Abstract
Rationale
The effects of nicotine on cognitive processes may play an important role in nicotine addiction. Nicotine withdrawal impairs hippocampus-dependent learning and genetic factors influence this effect. However, the neural changes that contribute to these impairments are unknown. Chronic nicotine upregulates hippocampal nicotinic acetycholine receptors (nAChRs), which may contribute to cognitive deficits when nicotine administration ceases. If nAChR upregulation underlies withdrawal deficits in learning, then strains of mice exhibiting withdrawal deficits in hippocampus-dependent learning should also show upregulation of hippocampal nAChRs.
Objectives
Here, we examined the effects of nicotine withdrawal on fear conditioning and [3H]epibatidine binding in the dorsal and ventral hippocampus in two inbred mouse strains and their F1 hybrids.
Methods
Male C57BL/6NTac, 129S6/SvEvTac, and B6129SF1/Tac mice were administered chronic nicotine (18 mg/kg/day) for 12 days through osmotic pumps and then were trained and tested in fear conditioning 24 h after cessation of nicotine treatment.
Results
Nicotine withdrawal impaired hippocampus-dependent contextual conditioning in C57BL/6NTac mice but not 129S6/SvEvTac or B6129SF1/Tac mice; no changes were observed in hippocampus-independent cued fear conditioning. Upregulated [3H]epibatidine binding was found in the dorsal, but not ventral, hippocampus of C57BL/6NTac mice and in the ventral hippocampus of B6129SF1/Tac mice after chronic nicotine.
Conclusions
Upregulation of high-affinity binding sites in the dorsal hippocampus of C57BL/6NTac mice, the only strain that exhibited nAChR upregulation in this region and withdrawal deficits in contextual conditioning, suggests that upregulation of high-affinity binding sites in the dorsal hippocampus mediates, in part, nicotine withdrawal deficits in contextual conditioning and genetic background modulates these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarette smoking is the leading preventable cause of death in the USA, resulting in approximately 443,000 deaths per year (CDC 2011). Despite known health consequences of smoking, 27.4 % of the population still continues to smoke (SAMHSA 2010). The aversive withdrawal symptoms a smoker may experience partially contribute to the low rates of long-term nicotine abstinence (Piasecki et al. 1998, 2000). These withdrawal symptoms include increased appetite, depressed mood, craving, anxiety, and changes in cognition (Benowitz 2008; Evans and Drobes 2009; Jacobsen et al. 2007; Kenny and Markou 2001; Patterson et al. 2010). In fact, changes in cognition during nicotine withdrawal predicted relapse (Patterson et al. 2010). Genetic factors also play an important role in nicotine addiction and nicotine withdrawal (Portugal and Gould 2008; Portugal et al. 2012a). Genetic factors accounted for approximately 50 % of the risk in smoking initiation and 70 % of the variance for continued smoking (True et al. 1997). With regard to nicotine withdrawal, heritability accounted for 26–53 % of nicotine withdrawal symptoms including irritability, depressed mood, and concentration problems (Pergadia et al. 2006). As disrupted cognition is often concomitant with nicotine withdrawal, examining the biological and genetic factors that contribute to changes in cognitive function during nicotine withdrawal is crucial to understanding the nicotine withdrawal phenotype.
Fear conditioning has been a useful animal model for examining changes in cognitive function due to nicotine withdrawal (Davis and Gould 2009; Davis et al. 2005; Portugal and Gould 2009). In this procedure, a tone conditioned stimulus is paired with an aversive footshock unconditioned stimulus. This pairing results in an association between the cue and the footshock (cued conditioning) as well as between the training context and the footshock (contextual conditioning). The association between the cue and the footshock is independent of the hippocampus while the association between the context and the footshock is dependent upon the hippocampus (Logue et al. 1997; Phillips and LeDoux 1992). Mice withdrawn from chronic nicotine administration showed impaired contextual conditioning while cued conditioning was unaffected (Davis et al. 2005). Importantly, the impairment in contextual conditioning was found to be specific to new contextual learning rather than memory recall (Portugal and Gould 2009). This suggests that chronic nicotine alters hippocampus function rather than producing a general learning and memory deficit. This supposition is supported by the finding that withdrawal from direct hippocampal infusion of nicotine also impaired contextual conditioning (Davis and Gould 2009).
Previous findings have shown that chronic nicotine upregulates nicotinic acetylcholine receptors (nAChRs) in the brain (Marks et al. 1983a; Schwartz and Kellar 1983), which return to control levels as the duration of withdrawal increases (Marks et al. 1985). Recent findings from our laboratory showed that the duration of upregulated high-affinity, cytisine-sensitive nAChRs in the hippocampus to return to control levels matched the duration of nicotine withdrawal deficits in contextual conditioning and suggested that upregulation of nAChRs in the hippocampus might underlie cognitive impairment during withdrawal (Gould et al. 2012). In addition, age-related differences in nicotine withdrawal deficits in contextual conditioning and high-affinity binding in the hippocampus were observed. Specifically, adult mice that showed nicotine withdrawal deficits in contextual conditioning had upregulated high affinity nAChRs in the hippocampus, whereas adolescent mice withdrawn from the same dose of chronic nicotine did not display learning deficits or upregulation of high-affinity nAChRs in the hippocampus (Portugal et al. 2012b). Together, these results strongly suggest that upregulation of high-affinity nAChRs in the hippocampus contribute to withdrawal deficits in contextual conditioning.
Comparing different inbred strains of mice is a common way to assess the influence of genetic background on behavioral and cognitive tasks as well as drug effects on these tasks (Crawley et al. 1997; Wehner et al. 2001). Mice within an inbred strain are genetically identical to each other after 20 generations of breeding (Committee on Standardized Genetic Nomenclature for Mice 1989); therefore, any observable differences within a strain reflect environmental and possibly epigenetic influences while differences between strains of mice using strain means reflect genetic differences. Genetic background modulates initial responses to nicotine and also contributed to the variability of upregulation in different brain regions (Collins et al. 1988; Marks et al. 1983b, 1991). A recent study from our laboratory, which characterized the effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning in a variety of inbred mouse strains, found that inbred mouse strains varied greatly in their susceptibility to nicotine withdrawal deficits in contextual conditioning (Portugal et al. 2012a). For example, withdrawal from doses of chronic nicotine that impaired contextual conditioning in C57BL/6J mice did not affect 129/SvEv mice (Portugal et al. 2012a). If upregulation of high-affinity binding sites in the hippocampus underlies nicotine withdrawal deficits in contextual conditioning, then strains of mice that do not exhibit withdrawal deficits should not show nAChR upregulation in the hippocampus. Therefore, the present study examined the relationship between hippocampal nAChR upregulation and withdrawal deficits in contextual conditioning in three different lines of mice.
Emerging evidence indicates that the hippocampus is not a homogenous structure but rather the dorsal and ventral regions mediate different behaviors (Fanselow and Dong 2010). Acute nicotine infused into the dorsal hippocampus enhanced hippocampus-dependent learning while nicotine infused into the ventral hippocampus impaired hippocampus-dependent learning (Davis et al. 2007; Kenney et al. 2012; Raybuck and Gould 2010), and repeated daily injections of nicotine differentially upregulated nAChRs in the dorsal and ventral regions (Abdulla et al. 1996). Therefore, the present study also examined changes in high-affinity binding from chronic nicotine in the dorsal and ventral hippocampus. Specifically, the present study examined the effects of nicotine withdrawal on fear conditioning and high-affinity nAChR binding in the dorsal and ventral hippocampi of C57BL/6NTac, 129S6/SvEvTac, and the pattern of inheritance in B6129SF1/Tac mice.
Method
Subjects
Male C57BL/6NTac, 129S6/SvEvTac, and B6129SF1/Tac hybrid mice (Taconic, Germantown, NY, USA) were 8–12 weeks of age at the beginning of all procedures. Mice were maintained on a 12-h light–dark cycle (lights on at 7:00 a.m.) and all behavioral procedures were conducted during the hours of 9:00 a.m. to 5:00 p.m. Mice were provided ad libitum access to food and water. All behavioral and surgical procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Drugs and administration
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9 % saline. Nicotine was administered subcutaneously via osmotic pumps (Alzet model 1002, Durect Co., Cupertino, CA, USA) at a dose of 18 mg/kg/day for 12 or 14 days. For nicotine withdrawal, nicotine was administered for 12 days after which pumps were removed. Training occurred 24 h later on day 13 and then testing occurred on day 14. For chronic nicotine, pumps remained implanted throughout training (day 13) and testing (day 14) of fear conditioning. Dose reported as freebase weight and based off of previous work (Turner et al. 2011a).
Surgeries
Pump implantation was performed as previously described (Davis et al. 2005). Mice were anesthetized with 5 % isoflurane gas and osmotic pumps were implanted subcutaneously via an incision in the lower back. Twelve days after pump implantation, a second, similar surgery was performed to remove osmotic pumps and induce spontaneous nicotine withdrawal.
Apparatus
Training of fear conditioning and testing of contextual conditioning took place in four identical clear Plexiglas chambers (26.5 × 20.4 × 20.8 cm) housed in sound attenuating boxes (Med-Associates, St. Albans, VT, USA). The floor of each chamber was made of metal bars connected to a shock generator and scrambler (Med Associates, Model ENV-414). Ventilation fans were mounted on the sides of each box to provide background noise. Illumination was provided by a 4-W light mounted above each box. The white noise cue and shock administration were controlled by a PC running LabView software. Testing for cued conditioning occurred in an altered context consisting of four chambers (20.3 × 22.9 × 17.8 cm) housed in sound attenuating boxes (Med-Associates) in a different room from the training room. The floor of each chamber was made of white plastic. Speakers were mounted on the left wall of each chamber to deliver the auditory cue. Vanilla extract was added to a tray beneath the floors to further distinguish the chambers from the training chambers. All chambers were cleaned with 70 % ethanol before and after all behavioral procedures.
Behavioral procedure
A modified delay fear conditioning training procedure that used a one 15-s conditioned stimulus (CS)–unconditioned stimulus (US) pairing was performed similar to previously described methods (Davis et al. 2007; Davis and Gould 2009; Gould et al. 2004). Freezing, defined as the complete absence of movement besides respiration, was sampled for 1 s every 10 s and was used as a measure of learning and memory (Gould and Wehner 1999b). On training day, mice were placed into training chambers and baseline freezing was scored for 120 s. An 85-dB auditory cue CS was then presented for 15 s which coterminated with a 2-s 0.57 mA footshock US. Mice remained in the chambers for an additional 30 s before being returned to their home cages. The next day, mice were returned to the original training chambers, and freezing to the context was scored for 5 min in the absence of the auditory cue CS. Approximately 1 h later, mice were placed in the altered context for a total of 6 min. Generalized freezing was scored for the first 3 min in the absence of the auditory cue CS. The auditory cue CS was then turned on and cued freezing was scored for 3 min.
Receptor binding
Tissue was harvested from mice immediately after testing of cued conditioning. Hippocampi were removed and dissected on ice into dorsal/ventral sections in a one third to two third ratio based on a previous work (Gresack et al. 2009; Moser et al. 1995). The samples were homogenized in 50 mM Tris–HCl (Sigma-Aldrich) buffer, pH 7.4 at 24 °C, and centrifuged twice at 35,000 × g for 10 min in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]epibatidine (PerkinElmer, Boston, MA, USA). Incubations were performed in Tris buffer at pH 7.4 for 2 h at 24 °C with [3H]epibatidine. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass fiber filters (Brandel, Gaithersburg, MD, USA) that were pretreated with 0.5 % polyethyleneimine (Sigma-Aldrich). The filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300 μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding. Binding data were expressed as femtomole per milligram tissue (Turner et al. 2011a, b).
Data analysis
Freezing and binding data were analyzed using two-way analysis of variance (ANOVA) tests. In the experiment examining the effects of chronic nicotine on fear conditioning in B6129SF1/Tac mice, one-way ANOVAs were used to analyze freezing. Independent samples t tests were used to determine specific group freezing differences within each significant interaction test. Binding data were followed by Bonferroni’s multiple comparison tests. Any animal that was 2.5 standard deviations from the mean was considered an outlier and excluded from data analysis. This criterion resulted in the removal of two animals.
Results
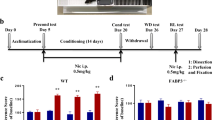
Figure 1 shows the effects of withdrawal from chronic nicotine on fear conditioning in C57BL/6NTac, 129S6/SvEvTac, and B6129SF1/Tac mice (n = 9–10 per group). A two-way ANOVA revealed a significant genotype by treatment interaction for contextual freezing [F(2, 52) = 3.409, p < 0.05] but no genotype by treatment interaction for baseline, generalized, or cued freezing (ps > 0.05). C57BL/6NTac mice withdrawn from chronic nicotine froze significantly less to the context than their saline withdrawn counterparts (p < 0.05). There were no significant differences between saline or nicotine withdrawn 129S6/SvEvTac mice or B6129SF1/Tac mice (ps > 0.05). Overall, the results of this experiment demonstrate that nicotine withdrawal disrupts contextual conditioning in C57BL/6NTac mice but not in 129S6/SvEvTac or B6129SF1/Tac mice.
The effects of nicotine withdrawal on fear conditioning in three strains of mice. Nicotine withdrawal disrupted contextual conditioning in C57BL/6NTac mice. WCS withdrawal from chronic saline, WCN withdrawal from chronic nicotine. *p < 0.05 compared to WCS C57BL/6NTac mice. Error bars represent ± SEM
It is possible that the absence of a withdrawal effect in B6129SF1/Tac mice was due to enhanced freezing from chronic nicotine that dropped to baseline levels during withdrawal. To explore this possibility, a second experiment was performed in a separate group of naïve B6129SF1/Tac mice (n = 6–10 per group) that compared mice withdrawn from chronic saline or nicotine to mice administered chronic saline or nicotine (Fig. 2). A one-way ANOVA revealed no significant effects of chronic or withdrawal from chronic nicotine or saline treatment on contextual, generalized, or cued freezing (all ps > 0.05). Thus, both chronic and withdrawal from chronic nicotine have no effect on fear conditioning in B6129SF1/Tac mice.
The effects of chronic and withdrawal from chronic nicotine on fear conditioning in B6129SF1/Tac mice. Neither chronic nor withdrawal from chronic nicotine affected fear conditioning in this strain of mouse. WCS withdrawal from chronic saline, WCN withdrawal from chronic nicotine. Error bars represent ± SEM
While previous research indicates that chronic nicotine upregulates nAChRs in the brain (Marks et al. 1983a; Schwartz and Kellar 1983), no study has examined if genetic background influences dorsal versus ventral hippocampus nAChR upregulation. Therefore, after testing in cued conditioning (48 h of withdrawal), mice were euthanized and their hippocampi were dissected on ice into dorsal and ventral sections for [3H]epibatidine binding experiments. For C57BL/6NTac mice, a two-way ANOVA revealed a significant main effect of treatment [F(1, 19) = 5.640, p < 0.05], whereas the main effect of region and the interaction between region and treatment was not significant (Fig. 3). Post hoc tests revealed that [3H]epibatidine binding was significantly higher in the dorsal hippocampus of C57BL/6NTac mice withdrawn from chronic nicotine treatment than mice withdrawn from chronic saline (p < 0.05). There was not a significant difference in [3H]epibatidine binding in the ventral hippocampus (p > 0.05). For 129S6/SvEvTac mice, there was a significant main effect of treatment [F(1, 34) = 5.941, p < 0.05] but no significant effect of region or interaction between region and treatment (Fig. 4). Post hoc tests did not reveal any significant differences in [3H]epibatidine binding in the dorsal or ventral hippocampus between 129S6/SvEvTac mice withdrawn from chronic saline and withdrawn from chronic nicotine (ps > 0.05), indicating no region-specific effects. Finally, for B6129SF1/Tac mice, a two-way ANOVA revealed a significant treatment by region interaction [F(1, 20) = 6.954, p < 0.01] (Fig. 5). Post hoc tests revealed that [3H]epibatidine binding was significantly higher in the ventral hippocampus of B6129SF1/Tac mice withdrawn from chronic nicotine than mice withdrawn from chronic saline (p < 0.001). There were no significant differences in [3H]epibatidine binding in the dorsal hippocampus of B6129SF1/Tac mice withdrawn from chronic saline or nicotine.
The effects of chronic nicotine on high-affinity [3H]epibatidine binding in the dorsal and ventral hippocampus of C57BL/6NTac mice measured after 48 h of withdrawal. Chronic nicotine upregulated high-affinity binding sites in the dorsal hippocampus. *p < 0.05 compared to C57BL/6NTac mice withdrawn from chronic saline within the same region. Error bars represent ± SEM
The effects of chronic nicotine on high-affinity [3H]epibatidine binding in the dorsal and ventral hippocampus of 129S6/SvEvTac mice measured after 48 h of withdrawal. Chronic nicotine did not significantly affect high-affinity binding sites in the dorsal or ventral hippocampus. Error bars represent ± SEM
The effects of chronic nicotine on high-affinity [3H]epibatidine binding in the dorsal and ventral hippocampus of B6129SF1/Tac mice measured after 48 h of withdrawal. Chronic nicotine upregulated high-affinity binding sites in the ventral hippocampus. *p < 0.05 compared to B6129SF1/Tac mice withdrawn from chronic saline within the same region. Error bars represent ± SEM
Discussion
The present study examined the effects of withdrawal from chronic nicotine on fear conditioning in three strains of mice as well as changes in dorsal and ventral hippocampal high-affinity nAChR binding following 48 h of withdrawal from chronic nicotine. Withdrawal from chronic nicotine impaired contextual conditioning in C57BL/6NTac mice but not in 129S6/SvEvTac mice or B6129SF1/Tac hybrids, which is likely due to impaired learning rather than memory recall (Portugal and Gould 2009). Cued conditioning was unaffected by nicotine withdrawal in all three strains of mice. These results parallel previous findings and indicate genetic background differences influence susceptibility to nicotine withdrawal deficits in contextual conditioning (Portugal et al. 2012a). In addition, chronic nicotine differentially upregulated high-affinity nAChR binding sites 48 h after withdrawal in the dorsal and ventral hippocampus across the strains of mice. Chronic nicotine upregulated high-affinity binding sites in the dorsal but not the ventral hippocampus of C57BL/6NTac mice. On the other hand, chronic nicotine did not significantly affect high-affinity binding sites in either the dorsal or the ventral hippocampus of 129S6/SvEvTac mice. Lastly, chronic nicotine upregulated high-affinity binding sites in the ventral but not the dorsal hippocampus of B6129SF1/Tac mice. These results suggest that chronic nicotine upregulation of high-affinity binding sites in the dorsal and ventral hippocampus is modulated by genetic background.
In the present study, the auditory cue CS might have been a stronger encoding stimulus than the context in which the mice were trained. A stronger stimulus presentation might result in a stronger CS–US association and overshadow any nicotine withdrawal-related changes in cued conditioning. As such, reducing the strength of the association between the auditory CS and the footshock US should increase sensitivity to nicotine withdrawal-related changes in cued conditioning and potentially unmask any learning impairments. To address this issue, Davis and Gould (2009) utilized a modified delay fear conditioning procedure in which the auditory CS was presented for 15 s and only paired once with the footshock US compared to a previous work with this paradigm that used two CS–US pairings with a 30-s CS (Davis et al. 2005). While the modified delay fear conditioning procedure reduced freezing to the auditory CS, nicotine withdrawal had no effect on cued conditioning. This finding suggests that the lack of an effect of nicotine withdrawal on cued conditioning is not due to the strength of the auditory cue CS as a stimulus. To this end, the present study utilized a modified fear conditioning procedure similar to Davis and Gould (2009) to reduce the effects of the strength of the auditory cue CS. Therefore, it is likely that the lack of an effect of nicotine withdrawal on cued conditioning was not due to the strength of the auditory cue CS as an encoding stimulus
One of the interesting findings from the current study is that the pattern of hippocampal subregion nAChR upregulation relates to the expression of withdrawal-associated learning deficits. Specially, C57BL/6NTac mice were the only strain to exhibit nicotine withdrawal deficits in contextual conditioning and upregulated high-affinity binding in the dorsal hippocampus. This suggests that upregulation of high-affinity binding sites in the dorsal hippocampus mediates, in part, nicotine withdrawal deficits in contextual conditioning. In support, the timing for nicotine withdrawal deficits in contextual conditioning to dissipate matched the return of upregulated high-affinity binding sites in whole hippocampus to control levels (Gould et al. 2012). Likewise, recent findings from our laboratory indicated that adult mice that exhibited nicotine withdrawal deficits in contextual conditioning displayed upregulated high-affinity binding in the hippocampus, while pre-adolescent mice did not exhibit nicotine withdrawal deficits in contextual conditioning or upregulated high-affinity binding in the hippocampus (Portugal et al. 2012b).
In addition to identifying regional variability in nAChR upregulation, it is also possible to estimate the subtype of nAChR involved in this upregulation. Epibatidine is a nAChR agonist that binds with high affinity to all heteromeric nAChRs including α4β2, α3β4, and α3β2 (Perry et al. 2002; Xiao and Kellar 2004). Previous data show that β2 knockout mice do not exhibit nicotine withdrawal deficits in contextual conditioning (Portugal et al. 2008). Likewise, infusions of DHβE, a high-affinity nAChR antagonist, into the dorsal hippocampus precipitated learning deficits in wild-type but not β2 knockout mice (Davis and Gould 2009). Thus, it is likely that chronic nicotine upregulated dorsal hippocampal high-affinity β2-containing nAChRs, possibly α4β2* (* indicates other subunits may be incorporated).
It is also possible to estimate the mechanism that caused this upregulation. Nicotine-induced upregulation has been well established in the literature both in vivo and in vitro, and multiple mechanisms have been offered to explain how upregulation occurs (for review, see Govind et al. 2009). A recent paper, however, indicates that upregulation is not a result of a single process but rather the result of at least two mechanisms (Govind et al. 2012). The first process was characterized by a fast onset and offset that was associated with changes in nAChR conformation. The second process was characterized by a slow onset and offset that required longer exposure to nicotine to initiate than the first and was associated with changes in nAChR number. In the present study, binding was measured 48 h post-withdrawal, which may reflect the second process rather than the first.
Multiple studies have highlighted inbred strain differences across a wide range of behaviors including cognitive tasks such as latent inhibition (Gould and Wehner 1999a), water maze performance (Owen et al. 1997; Wolff et al. 2002), and fear conditioning (Bothe et al. 2004, 2005; Nie and Abel 2001; Owen et al. 1997). In addition, strain differences in response to acute, chronic, and withdrawal from chronic nicotine across a wide range of measures have also been characterized (Collins et al. 1988; Damaj et al. 2003, 2007; Marks et al. 1983b, 1991; Portugal et al. 2012a). The results of these studies and the present study provide valuable insight into the selection of the proper strain to examine the effects of nicotine treatment on behavior. For example, 129/SvEv and C57BL/6J mice displayed different somatic and affective signs of precipitated nicotine withdrawal with the 129/SvEv strain being less sensitive to nicotine withdrawal than C57BL/6J mice (Damaj et al. 2003). Likewise, 129/SvEv mice were less sensitive to the effects of acute nicotine and withdrawal from chronic nicotine on contextual conditioning than C57BL/6J mice (Portugal et al. 2012a). The present study demonstrated nicotine withdrawal deficits in contextual conditioning in C57BL/6NTac, a different strain of C57BL/6 mice, while finding no withdrawal learning deficits in the 129S6/SvEvTac mice even with a higher dose of nicotine than previously examined. Thus, while there are phenotypic differences across C57BL/6 lines (Bothe et al. 2004, 2005; Bryant et al. 2008; Kiselycznyk and Holmes 2011; Stiedl et al. 1999), the current findings and prior results suggest that this strain may be more appropriate than the 129/SvEv strain to study nicotine withdrawal.
Another interesting finding from the present study was the pattern of inheritance of the nicotine withdrawal phenotype in B6129SF1/Tac mice. B6129SF1/Tac hybrids are generated by crossing C57BL/6NTac female mice to 129S6/SvEvTac male mice. Therefore, B6129SF1/Tac hybrid mice will be heterozygous at the loci where C57BL/6NTac and 129S6/SvEvTac differ and homozygous at the loci where they are the same. Any behavioral differences in response to nicotine withdrawal may then be attributed to inherited traits from one or both of the parental strains. The lack of a withdrawal phenotype in B6129SF1/Tac hybrid mice suggests a dominant inherited phenotype from 129S6/SvEvTac mice, which may be mediated by an absence of upregulated high-affinity binding sites in the dorsal hippocampus. Interestingly, chronic nicotine upregulated high-affinity nAChRs in the ventral hippocampus of B6129SF1/Tac hybrid mice while neither parental strain exhibited significant upregulation in this region. One cannot always predict a phenotype of an F1 hybrid based on the phenotypes of the parental strains alone (Owen et al. 1997). For example, in the present study, B6129SF1/Tac hybrid mice showed higher levels of contextual freezing than both parental strains. This is not surprising, however, as previous reports indicate that F1 hybrids have superior learning abilities compared to parental strains (Clapcote and Roder 2004; Owen et al. 1997), a phenomenon known as hybrid vigor. Therefore, the mechanisms that contribute to hybrid vigor might also underlie the upregulation of high-affinity nAChRs in the ventral hippocampus of B6129SF1/Tac mice. Taken together, these findings suggest the cognitive nicotine withdrawal phenotype in B6129SF1/Tac mice is inherited from 129S6/SvEvTac mice and also highlight the importance of understanding the phenotypes of both parental strains before interpreting results from F1 hybrids.
There is one issue that potentially limits the interpretation of the present results. High-affinity binding was measured 48 h after the cessation of chronic nicotine treatment. It is possible that chronic nicotine upregulated high-affinity binding sites in both the dorsal and ventral hippocampus of each strain at earlier time points, but for some strains, it rapidly returned to control levels. Indeed, it has previously been shown that chronic nicotine upregulates high-affinity binding sites in the hippocampus of 129SvJ;C57BL/6J F1 hybrid mice, but this upregulation returns to control levels within 24 h (Turner et al. 2011a). The absence of withdrawal deficits and upregulated high-affinity binding sites in the dorsal and ventral hippocampus of 129S6/SvEvTac mice and dorsal hippocampus of B6129SF1/Tac hybrid mice might be due to the time point chosen to measure binding, and thus if a shorter time period was examined after withdrawal, then nAChR upregulation and learning withdrawal deficits might be seen for the other strains. Even if this were the case, the current results would then suggest that genetic background influences the duration of nAChR upregulation although still supporting the hypothesis that nAChR upregulation relates to withdrawal deficits in hippocampus-dependent learning. While the time course for the emergence of withdrawal deficits is an issue for future studies, our results suggest that a threshold of high-affinity nAChR upregulation is needed to produce withdrawal impairments in learning and that these effects are influenced by genetics. Another area of investigation for future studies is whether this upregulation of high-affinity nAChRs in the dorsal hippocampus is a necessary and sufficient component for these withdrawal deficits.
References
Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD (1996) Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacol (Berl) 124:323–331
Benowitz NL (2008) Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 83:531–541
Bothe GWM, Bolivar VJ, Vedder MJ, Geistfeld JG (2004) Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav 3:149–157
Bothe GWM, Bolivar VJ, Vedder MJ, Geistfeld JG (2005) Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med 55:326–334
Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA (2008) Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet 22:315–331
CDC (2011) CDC health disparities and inequalities report—United States, 2011. MMWR 60:109–112
Clapcote SJ, Roder JC (2004) Survey of embryonic stem cell line source strains in the water maze reveals superior reversal learning of 129S6/SvEvTac mice. Behav Brain Res 152:35–48
Collins AC, Miner LL, Marks MJ (1988) Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol Biochem Behav 30:269–278
Committee on Standardized Genetic Nomenclature for Mice (1989) Rules for nomenclature of inbred strains. In: Lyon MF, Searle AG (eds) Genetic variants and strains of the laboratory mouse. Oxford University Press, New York, pp 632–635
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacol (Berl) 132:107–124
Damaj MI, Kao W, Martin BR (2003) Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther 307:526–534
Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR (2007) Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther 321:1161–1169
Davis JA, Gould TJ (2009) Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol 19:551–561
Davis JA, James JR, Siegel SJ, Gould TJ (2005) Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci 25:8708–8713
Davis JA, Kenney JW, Gould TJ (2007) Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci 27:10870–10877
Evans DE, Drobes DJ (2009) Nicotine self-medication of cognitive-attentional processing. Addict Biol 14:32–42
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19
Gould TJ, Wehner JM (1999a) Genetic influences on latent inhibition. Behav Neurosci 113:1291–1296
Gould TJ, Wehner JM (1999b) Nicotine enhancement of contextual fear conditioning. Behav Brain Res 102:31–39
Gould TJ, Feiro O, Moore D (2004) Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res 155:167–173
Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M (2012) The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology 62:2118–2125
Govind AP, Vezina P, Green WN (2009) Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78:756–765
Govind AP, Walsh H, Green WN (2012) Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci 32:2227–2238
Gresack JE, Schafe GE, Orr PT, Frick KM (2009) Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159:451–467
Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR (2007) Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacol (Berl) 193:557–566
Kenney JW, Raybuck JD, Gould TJ (2012) Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus 22(8):1681–1690
Kenny PJ, Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70:531–549
Kiselycznyk C, Holmes A (2011) All (C57BL/6) mice are not created equal. Front Neurosci 5:10–10
Logue SF, Paylor R, Wehner JM (1997) Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci 111:104–113
Marks MJ, Burch JB, Collins AC (1983a) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825
Marks MJ, Burch JB, Collins AC (1983b) Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther 226:291–302
Marks MJ, Stitzel JA, Collins AC (1985) Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther 235:619–628
Marks MJ, Campbell SM, Romm E, Collins AC (1991) Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther 259:392–402
Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995) Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A 92:9697–9701
Nie T, Abel T (2001) Fear conditioning in inbred mouse strains: an analysis of the time course of memory. Behav Neurosci 115:951–956
Owen EH, Logue SF, Rasmussen DL, Wehner JM (1997) Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience 80:1087–1099
Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C (2010) Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend 106:61–64
Pergadia ML, Heath AC, Martin NG, Madden PAF (2006) Genetic analyses of DSM-IV nicotine withdrawal in adult twins. Psychol Med 36:963–972
Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ (2002) Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem 82:468–481
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285
Piasecki TM, Fiore MC, Baker TB (1998) Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol 107:238–251
Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB (2000) Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol 109:74–86
Portugal GS, Gould TJ (2008) Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res 193:1–16
Portugal GS, Gould TJ (2009) Nicotine withdrawal disrupts new contextual learning. Pharmacol Biochem Behav 92:117–123
Portugal GS, Kenney JW, Gould TJ (2008) Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem 89:106–113
Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ (2012a) Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet 42:133–150
Portugal GS, Wilkinson DS, Turner JR, Blendy J, Gould TJ (2012b) Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem 97:482–494
Raybuck JD, Gould TJ (2010) The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem 94:353–363
SAMHSA (2010) Results from the 2010 National Survey on Drug Use and Health: summary of national findings. NSDUH Series H-41, HHS Publication No (SMA) 11-4658 Rockville, MD
Schwartz RD, Kellar KJ (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216
Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Sananbenesi F, Spiess J (1999) Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res 104:1–12
True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, Eisen SA, Lyons MJ, Tsuang MT (1997) Genetic and environmental contributions to smoking. Addiction 92:1277–1287
Turner JR, Castellano LM, Blendy JA (2011a) Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13:41–46
Turner JR, Ortinski PI, Sherrard RM, Kellar KJ (2011b) Cerebellar nicotinic cholinergic receptors are intrinsic to the cerebellum: implications for diverse functional roles. Cerebellum 10:748–757
Wehner JM, Radcliffe RA, Bowers BJ (2001) Quantitative genetics and mouse behavior. Annu Rev Neurosci 24:845–867
Wolff M, Savova M, Malleret G, Segu L, Buhot MC (2002) Differential learning abilities of 129T2/Sv and C57BL/6J mice as assessed in three water maze protocols. Behav Brain Res 136:463–474
Xiao Y, Kellar KJ (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107
Acknowledgments
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (NIDA, DA024787, DA017949, TJG; DA026236, JRT) and the National Cancer Institute (CA143187, JAB). DSW was supported by a NIDA diversity supplement (DA024787-01A1S1). All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and US laws.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilkinson, D.S., Turner, J.R., Blendy, J.A. et al. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology 225, 201–208 (2013). https://doi.org/10.1007/s00213-012-2808-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2808-8