Abstract
Rationale
Ecstasy (MDMA) is used predominately by adolescents and young adults. Young MDMA users are more likely than non-users to use other drugs, including cocaine. The response to stimulant drugs can be affected by environmental factors; however, little information exists about the role that housing plays in mediating effects of MDMA in adolescence.
Objectives
The present experiment examined whether social and environmental factors alter effects of MDMA on activity and cocaine reward.
Methods
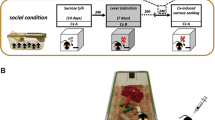
Male adolescent rats were housed on PND 23. Isolated rats were housed alone (1 rat/cage) in an impoverished environment with no toys (II) or enriched with toys (IE). Social rats were housed three/cage with (SE3) or without (SI3) toys. Starting on PND 29, 5 mg/kg MDMA or saline was injected and activity was measured for 60 min once daily for five consecutive days. On PND 36–40, cocaine CPP was conducted.
Results
Saline vehicle-induced activity of II rats was higher than other groups, and all groups became sensitized to the locomotor-stimulant effects of MDMA. In II rats, maximal CPP was increased after MDMA pre-exposure compared to vehicle. Environmental enrichment blocked this; however, dose–effect curves for cocaine CPP shifted to the left in both IE and SE3 rats. In rats with just social enrichment, there were no effects of MDMA on cocaine CPP.
Conclusion
Drug prevention and treatment strategies should take into account different environments in which adolescents live. These findings show that MDMA increases cocaine reward in male adolescents, and social enrichment diminishes, while environmental enrichment enhances this.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 3 million Americans over the age of 12 reported having used an illicit drug for the first time within the previous year. More than half of these first-time illicit drug users were younger than age 18, and among these teenagers, there has been an increase in ecstasy (3,4-methylenedioxymethamphetamine; MDMA) use compared to previous years (NSDUH 2009). In fact, the perceived risk associated with MDMA use has decreased in 8th graders and disapproval of using MDMA also has decreased (Johnston et al. 2008). A study in Australia showed that people with a high socioeconomic status were twice as likely to use MDMA as those in a low status (NSDHS 2000). Further, MDMA users were more likely to use other drugs compared to non-MDMA users, with 90 % of MDMA users and only 13.8 % of non-MDMA users reporting having used other illicit drugs. Specifically, cocaine was reported as being used by 43.8 % of MDMA users and 2.0 % of non-MDMA users (NSDUH 2005).
Previous studies showed that administration of 15 mg/kg MDMA twice daily for 4 days increased horizontal locomotor activity in male adult rats compared to rats that had been administered saline (Walker et al. 2007). In adolescent rats, 5 mg/kg MDMA once daily during PND 33–39 increased activity over saline (Aberg et al. 2007) and the rats developed sensitization to the locomotor-stimulant effect over several days, as had been reported in adult rats (e.g., Kalivas et al. 1998).
There is evidence that MDMA administration can alter later cocaine reward, especially in adolescence, suggesting that exposure to one drug may make another drug more rewarding. For example, we previously have shown that adolescent male rats that were pretreated with 2 or 5 mg/kg MDMA daily for 7 days developed a significant place preference to 10 mg/kg cocaine, whereas rats pretreated with saline showed no preference (Aberg et al. 2007). In contrast, adult male rats pretreated with saline had developed a significant preference to 10 mg/kg cocaine, but those pretreated with either dose of MDMA did not. These findings persisted in that the same results were observed during a re-test 2 weeks later.
It is well known that social and environmental factors are associated with early stages of adolescent drug involvement (e.g., Kandel 1985; Oetting and Beauvais 1987). In rats, environmental conditions can be manipulated by changing how many rats are housed in the same cage, and whether or not they have access to toys. Social and environmental enrichment have been shown to alter behavior and responses to drugs of abuse in animals, with some effects being sex and age dependent (e.g., Bowling and Bardo 1994). For example, environmental and social enrichment increased social investigation behavior in male adults (Pena et al. 2006), whereas social isolation for 5 days during adolescence enhanced all forms of social behavior (Varlinskaya and Spear 2008). In our laboratory, we have shown that environmental and/or social enrichment decreased cocaine conditioned reward in previously untreated rats (Zakharova et al. 2009b), and increased novelty-induced locomotor activity and increased cocaine-stimulated behavior in adolescent male rats. Others have shown that group housed adult male mice, which are sensitized to the locomotor-activating effects of cocaine, exhibited decreases in both the sensitized response to cocaine and cocaine conditioned place preference after subsequent environmental enrichment (Solinas et al. 2008). Similarly, environmental enrichment attenuated cue-induced cocaine seeking behavior in adult male rats (Thiel et al. 2011).
Clearly, the physical and social environment play an important role in initiation and maintenance of drug use, but since many studies were done in adult rats, it is not clear whether or not such changes will occur in adolescence. The goal of the present study was to investigate the effects of the environmental and social enrichment from weaning through adolescence on MDMA-stimulated locomotor activity and later cocaine reward in periadolescent male rats.
Methods
Subjects
The animals used in these studies were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996 and the Institutional Animal Care and Use Committee of the University of Miami. Adolescent male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) were housed in a temperature- and humidity-controlled environment under a 12 h light/dark schedule with lights on at 7 a.m. and off at 7 p.m. All behavioral testing was done during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour each day and the groups randomized over the course of the day. Food and water were available ad libitum.
Chemicals
(±) 3,4-Methylenedioxymethamphetamine (MDMA) HCl and cocaine HCl were obtained from NIDA (Rockville, MD, USA) and were dissolved in physiological saline (0.9 % NaCl).
Housing
Rats were housed in one of several conditions on postnatal day 23 (PND 23). Social (number of rats per cage) and environmental (availability of toys) conditions were manipulated (Table 1). Rats were housed alone with no toys (isolated, impoverished; II), alone with toys (isolated, enriched; IE), in groups of three without toys (social 3, impoverished; SI3), or in groups of three with toys (social 3, enriched; SE3). For rats in the environmentally enriched conditions, toys were made available in the home cage for 24 h per day and were changed twice per week. Toys included red and yellow transparent plastic tunnels and hollow balls that the animals could move in and out of or over, chew toys (non-toxic dog bones and other plastic toys), and nesting material. Animals remained in these conditions for the duration of the experiment.
MDMA-stimulated locomotor activity
Six days after housing began, the effects of MDMA on locomotor activity were measured daily for 60 min on five consecutive days (PND 29–33). The locomotor equipment consisted of clear acrylic chambers (40.64 × 40.64 cm) located within Digiscan activity monitors (Accuscan, Columbus, OH, USA) that were equipped with infrared light sensitive detectors mounted 2.5 cm apart along two perpendicular walls. Along the opposing walls, infrared light beams were mounted to aim at the detectors. Total distance traveled was calculated based upon the number of beam breaks, and was measured in centimeters. Each day, the rats were placed into the locomotor activity chambers for 15 min of habituation, after which they were injected with 5 mg/kg MDMA or vehicle (saline) i.p. Activity was monitored immediately after drug administration for 60 min. This dose of MDMA was chosen because it is a sub-toxic dose similar to the doses taken by humans (Boot et al. 2000). In addition, we reported previously that 5.0 mg/kg MDMA produced increases in locomotor activity and behavioral sensitization in adolescent male rats that were housed two per cage without producing toxicity (Aberg et al. 2007).
Cocaine conditioned place preference (CPP)
Cocaine CPP began the week following MDMA administration. Essentially, the procedures were done as described previously (Aberg et al. 2007; Zakharova et al. 2009a; Zakharova et al. 2009c). Pretest: On PND 36 (13 days after housing began and 3 days after last MDMA or saline pretreatment injection), rats were tested without the center barrier for 30 min to determine preference for one side of the apparatus. No differences were found between groups. Conditioning: The next day, cocaine conditioning began with the center barrier in place. For each rat, saline vehicle was paired with the rat’s preferred side during the morning session and a dose of cocaine (0, 0.3, 1, 3, 5, 10, or 20 mg/kg) was paired with the non-preferred side during the afternoon session. Rats were conditioned for 30 min during each session for 3 days (total of six sessions = three saline, three cocaine). Posttest: On the fifth day (PND 40), the amount of time spent on the cocaine-paired side with the center barrier removed was recorded during a 30-min session. Data are presented as the difference in time spent on the cocaine-paired side between the posttest and the pretest (Posttest minus Pretest).
Data analysis
Activity (total distance) in response to MDMA injections over the 5-day period was analyzed by a three-way (pretreatment × housing × day) analysis of variance (ANOVA) with repeated measures. Where appropriate, ANOVAs were followed by post hoc analyses with Fisher's protected least significant difference (PLSD). P values less than 0.05 were considered significant.
Conditioned place preference difference scores were analyzed for each dose of cocaine within each group using a Student’s t test compared to 0 to determine whether a preference or aversion to that dose of cocaine had occurred for that group. Within each housing condition, groups that received MDMA were compared to those that received saline using an independent samples t test for each dose to determine whether MDMA altered preference for specific doses of cocaine. A three-way ANOVA (pretreatment × housing × cocaine dose) was used to analyze data at the three doses of cocaine that were administered to all groups (3, 5, 10 mg/kg) to examine how MDMA pretreatment and housing conditions altered the dose–response curves for cocaine preference. Fisher’s PLSD post hoc analyses were used where appropriate. P values less than 0.05 were considered significant.
Results
Locomotor activity
Overall, across the 5 days of testing, there was a significant effect of housing on activity in rats administered saline vehicle (VEH) for 5 days prior to testing [F(3,705) = 36.617, P ≤ 0.0001]. Post hoc tests showed that male adolescent rats living in socially and environmentally impoverished conditions (II) exhibited higher activity in response to VEH compared to groups with either environmental enrichment (IE), social enrichment (SI3), or both social and environmental enrichment (SE3) on the first day of testing (P ≤ 0.05), and that the effect also was significant across all 5 days (Fig. 1, open symbols). These data also show that MDMA (filled symbols) increased activity compared to VEH (open symbols) in all of the groups of rats except the II rats. There was an overall significant effect of pretreatment [F(1,1476) = 588.7, P ≤ 0.0001], of housing [F(3,1476) = 5.49, P ≤ 0.001], and of pretreatment × housing [F(3,1476) = 10.32, P ≤ 0.0001]. Post hoc tests showed that MDMA significantly increased activity in all housing conditions, and that vehicle treated II rats had significantly greater activity levels than vehicle-treated rats in all other housing conditions.
Effect of environmental and social enrichment on MDMA-stimulated locomotor activity in adolescent male rats. Panels (a) and (b) show comparisons highlighting the effects of environmental enrichment. Panels (c) and (d) show the same data highlighting the effects of social enrichment. a Activity in isolated rats without environmental enrichment (II, circles) or with toys available (IE, squares) in response to saline vehicle (VEH, open symbols) or 5 mg/kg MDMA (filled symbols). b Socially housed rats without (SI3, circles) or with toys (SE3, squares). c Impoverished isolated rats (II, circles) compared to social impoverished rats (SI3, squares). d Enriched isolated rats (IE, circles) compared to social enriched rats (SE3, squares). Because of the differences in activity in response to VEH, the data are shown as % VEH in Fig. 2
Because of the significant differences in activity in VEH treated rats, the effects of MDMA are expressed as percent VEH activity in Fig. 2. For each housing condition, group mean activity on each day was used as the denominator to determine percent vehicle activity for each animal. Analysis of these data show that there is a significant overall effect of housing [F(3,726) = 62.2, P ≤ 0.0001], of day [F(4,726) = 27.28, P ≤ 0.001], and a significant interaction of housing × day [F(12,726) = 2.634, P ≤ 0.002]. Post hoc tests show that there were no significant differences across groups in the effects of MDMA on the first day of testing. However, housing conditions led to differential adaptation to the repeated MDMA administration in that there were significant differences across the 5 days of testing. Overall, MDMA stimulated activity to a lesser degree in II rats than in either IE, SI3, or SE3 rats (P ≤ 0.05). In addition, there were significant effects of both social conditions, with MDMA having a greater effect in the environmentally enriched social rats (SE3) than the environmentally enriched isolated rats (IE) and physical conditions, with a greater effect observed in SI3 than SE3 rats. Thus, there was an interaction between social and environmental conditions [F(1,726) = 12.48, P ≤ 0.0004], with environmental enrichment leading to a small but significant increased effect of MDMA on locomotor activity in isolated rats, but to a diminished effect in socially housed rats.
Effect of 5 mg/kg MDMA on locomotor activity in rats housed under differential conditions, expressed as % vehicle (VEH) activity. Data presented are for isolated rats without (II, open squares) and with environmental enrichment (IE, filled squares), and social rats without (SI3, open circles) and with environmental enrichment (SE3, filled squares). Sensitization to the locomotor-stimulating effect of MDMA occurred in all groups. *P < 0.05, compared to day 1
Sensitization to the effects of MDMA on locomotor activity occurred in all groups. In the socially housed rats (SI3 and SE3), there was significant sensitization to the locomotor-stimulant effects of MDMA by day 2 of testing (PND 30), and this persisted throughout the remainder of the 5-day period. The II rats were significantly sensitized by day 3 (PND 31) of MDMA administration and remained so throughout the 5 days of testing and the IE rats and there were significant increases on PND 32.
Conditioned place preference (CPP)
On PND 40, there were significant differences in cocaine CPP in the rats that had been exposed to MDMA or VEH during PND 29–33. In each group of rats, there was at least one dose of cocaine that produced a significant CPP, although the effective dose varied across groups (Fig. 3). An overall ANOVA of all of the CPP data showed that there was a significant pretreatment × cocaine dose × housing interaction [F(4,195) = 3.047, P ≤ 0.02]. In all four housing conditions, there were significant differences across doses in the MDMA vs. VEH pretreated groups. In the environmentally enriched rats, regardless of social condition (IE and SE3), there were shifts to the left of the dose–effect curve after MDMA administration (Fig. 3b, d), suggesting that cocaine is a more potent reward after MDMA exposure in enriched animals. This led to a significant difference in the effects of 3 mg/kg cocaine in both the IE rats [t(16) = 2.130, P ≤ 0.025] and the SE3 rats [t(16) = 2.135, P ≤ 0.024]. In contrast, in the environmentally impoverished groups there were differences across social conditions. In the isolated rats (II), there was a significant increase in the maximal CPP after MDMA compared to VEH pretreatment [t(9) = 2.483, P ≤ 0.017], but no difference in the SI3 rats. In the SI3 rats, the highest dose of cocaine (10 mg/kg) that was tested produced a significant aversion, with significantly less time spent in the cocaine-paired chamber after conditioning than during the pretest. Thus, it appeared that social enrichment blocked the increased cocaine reward observed after MDMA administration, but that this did not occur when environmental enrichment also was present.
Cocaine CPP subsequent to pretreatment with VEH (open symbols) or 5 mg/kg MDMA (filled symbols) in rats housed under different conditions. a Isolated impoverished rats (II). b Isolated enriched rats (IE). c Social impoverished rats (SI3). d Social enriched rats (SE3). Data are expressed as time spent in the cocaine-paired side during the posttest (after conditioning) minus the pretest. 0 represents no change in behavior. A positive value indicates more time spent in the cocaine-paired side after conditioning. *Significant CPP (significantly different from 0); #significantly greater than VEH pretreatment
Discussion
The present study shows that differential social and environmental housing conditions can alter the response to MDMA in adolescence. The data extend the literature showing that social and environmental enrichment work independently to alter drug effects in adolescents, extend the findings showing that enrichment alters the response to drugs in adults, and extend our previous findings showing the MDMA pre-exposure alters cocaine CPP in adolescent rats.
Locomotor activity
These data show that adolescent male rats living alone in an impoverished environment exhibit higher levels of activity, after habituation to the testing environment, than socially or environmentally enriched rats. The decrease in exploratory behavior of a familiar environment in enriched adolescent males is consistent with what has been reported in adult males (e.g., Bardo et al. 1995; Brenes and Fornaguera 2008). These findings are, however, in contrast to our previous study showing that when adolescent male rats are placed into a novel environment to which they have never been exposed, the rats living in a social condition explore more than the isolated rats and spend less time around the edges of the chamber (Zakharova et al. 2009b). Thus, housing conditions have different effects on the response to a novel environment than to a familiar one, suggesting that there may be differential anxiety levels associated with a novel environment in adolescent rats living in isolated conditions.
This dose of MDMA (5 mg/kg) did not significantly stimulate activity over that of vehicle upon first administration regardless of housing conditions. In addition, in response to the first administration of MDMA, there were no significant differences on locomotor activity across housing conditions. However, in socially housed animals there was a significant increase by day 2 of drug administration, and this persisted throughout the 5-day testing period. This sensitization was considerably greater than that seen in the isolated rats regardless of whether or not environmental enrichment was present. The sensitization observed in the socially housed rats was similar to that seen in our previous study in adolescent rats housed under standard conditions of two rats/cage with no environmental enrichment (Aberg et al. 2007) and also consistent with the finding that adolescent rats housed in groups rapidly exhibited sensitization to this dose of MDMA (Von Ameln and Von Ameln-Mayerhofer 2010). Together with the previous data, the current findings show that the isolated rats exhibit a diminished response to MDMA compared to adolescent male rats living in a social condition, regardless of the number of rats in the cage.
The isolated rats did become significantly sensitized to the locomotor-stimulant effects of MDMA, although to a much lesser degree than the social rats. Environmental enrichment did not significantly alter this effect, suggesting that social housing conditions play a more prominent role in mediating the development of sensitization to MDMA than does environmental enrichment.
It is interesting to note that an earlier study reported that acute administration of 5 mg/kg MDMA to adult rats increased social interaction and anxiety, shown by a decrease in the amount of time spent in the open arm of a plus maze (Morley and McGregor 2000). In adolescent mice injected with 5 mg/kg MDMA three times over a 6-day period beginning on PND 28, there were persistent increases in locomotor activity and social investigation observed on PND 80 (Morley-Fletcher et al. 2002). This was accompanied by an increase in serotonin in the hypothalamus. Thus, MDMA administered during this period of early adolescence can have long-lasting effects on both behavior and neurochemistry.
Conditioned place preference
Exposure to MDMA increased cocaine reward and this effect was altered depending upon housing conditions. In rats living with environmental enrichment, there was an increase in the potency of cocaine for producing CPP, regardless of the social conditions. In rats living in an impoverished condition (no environmental enrichment), this shift to the left of the dose–effect curve for cocaine CPP was not evident, although there was an increase in the maximal CPP observed in the isolated rats. Thus, the effects of MDMA on cocaine reward in male adolescent rats were manifest in different ways across housing conditions.
Previously, it has been reported that adult male rats administered 5 mg/kg MDMA and put into a familiar location with other rats later exhibited greater social interaction than rats injected with vehicle (Thompson et al. 2008). Further, the rats in the combined MDMA social group had greater increases in Fos expression in several brain regions, including the medial caudate putamen, the amygdala, and parts of the hypothalamus, compared to rats receiving MDMA but kept isolated. They also had increased Fos in the nucleus accumbens and ventral tegmental area compared to vehicle treated groups. These data suggest that MDMA and social conditions interact to cause unique changes in multiple brain regions, many of which are associated with social interaction. Although there were many differences between that study and the current study, we found that social housing interacted with MDMA administration to alter both the response to MDMA stimulation and the subsequent response to cocaine. Our data suggest that even when the rats are alone in the test chamber for 1 h after MDMA is administered, the drug effects are dependent upon the continued social housing of the animals.
The effects of MDMA on drug reward appear to differ not only depending upon housing condition but also depending upon the drug being tested. Twice-daily injections of MDMA or saline during adolescence (PND 29–31) in mice increased social interactions (Daza-Losada et al. 2008b), as it had in rats, but did not alter development of CPP to a single dose of morphine. This treatment did, however, reduce reinstatement of CPP by a priming dose of morphine (Daza-Losada et al. 2008a). In this study, the CPP was done 3 weeks later, at which time the mice were adults. Thus, there are marked differences between this study and the present study, but the findings suggest that further investigation is necessary to better understand the interaction between MDMA and subsequent drug use.
A previous study in our laboratory, done under the same conditions as the present study, showed that cocaine produced decreases in dopamine transporters and tyrosine hydroxylase in the nucleus accumbens of rats living in SE3 compared to II conditions (Zakharova et al. 2009b) and that this likely accounted for behavioral changes observed in rats that were not pretreated prior to cocaine CPP procedures. It is not known whether MDMA produced similar alterations; however, the increased potency of cocaine in the SE3 rats compared to the II rats after MDMA suggests that MDMA likely is having different effects on brain neurochemistry under different housing conditions.
A number of studies in rats show that social conditions can alter drug effects and that both the social condition and the drug can interact to influence drug reward or reinforcement (Thiel et al. 2009; Thiel et al. 2008). This appears to be true not only for cocaine in both adolescents (Zakharova et al. 2009b) and adults but also for nicotine in adolescent rats (Pentkowski et al. 2011; Thiel et al. 2009). In many studies, both social and environmental enrichment have been combined and this has been shown to have profound effects on adult rats (Bardo et al. 2001; Elliott and Grunberg 2005). In addition, in adult rats housed in a socially and environmentally enriched environment, amphetamine CPP was greater than in only social housing (no enrichment) (Bowling and Bardo 1994). Those results are consistent with our finding that social enrichment alone produces different effects than does a combination of social and environmental enrichment. The present data go further to suggest that adolescent males housed in a social condition without environmental enrichment are more sensitive to the aversive effects of cocaine than are isolated rats or social rats with environmental enrichment. At present, it is not clear why this occurs.
The present study shows that social and environmental enrichment have different and sometimes interacting effects on drug reward and on the effects of the psychostimulant MDMA, and that these effects occur soon after housing under these different conditions. In adolescent males, environmental enrichment (toys) increases the effects of MDMA on cocaine reward, whereas social enrichment alone does not have this effect. In contrast to environmental enrichment, social enrichment alone appears to block the enhanced cocaine reward seen after MDMA in isolated adolescent male rats. Further, these findings show that behavioral differences subsequent to drug exposure persist past the acute effects of the drug itself and these effects are altered by both social and environmental enrichment, albeit in different ways.
References
Aberg M, Wade D, Wall E, Izenwasser S (2007) Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol 29:37–46
Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP (1995) Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav 51:397–405
Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacol (Berl) 155:278–284
Boot BP, McGregor IS, Hall W (2000) MDMA (Ecstasy) neurotoxicity: assessing and communicating the risks. Lancet 355:1818–1821
Bowling SL, Bardo MT (1994) Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav 48:459–464
Brenes JC, Fornaguera J (2008) Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neuroscience Letters 436:278–282
Daza-Losada M, Rodríguez-Arias M, Aguilar MA, Miñarro J (2008a) Effect of adolescent exposure to MDMA and cocaine on acquisition and reinstatement of morphine-induce CPP. Prog Neuro-Psychopharmacol Biol Psychiatry 32:701–709
Daza-Losada M, Rodríguez-Arias M, Maldonado C, Aguilar MA, Miñarro J (2008b) Behavioural and neurotoxic long-lasting effects of MDMA plus cocaine in adolescent mice. Eur J Pharmacol 590:204–211
Elliott BM, Grunberg NE (2005) Effects of social and physical enrichment on open field activity differ in male and female Sprague–Dawley rats. Behav Brain Res 165:187–196
Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE (2008) Monitoring the Future national survey results on drug use, 1975–2007. Volume I: secondary school students (NIH Publication No. 08-6418A). Bethesda, MD: National Institute on Drug Abuse, p 707
Kalivas P, Duffy P, White S (1998) MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacol 18:469–479
Kandel DB (1985) On processes of peer influences in adolescent drug use: a developmental perspective. Adv Alcohol Subst Abuse 4:139–163
Morley KC, McGregor IS (2000) (+/−)-3,4-Methylenedioxymethamphetamine (MDMA, 'Ecstasy') increases social interaction in rats. Eur J Pharmacol 408:41–49
Morley-Fletcher S, Bianchi M, Gerra G, Laviola G (2002) Acute and carryover effects in mice of MDMA ("ecstasy") administration during periadolescence. Eur J Pharmacol 448:31–38
NSDHS (2000) AIHW 2000. National Drug Strategy Household Survey report. Drug statistics series no. 25. Cat. no. PHE 145. Canberra: AIHW.
NSDUH (2005) Substance Abuse and Mental Health Services Administration. (2005). Results from the 2004 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-28, DHHS Publication No. SMA 05-4062). Rockville, MD.
NSDUH (2009) Substance Abuse and Mental Health Services Administration. (2009). Results from the 2007 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194). Rockville, MD.
Oetting ER, Beauvais F (1987) Peer cluster theory, socialization characteristics, and adolescent drug use: a path analysis. J Counseling Psychol 34:205–213
Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM (2006) Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res 174:181–187
Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung THC, Deviche P, Adams M, Alba J, Neisewander JL (2011) Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats. Pharmacol Biochem Behav 100:1–7
Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M (2008) Reversal of cocaine addiction by environmental enrichment. Proc Nat Acad Sci 105:17145–17150
Thiel KJ, Okun AC, Neisewander JL (2008) Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend 96:202–212
Thiel K, Sanabria F, Neisewander J (2009) Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacol 204:391–402
Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL (2011) The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav 97:595–602
Thompson MR, Hunt GE, McGregor IS (2008) Neural correlates of MDMA ("Ecstasy")-induced social interaction in rats. Social Neuroscience 4:60–72
Varlinskaya EI, Spear LP (2008) Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res 188:398–405
Von Ameln N, Von Ameln-Mayerhofer A (2010) Atypical development of behavioural sensitization to 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) in adolescent rats and its expression in adulthood: role of the MDMA chirality. Addiction Biology 15:35–44
Walker QD, Williams CN, Jotwani RP, Waller ST, Francis R, Kuhn CM (2007) Sex differences in the neurochemical and functional effects of MDMA in Sprague–Dawley rats. Psychopharmacol (Berl) 189:435–445
Zakharova E, Leoni G, Kichko I, Izenwasser S (2009a) Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent rats. Behav Brain Res 198:45–50
Zakharova E, Miller JS, Unterwald EM, Wade D, Izenwasser S (2009b) Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neurosci 163:890–897
Zakharova E, Wade D, Izenwasser S (2009c) Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav 92:131–134
Acknowledgments
This work was supported by the National Institute on Drug Abuse (Grant # DA023996). The authors acknowledge Dean Wade and Sophie Khokhawalla for their help in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starosciak, A.K., Zakharova, E., Stagg, M. et al. Differential alteration of the effects of MDMA (ecstasy) on locomotor activity and cocaine conditioned place preference in male adolescent rats by social and environmental enrichment. Psychopharmacology 224, 101–108 (2012). https://doi.org/10.1007/s00213-012-2783-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2783-0