conditions
All 16 rats in the environmental enrichment group were housed together in a custom-built wire mesh cage (40 in. × 60 in. × 30 in.; Corners Limited, Kalamazoo, MI, USA) with a custom-built solid Plexiglas bottom filled with sawdust bedding. Inside the cage, animals had access to multiple water bottles, toys, running wheels (Wodent Wheels, Transoniq, USA), buckets, PVC tubes of various sizes and shapes, cardboard boxes, paper towels, climbing chains, and moveable shelves. The number, location, and type of all above mentioned objects were rearranged and/or exchanged every 1–2 days. Pair-housed or isolation-housed rats were housed in a standard Plexiglas cage (19 in. × 10.5 in. × 8 in.), which contained sawdust bedding, a small PVC tube, and a few paper towels.
Rat gambling task
Apparatus
Detailed descriptions of the testing chambers and training protocols have been provided in previous reports (Zeeb et al. 2009; Zeeb and Winstanley 2011). Briefly, the rGT took place in standard five-hole operant chambers contained within a ventilated and sound-attenuating box (Med Associates, St. Albans, VT, USA), with an array of five response holes located on one wall of the chamber and a food tray on the opposite wall, 2 cm above a metal bar floor. A stimulus light was located within each hole and the food tray, and sucrose pellets (45 mg, Bioserv, Frenchtown, NJ, USA) could be delivered into the food tray from an external pellet dispenser. Nose poke responses into the response holes or food tray were detected by a horizontal infrared beam. The entire chamber could be illuminated by a house light located at the top of the chamber on same wall as the food tray. Chambers were controlled by software written in Med PC by C.A.W., running on an IBM-compatible computer.
Training
Animals first received two 25-min habituation sessions, during which the chambers were turned on and sucrose pellets were placed in the response holes and food tray. Rats were then trained to make a nose poke response within 10 s to an illuminated response hole for a sucrose pellet reward. Once rats were completing these sessions with at least 80 % of trials correct and less than 20 % omissions (seven sessions), animals received seven forced choice sessions. Forced choice sessions were identical to the rGT except only one option was presented on each trial. This training ensured that all animals had equal experience with all four options to prevent a simple side bias from developing. Animals were then tested on the rGT for the duration of the experiment.
rGT
The trial structure and reinforcement schedule of the rGT have been previously described (Zeeb et al. 2009; Zeeb and Winstanley 2011) and a diagram is provided in Fig. 1. Each session was 30 min and animals were trained once daily. A trial was initiated when an animal made a nose poke response into the illuminated food tray. The tray light was then extinguished, initiating a 5-s inter-trial interval (ITI) period. A nose poke response into any of the holes at the array during the ITI was classified as a premature response—a measure of impulsive action—and punished with the illumination of the house light for 5 s after which the tray light was illuminated, allowing the rat to start another trial. This measurement is identical to the premature responses recorded on the five-choice serial reaction time task (5CSRTT) (see Robbins 2002, for a review of the 5CSRTT). Following the ITI, four holes (holes 1, 2, 4, and 5) were illuminated, and the animal had 10 s to choose any one of these options by making a nose poke response into the corresponding hole. A nose poke response into any of these holes extinguished all stimulus lights. If the trial was rewarded, the tray light turned on and the corresponding number of sucrose pellets was immediately delivered. Collection of this reward initiated the next trial. However, if the trial was not rewarded, the stimulus light within the hole that the animal chose flashed at 0.5 Hz for the duration of the corresponding timeout period. Responses made at the array or food tray during the timeout period or following reward delivery were recorded as perseverative responses. If the animal failed to make a response in 10 s, all stimulus lights were extinguished and the tray light was turned on, allowing the animal to start another trial.
Schematic diagram of the rGT. The magnitude of the reward or duration of the timeout period is indicated beside each option. The p values refer to the probability of a trial resulting in reward or a punishing timeout period. The maximum numbers of pellets that could be obtained if an animal chose a single option exclusively within a single session, assuming each trial lasted 5 s, are listed at the bottom of the diagram, indicating that the two-pellet option, P2, is the best option. A nose poke response in the illuminated food tray extinguished the tray light and initiated a new trial. After an inter-trial interval (ITI) of 5 s, four stimulus lights were turned on (holes 1, 2, 4, and 5) and the animal was required to respond in one of these holes within 10 s. If the animal was rewarded, all stimulus lights were extinguished and the animal received the corresponding number of pellets in the now illuminated food tray. If the animal was punished, the stimulus light in the corresponding hole flashed at a frequency of 0.5 Hz for the duration of the punishing timeout and all other lights were extinguished. At the end of the punishment period, the tray light was turned on and the animal could initiate a new trial. Failure to respond at the illuminated holes resulted in an omission. A response during the ITI was classified as a premature response and punished by a 5-s timeout during which the house light was turned on. Figure is modified from Zeeb et al. (2009) and Zeeb and Winstanley (2011)
Two versions of the rGT were used which differed only in the spatial location of the options and were counterbalanced across all animals. In version A, the order of the options presented from left to right were the one-pellet option (P1), the four-pellet option (P4), the two-pellet option (P2), and the three-pellet option (P3). In version B, the order of the options from left to right was P4, P1, P3, and P2. Similar to previous studies (Zeeb et al. 2009; Zeeb and Winstanley 2011), the rGT version used did not differentially affect decision-making during task acquisition or once behavior had stabilized.
The optimal choice in the rGT is P2, as this option results in the most reward earned per unit time due to the design of the reinforcement schedules (Fig. 1; Zeeb et al. 2009). The next best option is P1, and the two disadvantageous options are P3 and P4, due to the lower probability of receiving reward and the longer timeout periods incurred.
Pharmacological manipulations
All drugs were purchased from Sigma-Aldrich (Oakville, ON, Canada) and dissolved in sterile 0.9 % saline in a volume of 1 mL/kg. Doses were calculated as the salt and administered intraperitoneally. Once animals showed a statistically stable pattern of choice for at least three sessions on the rGT (i.e., where p > 0.05 in a repeated measures analysis of variance (ANOVA) for choice × session), animals were subjected to the following pharmacological challenges prior to rGT testing: d-amphetamine (0, 0.3, 1.0, and 1.5 mg/kg); the D2 receptor antagonist eticlopride hydrochloride (0, 0.003, 0.01, and 0.03 mg/kg); and the D1 receptor antagonist SCH 23390 (0, 0.001, 0.003, and 0.01 mg/kg).
Drug testing occurred in a 3-day cycle, beginning with an rGT training day, followed by a drug-testing day in which animals received an injection of either saline or drug. Following an injection, rats were placed individually in cages similar to the standard home cages for 10 min prior to rGT testing. On the third day, animals remained in their home cages. To determine whether administration of a dopamine D1 or D2 receptor antagonist would block the effects of amphetamine, the antagonist was administered 10 min prior to an injection of amphetamine. Ten minutes following the amphetamine injection, animals were tested on the rGT. The middle dose of amphetamine (1.0 mg/kg) was used as this dose had the largest effect on choice and premature responses across all groups. The 0.003 mg/kg dose of SCH 23390 and eticlopride was used, as this dose was the highest tested that did not affect the number of trials completed or premature responses in any group. Although the 0.003 mg/kg dose of eticlopride had a small effect on choice in the enriched group, the next highest dose (0.01 mg/kg) significantly affected choice in the pair-housed group; therefore, the 0.003 mg/kg dose was used.
All drug doses and saline injections for amphetamine, SCH 23390, and the two higher doses of eticlopride were administered according to a Latin square design. As both these doses of eticlopride were unexpectedly behaviorally active, the lowest dose of eticlopride was administered on a single drug-testing day following the Latin square in order to try and identify a behaviorally silent dose. The order of drug administration was amphetamine, eticlopride, amphetamine and eticlopride co-administration, SCH 23390, then amphetamine and SCH 23390 co-administration. A minimum of 1 week occurred between administrations of different compounds, during which animals were tested on the rGT but no drugs were administered.
Data analyses
The percent choice of each option, the percentage of premature responses made, the percentage of omissions, total number of trials completed, choice and collection latency, and perseverative responses made were also calculated and analyzed according to previously described methods (Zeeb et al. 2009). Variables calculated as a percentage (choice, premature responses, and omissions) were subjected to an arcsine transformation prior to statistical analysis to avoid a ceiling effect (McDonald 2009). ANOVAs and t tests were conducted using SYSTAT for Windows (version 12.00.08; SSI).
To analyze behavior during acquisition of the task, training sessions were divided into bouts of five sessions for the first 20 free-choice sessions (Zeeb and Winstanley 2011). Data from the last three stable sessions were analyzed to assess the animals’ baseline performance on the rGT. Data were analyzed using a repeated measures ANOVA with choice (four levels; P1–P4) and/or session/day (three or five levels) as within-subject factors and group (pair-housed, isolation-housed, and/or enriched) as a between-subjects factor. Additionally, using data from the last three stable training sessions, the sum of the best two options (P1 and P2) and the two worse options (P3 and P4) was determined. The difference between these two variables was then calculated to provide an overall performance score (see Zeeb and Winstanley 2011). Similar to studies on human subjects using the IGT (e.g., Denburg et al. 2005), a binomial test (McDonald 2009) was performed on the percent choice of the best and worst options for each subject to isolate subgroups of impaired or unimpaired rats that existed within each housing group. A very small group of rats were found to have a score significantly below 0 (p ≤ 0.05, one-tailed) (four rats from the enriched group, two rats from the pair-housed group, and one rat from the isolation-reared group; four rats were tested on version A and three rats were tested on version B). These rats were excluded from further analysis as the number of subjects within each housing group was too small to form a separate impaired subgroup. Additionally, only comparisons between the pair-housed group and the other two housing groups were conducted (i.e., a direct comparison between the isolation-reared rats and environmental enrichment group was not made). This method was employed as the pair-housed rats served as the appropriate control group in the present experiment, as rearing animals in isolated conditions has been shown to result in a number of negative behavioral consequences and may therefore not be a true control (see Simpson and Kelly 2011 for a discussion of this issue).
To determine whether the effects of amphetamine on the rGT were blocked by prior administration of either dopamine antagonist for each group, a repeated measures ANOVA was performed with antagonist (two levels; present or absent), dose (two levels; saline or amphetamine), and choice (four levels), if necessary, as within-subject factors. If the outcome of an ANOVA was significant, further ANOVA or post hoc analyses were conducted. To compare data within a group, paired sample t tests were used, whereas independent sample t tests were used to compare data between groups. A significance level of p ≤ 0.05 was used for all analysis.
Results
Baseline performance
Choice behavior
Following seven sessions of forced choice training, animals in all three groups initially showed a preference for P2, the optimal choice, which increased with further training—a pattern similar to the previous results (Zeeb et al. 2009; Zeeb and Winstanley 2011). During rGT training, all rats developed a significant choice preference over time (Fig. 2; all rats, days 1–20—day: F 19, 36 = 4.691, p < 0.001; choice: F 3, 57 = 37.834, p < 0.001; day × choice: F 57, 1,083 = 5.313, p < 0.001). However, this effect was most pronounced in the pair-housed group (Fig. 2). Compared to pair-housed animals, rats reared in the enriched environment chose P2 significantly less during the initial training days (P2, day × housing—days 1–5: F 4, 56 = 4.712, p = 0.002). The initial reduction in choice of P2 in the enriched group was also accompanied by a small increased preference for P4, the worst option (P4, day × housing—days 1–5: F 4, 56 = 5.156, p = 0.001). A similar decreased preference for the optimal option was also observed in animals reared in isolation, compared to pair-housed rats (P2, day × housing—days 1–5: F 4, 36 = 3.142, p = 0.03).
Enriched and isolation-reared rats are similarly impaired in acquisition of the rGT. a Within the first five rGT sessions, pair-housed rats drastically increase choice of P2 and decrease choice of P1. Both isolation-reared (b) and environmentally enriched (c) rats are slower to learn the optimal strategy, choosing the optimal option, P2, less often during the first five rGT sessions. Data are represented as the mean percent choice for each option (±SEM)
No further significant interactions between day and housing for the isolation-reared or enriched rats compared to the pair-housed group were observed during task acquisition. Animals were trained on the rGT for a total of 24 sessions prior to pharmacological challenges. Data from the last three stable training sessions were analyzed as a measure of baseline performance.
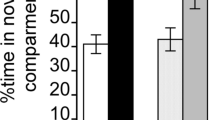
Once the task had been acquired, animals reared in isolation, compared to pair-housed rats, tended to perform worse on the rGT. Although a main effect of choice × housing condition just failed to reach significance, further analysis demonstrated that animals reared in isolation chose P2, the most advantageous option, significantly less than the pair-housed rats (Fig. 3; choice × housing: F 3, 27 = 2.628, p = 0.07; P2: t(11) = −2.318, p = 0.05). Furthermore, an increased choice of the disadvantageous options by the isolation-reared rats was also evident in the significantly smaller difference score compared to pair-housed animals, again indicating that animals reared in isolation were not making optimal choices on the rGT (Fig. 3; t(7) = −2.731, p = 0.03). In contrast, environmentally enriched rats did not differ significantly from pair-housed rats in their choice patterns and preference for the advantageous options over the disadvantageous options following sufficient training (Fig. 3). In sum, although both environmental enrichment and isolation-rearing caused animals to choose the best option less often during the initial training stages, only rats housed in isolation chose the advantageous options less once the task had been acquired.
Baseline performance of the rGT. a Following sufficient training on the rGT, enriched rats did not differ from pair-housed animals in choice of each option. However, animals reared in isolation chose the best option, P2, significantly less than the pair-housed control rats. b The decision-making deficit in isolation-reared rats is also reflected in a significantly lower difference score compared to pair-housed controls. c Compared to pair-housed rats, isolated rats made less premature responses whereas enriched animals made more premature responses, although this was not statistically significant. Data are shown as the mean (±SEM) of the last three sessions prior to pharmacological challenges. *p ≤ 0.05 indicates a significant difference compared to pair-housed rats according to a repeated measures ANOVA for both choice and premature responses, whereas the difference score was analyzed using an independent sample t test
Other behavioral measurements
Visual inspection of the data indicated that enriched animals made more premature responses than pair-housed rats, whereas animals reared in isolation exhibited the lowest level of impulsive action; however, these observations were not significant (Fig. 3). Measurements for the other variables analyzed during rGT testing are presented in Table 1. Similar to previous studies (Zeeb et al. 2009; Zeeb and Winstanley 2011), omissions and perseverative responses remained relatively low in pair-housed rats, animals completed about 100 trials within each session, and premature responses were just under 20 %. Both isolation-reared and enriched animals received fewer sugar pellet rewards per session, although this difference only reached significance in the enriched group. A reduction in the total reward earned may have been influenced by choice preference as well as the number of trials completed. Both enriched and isolation-reared rats completed less trials than pair-housed rats, although this difference was not itself significant. Furthermore, there were no differences between groups for collection latency, omissions, or in the number of perseverative responses made either during punishment or following a rewarded trial. Additionally, isolation-reared and environmentally enriched rats took longer to make a choice compared to pair-housed rats, but these differences were also not significant. Therefore, although housing environment affected the animals’ decision-making patterns during training and performance of the rGT, there were little differences between housing groups with regards to the other variables measured by the rGT.
Effects of amphetamine on rGT performance
Choice behavior
An acute dose of amphetamine disrupted animals’ ability to perform the rGT optimally (dose: F 3, 57 = 3.369, p = 0.02; dose × choice: F 9, 171 = 8.094, p < 0.001). The ability for amphetamine to alter decision-making on the rGT was dependent on housing condition (dose × choice × housing: F 18, 171 = 2.117, p = 0.007). Amphetamine significantly impaired decision-making only in the socially housed rats (both pair-housed and enriched groups), but not in the isolation-reared animals (Fig. 4; dose × choice—pair-housed: F 9, 36 = 10.136, p < 0.001; enriched: F 9, 90 = 2.343, p = 0.02).
Effects of amphetamine on rGT performance. a Pair-housed rats decrease choice of P2 in response to all doses of amphetamine and increased choice of P1 following administration of the 1.0 and 1.5 mg/kg dose of amphetamine. A small but significant increased choice of P4 was also observed following the 1.5 mg/kg dose. c Isolation-rearing blocked the ability of amphetamine to alter choice preference. e Enriched rats chose P2 less and P4 more following the 1.0 mg/kg dose. A small, but significant, increase in P3 was observed following the 0.3 or 1.5 mg/kg dose of amphetamine. However, enriched rats did not increase in choice of P1 following any dose of amphetamine. A similar increase in premature responding following all doses of amphetamine was observed in the pair-housed (b), isolation-reared (d), and enriched (f) rats. Data are shown as the mean (±SEM). *p ≤ 0.05 indicates a significant difference compared to saline according to a paired sample t test
Specifically, pair-housed rats decreased their choice of the optimal option, P2, and increased in choice of P1, the option which offers the smallest punishment duration and the highest frequency of reward, similar to previous results (Zeeb et al. 2009) (Fig. 4; dose × choice—saline vs 0.3 mg/kg: F 3, 12 = 7.414, p = 0.005; saline vs 1.0 mg/kg: F 3, 12 = 11.218, p < 0.001; saline vs 1.5 mg/kg: F 3, 12 = 18.272, p < 0.001). It is interesting to note that, similar to previous results (Zeeb et al. 2009), there was also a very small, but statistically significant, increase in choice of P4 in the pair-housed rats when challenged with the 1.5 mg/kg dose of amphetamine.
Animals raised in an enriched environment also decreased their choice of P2 when challenged with an acute dose of amphetamine, yet these rats did not show a significant increase in P1. Instead, enriched rats showed a small, but significant, increase in the disadvantageous options (Fig. 4; dose × choice—saline vs 0.3 mg/kg: F 3, 30 = 3.322, p = 0.03; saline vs 1.0 mg/kg: F 3, 30 = 4.467, p = 0.01; saline vs 1.5 mg/kg: F 3, 30 = 2.906, p = 0.05).
Other behavioral measurements
All groups of rats increased the number of premature responses made when challenged with any dose of amphetamine, again replicating our previous results (Zeeb et al. 2009). This increase in impulsive action in response to amphetamine was independent of housing condition (Fig. 4; dose: F 3, 57 = 16.408, p < 0.001; dose × housing: F 6, 37 = 0.968, not significant [NS]). Therefore, although isolation housing blocked amphetamine’s ability to alter decision-making, amphetamine resulted in a similar increase in impulsive action for all groups.
Statistical analysis and values for the other rGT variables are presented in Supplementary Information (Table S1). Amphetamine decreased the number of trials completed following all doses in both the pair-housed and enriched groups. A similar effect was observed in the isolation reared rats. However, omissions and the latency to collect reward were not affected by amphetamine at any dose. Therefore, similar to Zeeb et al. (2009), the decreased number of trials completed may be somewhat attributed to a loss in playing time cause by high levels of premature responding.
In summary, isolation-rearing blocked the ability of amphetamine to alter choice patterns on the rGT. However, rearing animals in an enriched environment altered amphetamine’s actions, causing animals to demonstrate an increased choice of the disadvantageous “high-risk high-reward” options, rather than P1, following amphetamine administration. Despite these differences, amphetamine similarly altered the other variables measured during rGT performance, notably increasing the number of premature responses made in all groups.
Effects of the D1 receptor antagonist SCH 23390 on rGT performance
Choice behavior
Similar to previous results (Zeeb et al. 2009), SCH 23390 did not alter decision-making patterns on the rGT in any group. Although there was a significant interaction between dose and choice when all groups were combined, there were no significant effects of SCH 23390 on choice when each group was analyzed independently (Fig. 5).
Effects of dopamine antagonists on choice preferences. SCH 23390 failed to alter choice in the pair-housed (a), isolated (c), or enriched (e) groups. b Eticlopride increased choice of P2 in the pair-housed rats following the 0.01 mg/kg dose. d An increased preference for P1 was observed in the isolation-reared rats following administration of the highest dose of eticlopride, whereas f a decreased choice of P1 and P4 was observed in the enriched rats following administration of the lowest dose. Data are shown as the mean (±SEM). *p ≤ 0.05 indicates a significant difference compared to saline according to a paired sample t test
Other behavioral measurements
Data and results from the statistical analyses for the other variables analyzed are presented in Supplementary Information (Table S2). A general decrease in motor output was observed following the highest dose of SCH 23390 administered, again replicating previous results (Zeeb et al. 2009), in that the number of trials completed decreased, and the number of omissions increased as did all response latencies across the three groups. Premature responses also significantly decreased in response to the highest dose of SCH 23390, but only in the pair-housed and enriched group, again indicative of decreased motor output. A lack of effect of this drug on premature responding for isolation-reared rats can likely be attributed to the fact that premature responses were already quite low in this group. In sum, housing environment did not significantly alter the actions of SCH 23390 on rGT performance.
Effects of the D2 receptor antagonist eticlopride on rGT performance
Choice behavior
In line with previous results (Zeeb et al. 2009), the 0.01 mg/kg dose of eticlopride improved performance on the rGT by increasing choice of P2, the optimal option. However, this effect was only observed in pair-housed rats (Fig. 5; dose × choice—saline vs 0.01 mg/kg: F 3, 12 = 4.403, p = 0.03). Animals reared in isolation showed a small, but significant, increase in P1—the option associated with the shortest punishment time and smallest, but most frequent, reward delivery—in response to the largest dose of eticlopride (Fig. 5; dose × choice—saline vs 0.03 mg/kg: F 3, 15 = 3.701, p = 0.04). Similarly, animals reared in an enriched environment exhibited a minor, but significant, decrease in choice of P1 and P4, the worst option, in response to the lowest dose of eticlopride (Fig. 5; dose × choice—saline vs 0.003 mg/kg: F 3, 30 = 5.827, p = 0.003). To summarize, eticlopride improved rGT performance following administration of the 0.01 mg/kg dose in the pair-housed group, but only altered choice patterns for the isolated group following administration of the highest dose (increase choice of P1) and failed to largely change decision-making preferences in the enriched group.
Other behavioral measurements
Data and statistical analyses for the other rGT variables in response to eticlopride are present in Supplementary Information (Table S3). The highest dose of eticlopride increased omissions and increased the latency to choose an option, which may indicate a general decrease in motor output. Furthermore, this dose of eticlopride also decreased the number of trials completed in both the pair-housed and isolated rats and increased the latency to collect reward in the pair-housed group. Additionally, there was a significant decrease in premature responses in the enriched group following administration of the 0.01 mg/kg and 0.03 mg/kg doses of eticlopride and a strong trend for a decreased number of premature responses in response to the highest dose of eticlopride for the pair-housed rats. A decrease in the number of perseverative responses during the punishment period in the enriched and isolated groups was observed. In contrast, there was no effect of eticlopride on the number of perseverative responses following reward delivery. Therefore, similar to the effects of SCH 23390, housing environment did not largely alter the actions of eticlopride on the other variables measured during rGT performance.
Effects of amphetamine and dopamine antagonist co-administration
Choice behavior
The ability of amphetamine to increase choice of P1 and decrease choice of P2 in the pair-housed rats was not blocked by prior administration of either the dopamine D1 or D2 receptor antagonists (Fig. 6). Likewise, amphetamine still decreased choice of P2 in the environmentally enriched rats when either SCH 23390 or eticlopride was administered prior to amphetamine (Fig. 6). Furthermore, as there was no significant effect of amphetamine on choice in the isolated group, there was also no effect of prior administration of either the D1 or D2 receptor antagonists on decision-making in this group (Fig. 6).
Prior administration of a dopamine antagonist blocks the effects of amphetamine on impulsive action, but not decision-making. Prior administration of a dopamine D1 or D2 receptor antagonist failed to block the ability of amphetamine to alter decision-making in the pair-housed (a) and enriched group (e), and no effect was observed in the isolation-reared rats (c). In contrast, both antagonists similarly blocked the ability of amphetamine to increase premature responding in the pair-housed (b), isolated (d), and enriched (f) groups. Data are shown as the mean (±SEM)
Other behavioral measurements
Prior administration of either the D1 or D2 receptor antagonists blocked amphetamine’s ability to increase premature responses similarly in all groups (Fig. 6; SCH 23390 co-administration—dose × antagonist: F 1, 19 = 15.251, p = 0.001; dose × antagonist × housing: F 2, 19 = 0.440, NS; eticlopride co-administration—dose × antagonist: F 1, 19 = 5.763, p = 0.03; dose × antagonist × housing: F 2, 19 = 0.087, NS).
Values and statistical analyses for the other variables analyzed are provided in Supplementary Information (Table S4). Both the dopamine D1 and D2 receptor antagonists were able to significantly block the ability of amphetamine to decrease the number of trials completed and amount of reward received in the enriched group. Interestingly, only the dopamine D1 receptor antagonists was able to block the effects of amphetamine on trials completed and pellets received for the isolation-reared rats. Although prior administration of both antagonists increased the number of trials completed in the pair-housed group, this observation was not significant.
Discussion
These findings illustrate that different rearing environments affect decision-making processes and motor impulsivity, measured concurrently using the rGT. Compared to pair-housed rats, animals reared in an enriched environment or in isolation were slower to learn the optimal strategy; however, only isolation-reared rats chose the advantageous options less once the task had been acquired. Although there were some minor and inconsistent effects of eticlopride in the enriched or isolation-reared groups, pair-housed rats chose the best option more often when the dopamine D2 receptor antagonist was administered, similar to previous results (Zeeb et al. 2009).
Furthermore, isolation-rearing blocked amphetamine’s ability to alter decision-making on the rGT, yet amphetamine similarly increased premature responding in all rats. Although the effects of amphetamine on decision-making were not affected by prior administration of a dopamine D1 or D2 receptor antagonist, pretreatment with either antagonist blocked the ability of amphetamine to increase impulsive action. Therefore, amphetamine’s effects on premature responding appear to be mediated by the dopamine system, in accordance with previous findings using the 5CSRTT (Robbins 2002), whereas its ability to alter decision-making on the rGT may be mediated by another neurotransmitter.
Housing environment differentially alters rGT acquisition and performance
During task acquisition, rats reared in an enriched environment or in isolation were impaired at determining which of the two advantageous options (P1 or P2) was best. However, with further training, environmentally enriched rats did not differ from pair-housed rats, yet isolated animals chose the disadvantageous more often. Therefore, isolation-reared rats were tempted by larger rewards, despite the greater loss associated with these options.
Insight into the nature of these impairments may be obtained by comparing our results to studies employing other paradigms. Rats reared in isolated conditions are impaired on simpler cognitive tasks, such as reversal learning and other tests of behavioral flexibility (Jones et al. 1991; Krech et al. 1962; Schrijver and Würbel 2001). Likewise, patients with damage to the ventromedial prefrontal cortex are impaired on reversal learning tasks and performance of the IGT (Bechara et al. 1994, 1999; Fellows and Farah 2003; Rolls et al. 1994). The inability of subjects with ventromedial prefrontal cortex lesions to optimally perform the IGT may in fact be caused by a reversal learning deficit, leading to an inability to overcome an initial preference for the disadvantageous decks (Fellows and Farah 2005). Additionally, we have shown that rats with orbitofrontal cortex damage acquire the optimal strategy on the rGT at a slower rate, which may reflect an inability to appropriately learn contingencies of the task during the first few training sessions (Zeeb and Winstanley 2011).
The delay-discounting task, in which subjects choose between a small reward delivered immediately or a larger reward delivered after a delay, is another laboratory test used to assess decision-making processes (Evenden and Ryan 1996). In such a task, isolation-reared rats chose the larger reward more often despite the greater cost (delay) associated with this option (Hellemans et al. 2005). Together with the findings from the present study, these results suggest that isolation-reared rats may be biased toward options associated with greater reward magnitude despite their adverse consequences, potentially due to a lack of flexibility when learning reinforcement contingencies.
In contrast, environmentally enriched rats initially preferred the smaller, sooner reward over a larger, delayed reward on a delay-discounting task, although these rats did not differ from pair-housed animals once performance stabilized (Hellemans et al. 2005). However, using an adjusting delay task, in which the delay to the large reward is increased with each selection of the large reward option, animals reared in an enriched environment were less impulsive (i.e., chose the large reward more often) compared to isolation-reared rats (Perry et al. 2008). Although these results are somewhat contradictory, it is worth noting that the study by Perry et al. (2008) failed to include a socially housed control group (such as pair-housed rats reared in standard conditions), which may confound the interpretation of these results (see Simpson and Kelly 2011). Furthermore, the number of training sessions for the adjusting delay procedure was substantially less than the number of sessions in the study by Hellemans et al. (2005) that utilized a delay-discounting task during which the delay to the large reward is systematically increased within a session.
Additionally, animals reared in an enriched environment demonstrate a decreased sensitivity to sucrose reward (van der Harst et al. 2003). Therefore, in the present study, enriched rats may have been unable to distinguish between the two best options due to altered reward processing mechanisms. However, with further exposure to the task contingencies, choice preferences of the enriched rats did not differ from control subjects. Hence, enriched animals may have overcome their initial deficit in reward processing as training progressed. A decreased sensitivity to reward may have emerged in the environmental enrichment group due to constant stimulation present within the enriched environment, including new toys, multiple animals to interact with, and different environments to explore; however, this hypothesis is purely speculative and has not been empirically tested.
Future studies employing the environmental enrichment or isolation-rearing paradigms may want to consider additional control groups of rats to determine which facets of the environment are contributing to alterations in behavior. Substantial variation exists across different laboratories employing environmental enrichment paradigms (see Simpson and Kelly 2011 for a review); therefore, it may be worth considering a specific control group for cage size and/or the number of objects or toys present within the enrichment cage. In the present study, the enriched cage differed from the pair-housed environment on a number of levels, whereas the pair- and isolation-reared rats were housed in identical cages. To control for some of the variation in the environment, it would be interesting to house a group of animals in the same cage used for the environmental enrichment group, but provide no additional objects or toys and maintain an unchanging environment throughout the experiment. It could then be determined whether social housing or the novelty present within the environment primarily contributes to the behavioral changes observed.
Housing environment alters the effects of dopamine antagonists on rGT performance
Similar to previous results (Zeeb et al. 2009), acute administration of a dopamine D1 receptor antagonist did not affect decision-making on the rGT, and a dopamine D2 antagonist promoted choice of the optimal strategy. However, the effect of the dopamine D2 antagonist was only observed in the pair-housed group. These results suggest that different rearing conditions may have altered the function or expression of dopamine D2 receptors.
We were unable to find any previous research on how environmental enrichment alters dopamine D2 receptor function or expression. Therefore, although we can hypothesize that animals reared in an enriched environment may have a reduced sensitivity to dopamine D2 receptor antagonists, the reason for this is unclear. Studies aiming to clarify the effect of isolation-rearing on dopamine D2 receptors have reported mixed results (see Djouma et al. 2006; Bardo and Hammer 1991; King et al. 2009; Malone et al. 2008). Interestingly, animals in studies that found increases in dopamine D2 receptor density were exposed to behavioral manipulations or handled extensively for vehicle or drug injections (Djouma et al. 2006; King et al. 2009). Experience on cognitive tasks has been shown to alter receptor expression (for, e.g., Hellemans et al. 2005). It can therefore be hypothesized that behavioral testing introduced or unmasked differences in receptor expression in the current study. In sum, a better understanding of the effects of isolation-rearing and environmental enrichment on dopamine receptor availability and function following training on cognitive tasks is required before any further conclusions can be drawn.
Dopamine antagonists block the effects of amphetamine on impulsive action
Amphetamine increases impulsive action, as measured by the number of premature responses made on the 5CSRTT (for e.g., Cole and Robbins 1987; Harrison et al. 1997; Pattij et al. 2007). Additionally, in line with previously reported results (Zeeb et al. 2009), amphetamine increased this measure of impulsive action on the rGT. Similar to the 5CSRTT (Robbins 2002), the effect of amphetamine on motor impulsivity can be likewise attributed to increased dopamine activity, as prior administration of a dopamine D1 or D2 antagonist similarly blocked amphetamine’s ability to increase premature responding in all groups.
It has previously been shown that isolation-reared rats also exhibited a mild reduction in motor impulsivity (Dalley et al. 2002), similar to the effects observed in the present study. However in the latter study, amphetamine did not increase premature responding in isolation-reared rats, in contrast to the results presented here. Amphetamine may have had different behavioral effects in the two experiments due to differences in the route of administration. In the study by Dalley et al. (2002), amphetamine was administered intravenously at 15 min intervals. The behavioral effects of intravenous injections appear to be smaller compared to systemic administration (for e.g., Cole and Robbins 1987). Hence, a direct comparison between amphetamine’s effects on motor impulsivity in the current study and the study by Dalley et al. (2002) may not be appropriate.
Dopamine antagonists do not block the effects of amphetamine on decision-making
In pair-housed rats, amphetamine decreased optimal choice and increased choice of P1—associated with the highest frequency of reward and the least amount of punishment—similar to previous results (Zeeb et al. 2009). Interestingly, amphetamine also increased choice of an option associated with less punishment (foot-shock) on a risky decision-making task (Simon et al. 2009). Therefore, amphetamine may cause animals to be more sensitive to punishment or loss. However, although amphetamine disrupted decision-making in enriched rats by decreasing their choice of P2, these rats also increased their choice of the disadvantageous options (P3 or P4). As previously mentioned, animals raised in an enriched environment may be more sensitive to an acute dose of amphetamine (Bardo et al. 1995; Bowling et al. 1993). Although this effect is attributed to increased dopamine release, the release of dopamine caused by amphetamine administration does not appear to contribute to decision-making processes on the rGT, at least with regards to activity at either the dopamine D1 or D2 receptors.
In addition to facilitating dopamine release, amphetamine also increases the levels of other monoamines, such as serotonin (5-HT) and norepinephrine (Floor and Meng 1996; Jones et al. 1998; Kuczenski and Segal 1989; Parada et al. 1988; Rothman et al. 2001). It has been hypothesized that 5-HT plays a significant role in the processing of punishment signals (Cools et al. 2008; Robinson et al. 2012). Interestingly, acute administration of a 5-HT1A receptor agonist generally impaired animals’ decision-making ability, increasing rats’ choice of both a more conservative option (P1) and a more “risky” option (P3) (Zeeb et al. 2009). Additionally, environmental enrichment increased the density of 5-HT1A receptors in the frontal cortex of rats performing a delay-discounting task (Hellemans et al. 2005). Therefore, alterations in the 5-HT system may have contributed to amphetamine’s ability to increase choice of the disadvantageous options in this group.
Conversely, rearing animals in isolation blocked amphetamine’s ability to alter decision-making, which may also be due to alterations within the 5-HT system. Rats reared in isolated conditions exhibited a reduction in 5-HT release in the medial prefrontal cortex following intravenous amphetamine administration (Dalley et al. 2002) and demonstrated decreased forebrain 5-HT release and increased turnover (Bickerdike et al. 1993; Brenes et al. 2008). Additionally, compared to pair-housed controls, isolated rats also have a reduced density of 5-HT axons, specifically within the basolateral amygdala (Kuramochi and Nakamura 2009), an area known to be involved in modulating decision-making on the IGT (Bechara et al. 1999) and rGT (Zeeb and Winstanley 2011).
Relationship between impulsive action and risky decision-making
Recent studies have suggested that patients with pathological gambling show elevated levels of motor impulsivity (see Verdejo-Garcia et al. 2008 for a review). Although studies comparing baseline levels of impulsive action and delay-discounting in rodents or humans failed to find a correlation between these two measures (Winstanley et al. 2004; Crean et al. 2000), animals selected for high levels of motor impulsivity also demonstrate increased rates of delay-discounting (Robinson et al. 2009). These findings suggest that within the normal population, different forms of impulsivity may not correlate within individuals, but in extreme conditions—such as pathological gambling or the animals selected in the study by Robinson et al. (2009)—highly impulsive individuals may exhibit multiple forms of impulse control deficits.
In the current study, both motor impulsivity and decision-making processes were measured concurrently using the rGT (Zeeb et al. 2009). Here, we showed that rearing animals in distinct housing conditions differentially affects decision-making and impulsive action; isolation-reared rats demonstrated decreased levels of motor impulsivity but also increased choice of the disadvantageous options. Although it could be hypothesized that an opposing relationship exists between impulsive action and risky decision-making, both pair-housed rats and animals reared in an enriched environment showed similar decision-making preferences, yet enriched animals tended to make more premature responses on the rGT. Therefore, alterations in housing environment may affect the association between impulsive action and risky decision-making. Hence, in line with the findings from previous research utilizing the delay-discounting task (Winstanley et al. 2004; Crean et al. 2000), a universal relationship between baseline levels of impulsive action and decision-making may not exist.
Conclusion
In summary, the results from the current study suggest that housing environment influences decision-making with regards to learning response contingencies and selection of the best strategy. Furthermore, the ability of amphetamine to alter decision-making processes on the rGT is unlikely to be mediated by the drug’s effects on the dopamine system. Therefore, future studies should be aimed at determining the role of other neurotransmitters, such as 5-HT, on decision-making. Additionally, these results highlight the capability of the rGT to concurrently measure—and dissociate—impulsive action and decision-making under risk and uncertainty.
References
Bardo MT, Hammer RP (1991) Autoradiographic localization of dopamine D1 and D2 receptors in rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience 45:281–290
Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP (1995) Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav 51:397–405
Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 155:278–284
Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50:7–15
Bechara A, Damasio H, Damasio AR, Lee GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19:5473–5481
Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39:376–389
Bickerdike M, Wright I, Marsden C (1993) Social isolation attenuates rat forebrain 5-HT release induced by KCI stimulation and exposure to a novel environment. Behav Pharmacol 4:231–236
Bowling SL, Rowlett JK, Bardo MT (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893
Brenes JC, Rodríguez O, Fornaguera J (2008) Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav 89:85–93
Brown AS (2011) The environment and susceptibility to schizophrenia. Prog Neurobiol 93:23–58
Cella M, Dymond S, Cooper A (2010) Impaired flexible decision-making in major depressive disorder. J Affect Disord 124:207–210
Cole BJ, Robbins TW (1987) Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 91:458–466
Cools R, Robinson OJ, Sahakian B (2008) Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology 33:2291–2299
Crean JP, de Wit H, Richards JB (2000) Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharmacol. 8(2):155–162
Dalley JW, Theobald DE, Pereira EAC, Li PMMC, Robbins TW (2002) Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 164:329–340
Denburg NL, Tranel D, Bechara A (2005) The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia 43:1099–1106
Djouma E, Card K, Lodge DJ, Lawrence AJ (2006) The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci 23:3319–3327
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 128:161–170
Fellows LK, Farah MJ (2003) Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain 126:1830–1837
Fellows LK, Farah MJ (2005) Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex 15:58–63
Floor E, Meng L (1996) Amphetamine releases dopamine from synaptic vesicles by dual mechanisms. Neurosci Lett 215:53–56
Fone KCF, Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32:1087–1102
Garon N, Moore C, Waschbusch DA (2006) Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J Atten Disord 9:607–619
Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W (2005) Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res 23:137–151
Halperin JM, Healey DM (2011) The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neurosci Biobehav Rev 35:621–634
Harrison AA, Everitt BJ, Robbins TW (1997) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133:329–342
Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768
Hellemans KGC, Nobrega JN, Olmstead MC (2005) Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res 159:207–220
Hoffmann LC, Schütte SRM, Koch M, Schwabe K (2009) Effect of “enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience 158:1589–1598
Jones GH, Marsden CA, Robbins TW (1991) Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res 43:35–50
Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18:1979–1986
King MV, Seeman P, Marsden CA, Fone KCF (2009) Increased dopamine D2High receptors in rats reared in social isolation. Synapse 63:476–483
Krech D, Rosenzweig MR, Bennett EL (1962) Relations between chemistry and problem-solving among rats raised in enriched and impoverished environments. J Comp Physiol Psychol 55:801–807
Kuczenski R, Segal D (1989) Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci 9:2051–2065
Kuramochi M, Nakamura S (2009) Effects of postnatal isolation rearing and antidepressant treatment on the density of serotonergic and noradrenergic axons and depressive behavior in rats. Neuroscience 163:448–455
Laviola G, Hannan AJ, Macrì S, Solinas M, Jaber M (2008) Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis 31:159–168
Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA (2008) Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience 152:265–272
McDonald J (2009) Handbook of biological statistics. Sparky House, Baltimore
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709
Parada M, Hernandez L, Schwartz D, Hoebel BG (1988) Hypothalamic infusion of amphetamine increases serotonin, dopamine and norepinephrine. Physiol Behav 44:607–610
Pattij T, Janssen MCW, Vanderschuren LJMJ, Schoffelmeer ANM, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 191:587–598
Perry JL, Stairs DJ, Bardo MT (2008) Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res 193:48–54
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW (2009) Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus 'stopping'. Behav Brain Res 196(2):310–316
Robinson OJ, Cools R, Sahakian BJ (2012) Tryptophan depletion disinhibits punishment but not reward prediction: implications for resilience. Psychopharmacology (Berl) 219(2):599–605
Rolls ET, Hornak J, Wade D, McGrath J (1994) Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatr 57:1518–1524
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41
Schrijver NC, Würbel H (2001) Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci 115:437–442
Shurman B, Horan WP, Nuechterlein KH (2005) Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res 72:215–224
Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B (2009) Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology 34:2208–2217
Simpson J, Kelly JP (2011) The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav Brain Res 222:246–264
Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR (2008) Decision-making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry 39:567–576
Solinas M, Thiriet N, Chauvet C, Jaber M (2010) Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 92:572–592
van der Harst JE, Baars A-M, Spruijt BM (2003) Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res 142:151–156
Verdejo-Garcia A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32(4):777–781
Vitaro F, Arseneault L, Tremblay RE (1999) Impulsivity predicts problem gambling in low SES adolescent males. Addiction 94(4):565–575
Welte JW, Barnes GM, Wieczorek WF, Tidwell M-CO, Parker JC (2004) Risk factors for pathological gambling. Addict Behav 29:323–335
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004) Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 29(7):1331–1343
Zeeb FD, Winstanley CA (2011) Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci 31:2197–2204
Zeeb FD, Robbins TW, Winstanley CA (2009) Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34:2329–2343
Acknowledgments
This work was supported by an operating grant awarded to CAW from the Canadian Institutes for Health Research (CIHR). CAW also receives salary support through the Michael Smith Foundation for Health Research and the CIHR New Investigator Award program. We would like to thank Chris McKinnon for his enthusiasm and invaluable technical assistance.
Conflict of interest
CAW has previously consulted for Theravance on an unrelated matter. No authors have any other conflicts of interest or financial disclosures to make.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Zeeb, F.D., Wong, A.C. & Winstanley, C.A. Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: Dissociations between impulsive action and risky decision-making. Psychopharmacology 225, 381–395 (2013). https://doi.org/10.1007/s00213-012-2822-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2822-x