Abstract
Rationale
The striatopallidal medium spiny neurons have been viewed as a final common path for drug reward, and the ventral pallidum (VP) as a convergent point for hedonic and motivational signaling. The medium spiny neurons are GABAergic, but they colocalize enkephalin.
Objective
The present study investigated the role of the GABAergic mechanisms of the VP in ethanol consumption.
Methods
The effects of bilateral microinjections of GABAA and GABAB receptor agonists and antagonists into the VP on voluntary ethanol consumption were monitored in alcohol-preferring Alko alcohol rats given 90 min limited access to ethanol in their home cages every other day. The influences of coadministration of GABA and opioid receptor modulators were also studied.
Results
The GABAA receptor agonist muscimol (1–10 ng/site) decreased ethanol intake dose-dependently, while administration of the GABAA receptor antagonist bicuculline (10–100 ng) had an opposite effect. The GABAB receptor agonist baclofen (3–30 ng) also suppressed ethanol intake, but the GABAB receptor antagonist saclofen (0.3–3 μg) failed to modify it. Animals coadministered with bicuculline (30 ng) and baclofen (30 ng) consumed ethanol significantly less than those treated with bicuculline alone. Coadministration of the μ-receptor agonist D-Ala2,N-Me-Phe4,Glyol5-enkephalin (DAMGO, 0.1 μg) with bicuculline counteracted, whereas the μ-receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP, 1 μg) enhanced the bicuculline-induced increase of ethanol intake. When given alone, DAMGO decreased while CTOP increased ethanol intake.
Conclusions
The study provides evidence for the ventral pallidal GABAergic mechanisms participating in the regulation of ethanol consumption and supports earlier work suggesting a role for pallidal opioidergic transmission in ethanol reward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An understanding of the neuronal mechanisms underlying ethanol consumption is a key in the development of drugs to treat alcohol dependence in humans. Intake of ethanol and other drugs of abuse is generally believed to be based on their reinforcing effects, and the mesolimbic dopamine neurons have been hypothesized to be the neural substrate for the reinforcement produced by these substances (Koob et al. 1998; Wise 1998). The cell bodies of the mesolimbic dopamine neurons are located in the ventral tegmental area and their axons project to the nuclei of the forebrain, mainly to the nucleus accumbens (NAc), the septal area, the amygdala, the hypothalamus, the hippocampus, and the frontal cortex (Koob 1992).
Drug-induced increase in dopamine release in the NAc is proposed to be a critical condition to produce reward (Bardo 1998; Koob 1992). Many drugs of abuse including ethanol enhance dopamine neurotransmission in the NAc (Koob 1992; Kiianmaa et al. 1995). Numerous studies, however, suggest that the reinforcing effects of ethanol are probably not mediated exclusively through the dopaminergic system. For instance, destruction of the mesolimbic dopamine pathways or dopaminergic terminals in the NAc does not attenuate acquisition or maintenance of ethanol self-administration behavior (Kiianmaa et al. 1979; Koistinen et al. 2001; Rassnick et al. 1993; Shoemaker et al. 2002).
A mechanism downstream of the NAc may also have a role in the mediation of reinforcement from ethanol. The mesolimbic dopamine neurons synapse on GABAergic medium spiny neurons in the NAc. One population of the medium spiny neurons also expressing enkephalin projects to the ventral pallidum (VP) and has been viewed as a final common path for drug reward, and consequently, the VP as an essential convergent point for hedonic and motivational signaling in the brain (Smith et al. 2009; Wise 2002; Zahm et al. 1985). It would appear to be depression of medium spiny neurons’ output, i.e., suppression of GABA release and activation of VP that is common to various drugs of abuse and could be important for their reinforcing properties (Wise 2002).
In accordance with this view, ethanol, amphetamine, cocaine, and heroin have been shown to suppress extracellular levels of GABA in the VP (Bourdelais and Kalivas 1990; Caille and Parsons 2004; Kemppainen et al. 2010; Li et al. 2009; Tang et al. 2005). Furthermore, cocaine injected into the VP increases locomotor activity (Gong et al. 1996), and excitotoxic lesions of the VP produce changes in intravenous self-administration of cocaine and heroin (Hubner and Koob 1990; Robledo and Koob 1993). Ethanol-maintained responding has been found to be reduced by infusions of a ligand binding at GABAA1 receptors into the VP (June et al. 2003), and in a recent study, the present group showed that intake of ethanol was decreased and increased by intrapallidal injections of μ-opioid receptor agonists and antagonists, respectively (Kemppainen et al. 2012).
The purpose of the present study was to investigate further the role of the GABAergic mechanisms of the VP in voluntary ethanol consumption. This was done by monitoring intake of ethanol after bilateral microinjection of GABAA and GABAB receptor agonists and antagonists into the VP. Since the effects of μ-opioid receptor agonists and antagonists on ethanol consumption may be based on the modulation of GABAergic neurotransmission, the influence of coadministration of GABA and μ-opioid receptor modulators was also studied (Kemppainen et al. 2012; Napier and Mitrovic 1999). The experiments were conducted using the alcohol-preferring Alko alcohol (AA) rats that consume voluntarily high amounts of ethanol. The AA and non-preferring Alko non-alcohol (ANA) rat lines have been produced by bi-directional, selective outbreeding for differential ethanol intake for unraveling the biological factors underlying predisposition for high or low ethanol consumption (Eriksson 1968; Sommer et al. 2006).
Materials and methods
Subjects
A total of 94 male alcohol-preferring AA rats (National Institute for Health and Welfare, Helsinki, Finland), housed in single wire mesh cages (21 cm × 38 cm × 19 cm), were used in this study. The rats were from generations F98-100, and they were at least 3 months of age at the beginning of the experiments. They were given food (SDS RM1 (E) SQC, Witham, Essex, England) and water ad libitum at all times. All rats used in the experiments were ethanol naïve. Ambient temperature was maintained at 22 ± 1°C and humidity at 55 ± 10 %. The light/dark cycle was 12/12 h (lights on at 0600 h). Prior to surgery, all rats were weighed between 250 and 400 g. All experimental procedures using animals were carried out in accordance with the European Communities Council Directive (2007/526/EEC) and the National Animal Welfare Act and were reviewed and approved according to the Act on the Use of Animals for Experimental Purposes (62/2006) in the National Animal Ethics Committee in Finland.
Surgery and microinjections
The rats were anesthetized with halothane (4 % during induction for 4 min and 1.5–2 % during the surgery) and attached to a stereotaxic frame for bilateral implantation of guide cannulas (o.d. 1.0 mm) into the brain just above the VP. Before operation, rats were applied lidocaine to surgical site. The injections were given using an injection cannula (o.d. 0.3 mm). The coordinates for the injection site, as given in the atlas of the rat brain by Paxinos and Watson (1998), were 0.8 mm posterior to bregma, 3.2 mm lateral to midline, and 5.1 mm below the dura. They were selected on the basis of the neurochemical work on the VP and hedonic reward (Smith and Berridge 2007). The guide cannulas were fastened to the skull with three stainless steel screws and dental cement. Body temperature was kept constant at 37°C during the procedure with a thermostatically controlled thermal mattress. After the surgery, the rats were placed in their home cages. The rats were administered carprofen (Rimadyl®, 0.1 mg/kg, s.c.) before and the day after the surgery. They were allowed to recover at least for 7 days.
After the recovery period, each rat was habituated to all the experimental procedures in four sessions conducted every other day. During the first three habituation sessions, dummies were removed from the guide cannulas without inserting injection cannulas, and during the fourth session, injection cannulas were inserted into the guides, but no injections were given. After each session, rats were returned to their home cages and given access to ethanol as described below. On the test days, each rat received simultaneous bilateral injections of 0.3 μl of a drug, a mixture of two drugs, or the vehicle at a rate of 0.3 μl/min delivered with a microinfusion pump (CMA, Stockholm, Sweden). After the injections, the injection cannulas were left in place for 1 min in order to minimize leakage up the cannula track. Drug dosages including the vehicle control, four in total, were given in a random order to all rats and at least 48 h were allowed between injections.
Ethanol intake
To establish a stable baseline of ethanol intake before surgery, two 100-ml drinking tubes containing water or 10 % (v/v) ethanol were placed on the front wall of the cage. The left–right position of the tubes was changed three times a week to avoid any side preference. Consumption of both liquids was recorded (in milliliter) daily, while measurements for food consumption and body weight were taken twice a week. All measurements were continued for 4 weeks.
The effects of GABAA, GABAB, and μ-opioid receptor agonists and antagonists on ethanol intake were studied using a limited access paradigm. Access to ethanol was limited daily to 2 h a day (0900–1100 h) for 2 weeks and then to 90 min every other day till the end of the experiment. When a stable level of ethanol intake had been established (about 2 weeks), the guide cannulas were implanted. The surgery was followed by a recovery period of about 7 days, during which intake of ethanol returned to the pre-surgery level, and habituation to the experimental procedures. After each habituation or microinjection session, rats were returned to their home cages and given access to ethanol. Intake of ethanol was then recorded at 10-min intervals for 90 min. Consumption of food and water was recorded 90 min and 24 h after the beginning of the session. Possible unspecific effects of the injections on ethanol intake were studied by giving the rats an extra limited access session without any injections 48 h after the final microinjection.
Drugs
Ethanol (Etax A, 96 % v/v, Altia, Rajamäki, Finland) was diluted with tap water. Halothane was from Nicholas Piramal India (Chennai, India), karprofen (Rimadyl Vet) from Vericore (Dundee, UK), and lidocaine from Orion Pharma (Espoo, Finland). (–)-Bicuculline methiodide (GABAA receptor antagonist), muscimol (GABAA receptor agonist), saclofen (GABAB receptor antagonist), baclofen (GABAB receptor agonist), D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) (μ-opioid receptor antagonist), and D-Ala2,N-Me-Phe4,Glyol5-enkephalin (DAMGO) (μ-opioid receptor agonist) were obtained from Tocris (Bristol, UK). Drugs for the microinjection were dissolved in a modified Ringer’s solution (148 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2⋅6H2O, pH ~ 7.00).
The injection doses (per site) used in the experiments were as follows: bicuculline, 10, 30, and 100 ng; muscimol, 1, 3, and 10 ng; baclofen, 3, 10, and 30 ng; and saclofen 0.3, 1, and 3 μg. Bicuculline (30 ng) was also coadministered as a mixture with baclofen (30 ng), DAMGO (0.1 μg), or CTOP (1 μg). The doses were selected on the basis of preliminary experiments and earlier reports by us and others (Stratford and Kelley 1997; Stratford et al. 1999; Kandov et al. 2006; Shimura et al. 2006; Moore and Boehm 2009; Kemppainen et al. 2010).
Histology
The positions of the injection cannulas were verified by fixing the brain in 10 % formalin and making frozen 100 μm coronal sections stained with thionine after completion of the experiments. Only the rats where the tip of the cannula was verified to be in the VP were included in the results.
Statistical analysis
Data on cumulative intake of ethanol recorded at 10-min intervals were analyzed with mixed-design, two-way ANOVA with treatment (drug dosages) as the between-subjects factor and measuring interval (time) as the within-subjects repeated measure. Data were also summarized in 30, 50, and 90 min periods and the total cumulative ethanol consumption for these specific time points was analyzed with one-way ANOVA. Dunnett’s test was used as a post hoc test to identify group differences. Criterion for significance was set at p = 0.05. Ethanol intake (milliliter) was converted into grams of 100 % ethanol/kg body weight for data analyses.
Results
Histology
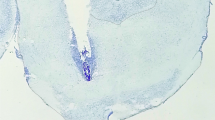
Histological examination of the probe placements showed that microinjections were given in the central or caudal portions of the VP (Fig. 1). Eleven rats were excluded from the experiments because of inappropriate placement of cannulas. Ethanol consumption of the excluded rats was not modified by the receptor ligands either.
Schematic diagram of representative injection sites in the VP. Each circle represents two individual rats and is fitted to the coronal section closest to the actual anterior–posterior site of the injection. Anterior–posterior placement of the sections is indicated relative to bregma. The coronal sections were adapted from the atlas of Paxinos and Watson (1998)
Acquisition of ethanol intake
During the period of continual access to ethanol (25 days), ethanol consumption rapidly increased from 0 to 4.15 g/kg (average of intake on days 23–25). During the last five sessions of limited access to ethanol, the average intake was 0.84 g/kg/90 min. Intake of ethanol on this level by AA rats has earlier been shown to result in brain ethanol level of about 15 mM (Nurmi et al. 1999). No significant change in intake of ethanol was observed following the surgical operation, and the level remained stable during habituation to the experimental procedures. Measurements performed after the experiments revealed that the injection procedures (four injections) did not have an effect on the basal level of ethanol intake.
Effects of GABAA receptor agonist and antagonist on ethanol intake
Muscimol
Microinjections of muscimol decreased ethanol intake in a dose-dependent manner (F 27, 288 = 6.84, p = 0.000, for drug × time interaction; F 3, 32 = 9.75, p = 0.000, for drug) (Fig. 2a). Ethanol intake was significantly suppressed after the two highest doses, 3 and 10 ng, at 30, 50, and 90 min (F 3, 32 = 8.33, p = 0.000; p < 0.01 (3 ng), p < 0.001 (10 ng) for 30 min, Dunnett’s test; F 3, 32 = 9.53, p = 0.000, p < 0.01 (3 ng); p < 0.001 (10 ng) for 50 min; F 3, 32 = 8.84, p = 0.000, p < 0.001 (3 ng); p < 0.001 (10 ng) for 90 min) (Fig. 2c).
Cumulative intake of ethanol during the 90-min session after bilateral microinjections of muscimol (n = 9) (a, c) or bicuculline (n = 14) (b, d) into the VP of AA rats. Doses are given in nanogram per site. Intake of ethanol is given in gram per kilogram (mean ± SEM at 10-min intervals). *p < 0.05, **p < 0.01, ***p < 0.001 relative to the vehicle (Dunnett’s test)
Bicuculline
There was a significant, dose-dependent increase in consumption of ethanol after intrapallidal administration of bicuculline (F 27, 468 = 4.67, p = 0.000, for drug × time interaction) (Fig. 2b). When tested, the groups differed significantly at individual time points (30, 50, and 90 min) (F 3, 52 = 4.96, p = 0.004, for 30 min; F 3, 52 = 4.34, p = 0.008, for 50 min; F 3, 52 = 3.78, p = 0.016, for 90 min). Maximal effect was found after the administration of the highest doses, 30 and 100 ng, from 30 to 90 min (p < 0.01 (30 ng), p < 0.05 (100 ng) for 30 min; p < 0.01 (30 ng), p < 0.01 (100 ng) for 50 min; and p < 0.01 (30 ng), p < 0.01 (100 ng) for 90 min) (Fig. 2d).
Effects of a GABAB receptor agonists and antagonist on ethanol intake
Baclofen
Microinjections of baclofen decreased ethanol intake significantly (F 3, 28 = 3.43, p = 0.028, for drug; F 3, 28 = 4.26, p = 0.013, for drug × time interaction) (Fig. 3a). Further analysis revealed a dose-dependent decrease of ethanol intake, which was significant after 10 (p < 0.05) and 30 ng (p < 0.01) at 30 min (F 3, 28 = 4.24, p = 0.014) and after 30 ng (p < 0.01) at 50 (F 3, 28 = 3.49, p = 0.029) and 90 min (F 3, 28 = 3.84, p = 0.003) (Fig. 3c).
Cumulative intake of ethanol during the 90-min session after bilateral microinjections of baclofen (n = 8) (a, c) or saclofen (n = 8) (b, d) into the VP of AA rats. Doses are given in nanogram per site for baclofen and in microgram per site for saclofen. Intake of ethanol is given in gram per kilogram (mean ± SEM) at 10-min intervals. *p < 0.05, **p < 0.01 relative to the vehicle (Dunnett’s test)
Saclofen
The rats receiving injections of saclofen into the VP did not differ from the vehicle-treated controls in intake of ethanol (Fig. 3b, d).
Effects of coadministration of GABAergic and opioidergic agents on ethanol intake
Bicuculline and baclofen
In this experiment, the animals received bicuculline (30 ng), baclofen (30 ng), or bicuculline (30 ng) and baclofen (30 ng). There was a significant difference among the different treatments in their effects on ethanol intake (F 3, 44 = 6.44, p = 0.001, for drug; F 27, 396 = 3.99, p = 0.000, for drug × time interaction) (Fig. 4). Post hoc analysis of the data revealed that bicuculline had only a tendency to increase ethanol intake and that baclofen failed to suppress consumption of ethanol significantly relative to the vehicle. The level of ethanol intake after coadministration of bicuculline and baclofen was, however, significantly below the level after bicuculline alone (p < 0.05 for 30 min, p < 0.01 for 50 and 90 min) but not significantly above that after baclofen alone.
Cumulative intake of ethanol during the 90-min session after bilateral microinjections of bicuculline (Bi) (30 ng), baclofen (Bf) (30 ng), or bicuculline (30 ng) and baclofen (30 ng) (Bi + Bf) into the VP of AA rats. Doses are per site, and intake of ethanol is given in gram per kilogram (mean ± SEM; n = 12) at 10-min intervals. *p < 0.05, **p < 0.01 relative to bicuculline (Dunnett’s test)
Bicuculline and DAMGO
The animals were administered here bicuculline (30 ng), DAMGO (0.1 μg), or bicuculline (30 ng) and DAMGO (0.1 μg). These treatments modified intake of ethanol significantly (F 3, 28 = 23.23, p = 0.000, for drug; F 27, 252 = 9.31, p = 0.000, for drug × time interaction) (Fig. 5a). As shown by cumulative data on ethanol consumption at 30, 50, and 90 min (Fig. 5c) (F 3, 28 = 24.23, p = 0.000, for 30 min; F 3, 28 = 27.05, p = 0.000, for 50 min; F 3, 28 = 11.04, p = 0.000, for 90 min), bicuculline increased ethanol intake at 30 and 50 min (p < 0.05) relative to the vehicle treatment, whereas DAMGO decreased it at all time points (p < 0.01). The level of ethanol intake after coadministration of bicuculline and DAMGO was significantly lower than that after the vehicle (p < 0.01) or bicuculline alone (p < 0.001) at 30 and 50 min and significantly higher than that after DAMGO alone at 90 min (p < 0.05).
Cumulative intake of ethanol during the 90-min session after bilateral microinjections of bicuculline (Bi) (30 ng), DAMGO (Da) (1 μg), or bicuculline (30 ng) and DAMGO (Bi + Da) (n = 8) (a, c), or bicuculline (Bi) (30 ng), CTOP (Ct) (1 μg), or bicuculline (30 ng) and CTOP (1 μg) (n = 12) (b, d) into the VP of AA rats. Doses are per site, and intake of ethanol is given in gram per kilogram (mean ± SEM) at 10-min intervals. *p < 0.05, **p < 0.01 relative to the vehicle; #p < 0.05, ###p < 0.001 relative to bicuculline; +p < 0.05 relative DAMGO (c); ++p < 0.01 relative to CTOP (d) (Dunnett’s test)
Bicuculline and CTOP
The animals received bicuculline (30 ng), CTOP (1 μg), or bicuculline (30 ng) and CTOP (1 μg). The treatments resulted in increased intake of ethanol as shown by the cumulative intake of ethanol over the 90-min session (F 3, 46 = 8.35, p = 0.000, for drug; F 27, 396 = 7.16, p = 0.000, for drug × time interaction) (Fig. 5b). One-way ANOVA on cumulative data at 30, 50, and 90 min (F 3, 44 = 9.63, p = 0.000, for 30 min; F 3, 44 = 8.52, p = 0.000, for 50 min; F 3, 44 = 7.58, p = 0.000, for 90 min) revealed that bicuculline increased ethanol intake (p < 0.05) at all time points, but there was only a tendency for increased consumption of ethanol after administration of CTOP (Fig. 5d). Coadministration of bicuculline and CTOP increased ethanol consumption relative to the vehicle (p < 0.01) at all three time points. Furthermore, the level of ethanol intake after bicuculline and CTOP was also significantly above that after CTOP alone (p < 0.01) at 30, 50, and 90 min and that after bicuculline alone (p < 0.05) at 30 min.
Effects of GABAergic agents and coadministration of GABAergic and opioidergic agents on food and water intake
Food and water intake recorded 90 min and 24 h after bilateral microinjections of GABAergic agents alone, or bicuculline coadministered with baclofen, DAMGO, or CTOP into the VP is given in Table 1. None of the GABAergic agents could modify water or food intake significantly when administered alone. The animals administered bicuculline, DAMGO, or bicuculline and DAMGO, however, showed a significant increase in food intake (F 3, 28 = 4.47, p = 0.011) during the 90-min session. Intake of food by the animals receiving DAMGO or bicuculline and DAMGO was significantly above the level of the vehicle-treated controls (p < 0.05). There was a significant suppression of water intake in the experiment where the animals received bicuculline, baclofen, or bicuculline and baclofen during the 90-min session (F 3, 44 = 4.26, p = 0.01). The reduction was significant in the animals receiving baclofen (p < 0.01) or bicuculline and baclofen (p < 0.05).
Discussion
The present study clarified the importance of GABAergic mechanisms of the VP in the mediation of voluntary ethanol intake by investigating the effects of intrapallidal administration of GABA receptor agonists and antagonists on the intake of ethanol by alcohol-preferring AA rats using a limited access paradigm. The results showed that microinjections of the GABAA receptor agonist muscimol into the VP decreased ethanol intake dose dependently, while administration of the GABAA receptor antagonist bicuculline had an opposite effect. Administration of the GABAB receptor agonist baclofen also suppressed intake of ethanol, but the GABAB receptor antagonist failed to modify it. In contrast, coadministration of bicuculline with baclofen could not attenuate the baclofen-induced suppression of ethanol intake. Animals coadministered the μ-opioid receptor agonist DAMGO with bicuculline consumed ethanol significantly less than those administered bicuculline alone, whereas enhanced intake of ethanol was seen after coadministration of μ-opioid receptor antagonist CTOP with bicuculline. Histological verification showed that the injections had been given into the central or posterior VP.
There is strong evidence that GABAergic neurotransmission is important for many behavioral actions of ethanol including its acute reinforcing effects (cf. Kumar et al. 2009). There are reports showing that low to moderate concentrations of ethanol enhance GABAergic neurotransmission (Ticku 1980; Ticku et al. 1986), and at the pharmacological level, one can antagonize the effects of ethanol with GABA antagonists, particularly its sedative, anxiolytic-like, and acute reinforcing actions (cf. Koob 2004). Besides the GABAA receptor complex, the GABAB receptor system has also been implicated in the modulation of ethanol’s reinforcing properties. For example, GABAergic drugs targeting GABAB receptors have efficacy in modulating ethanol intake in rodents (cf. Vlachou and Markou 2010).
The neuronal mechanisms underlying GABAergic involvement in the pharmacology of ethanol self-administration are not, however, known in detail. Besides the mesolimbic dopamine neurons, which seem to play a central role in the reinforcing effect of ethanol and other drugs of abuse, the GABAergic afferents and efferents of these neurons have also been seen as important elements of drug reward circuitry (Wise 1998). Since destruction of the mesolimbic dopamine pathways or dopaminergic terminals in the NAc does not attenuate acquisition or maintenance of ethanol consumption (Kiianmaa et al. 1979; Koistinen et al. 2001; Rassnick et al. 1993; Shoemaker et al. 2002), it has been speculated that reinforcement from ethanol may also be mediated by a mechanism downstream of the NAc, i.e., by the accumbal GABAergic medium spiny neurons that colocalize enkephalin and project to the VP (Wise 2002; Zahm et al. 1985). It has been pointed out that depression of medium spiny neurons’ output and activation of VP is common to various drugs of abuse and could be important for their reinforcing properties (Wise 2002). Furthermore, because of the abundance of dopamine, GABA, and opioid receptors, VP has been viewed as a potent substrate for hedonic reward and drug self-administration (Bardo 1998; Smith et al. 2009). It has also been emphasized that the VP is an essential convergent point for hedonic and motivational signaling in the brain and a final pathway for reward (Smith et al. 2009). In accord with these hypotheses, ethanol, amphetamine, cocaine, and heroin have been shown to suppress extracellular levels of GABA and elevate extracellular enkephalin in the VP (Bourdelais and Kalivas 1990; Caille and Parsons 2004; Kemppainen et al. 2010; Li et al. 2009; Olive and Maidment 1998; Tang et al. 2005).
In the present study, intrapallidal administration of the GABAA receptor agonist muscimol suppressed the intake of ethanol in a limited access paradigm, while administration of the GABAA receptor antagonist bicuculline was followed by an increase in ethanol consumption. This is in line with earlier studies showing that specific and unspecific procedures targeted at neurons of VP can modify self-administration of drugs of abuse. Intravenous self-administration of cocaine and heroin could be altered by excitotoxic lesions of the VP (Hubner and Koob 1990; Robledo and Koob 1993), and ethanol-maintained responding was reduced by drug infusions targeted to GABAA1 receptors in the VP (June et al. 2003). Since suppression of GABA release in the VP and the resulting disinhibition of pallidal neurons has been hypothesized to be important for the reinforcing properties of various drugs of abuse (Wise 2002), one may speculate that the increase in intake of ethanol after administration of bicuculline results from blockade of postsynaptic sites in the VP by bicuculline (Chrobak and Napier 1993). Application of muscimol, in contrast, might elicit a decrease in ethanol intake via direct inhibition of ventral pallidal neurons.
The selective GABAB receptor agonist baclofen suppressed here intake of ethanol when administered into the VP. Baclofen could also effectively inhibit the bicuculline-induced increase in consumption of ethanol. Administration of the GABAB receptor antagonist saclofen could, however, not modify ethanol intake. Numerous preclinical and clinical studies have reported earlier that GABAB receptor agonists suppress ethanol reinforcement and intake as well as reduce the use of cocaine, heroin, and nicotine (Cousins et al. 2002; Heilig and Egli 2006; Vlachou and Markou 2010). Baclofen administered intraperitoneally has been found to inhibit acquisition and motivation of ethanol consumption in rats (Colombo et al. 2000; 2003) and when microinjected into the ventral tegmental area to reduce binge-like ethanol intake (Moore and Boehm 2009). Furthermore, intrategmental and intra-NAc injections have been reported to decrease heroin reinforcement, morphine self-administration, and morphine-induced conditioned place preference (Tsuji et al. 1996; Xi and Stein 1999; Yoon et al. 2009), whereas peripheral administration of baclofen attenuates self-administration of cocaine and morphine across a wide range of conditions (Brebner et al. 2000; Roberts et al. 1996; Shoaib et al. 1998). Moreover, the GABAB receptor antagonist saclofen does not have effects on food intake when administered into the ventral tegmental area, but it induces a short-term decrease of food intake when injected into the NAc (Echo et al. 2002; Kandov et al. 2006). The lack of saclofen’s effect on ethanol intake in the present study remains unclear. In an earlier study, intrapallidal injections of another GABAB receptor antagonist, phaclofen, were without effect on locomotor activity (Austin and Kalivas 1990). The inhibitory effect of GABAB receptor agonists on the self-administration of drugs of abuse is often interpreted through a modulation of dopaminergic neurotransmission on the level of the ventral tegmental area or the NAc (Cousins et al. 2002; Xi and Stein 1999; Yoon et al. 2009). According to the results obtained here, baclofen-induced suppression of ethanol intake could also be based on baclofen blocking ethanol reward through activation of GABAB receptors on ventral pallidal neurons. Whether the attenuation of bicuculline-induced increase in ethanol consumption after intrapallidal baclofen could also be explained by modulation of the effects produced by GABA postsynaptically is a matter of speculation. The possibility of baclofen interacting with other, e.g., dopaminergic or glutamatergic, inputs of the VP cannot, however, be excluded.
In a previous study by the present group, it was shown that intrapallidal injections of the μ-opioid receptor agonists DAMGO reduced intake of ethanol in a dose-dependent manner, while the μ-opioid receptor antagonist CTOP increased ethanol intake (Kemppainen et al. 2012). These findings provided evidence for the involvement of μ-opioid receptors of the VP in the regulation of ethanol consumption. Interestingly, DAMGO counteracted the bicuculline-induced increase in ethanol intake in the present study, and coadministration of CTOP and bicuculline had an additive effect on consumption of ethanol beyond that of CTOP or bicuculline alone. The observations do not, however, clearly indicate GABAergic involvement in the effects of DAMGO and CTOP on bicuculline-induced increase in ethanol consumption. It seems possible that the changes in ethanol intake may involve both GABAA and μ-opioid receptors located postsynaptically on ventral pallidal neurons: disinhibition of the neurons by bicuculline could be attenuated by DAMGO or enhanced by CTOP producing a reduction or an increase in ethanol reward, respectively. Other investigators have suggested presynaptic μ-opioid modulation of GABAergic transmission in the VP. For instance, increases in motor activity induced by intrapallidal μ-opioid receptor stimulation might be partly mediated by reducing presynaptic release of GABA (Austin and Kalivas 1990; Tang et al. 2005). In support for this view, electrophysiological studies have shown that μ-opioid receptor activation is functionally antagonistic to GABAergic neurotransmission in the VP (Chrobak and Napier 1993; Napier and Mitrovic 1999) and that μ-opioid receptors have been localized on pallidal GABAergic afferents from the NAc (Olive et al. 1997).
The microinjections of GABAergic agents given before the 90-min access to ethanol had no effects on food or water intake when given alone. The lack of effect may be related to the animals having unlimited access to food and water at all times. In earlier studies, blockage of GABAA receptors in the VP with bicuculline has, however, been found to stimulate food intake and eating behavior and increase ingestion of saccharin solution, but does not modify water intake (Shimura et al. 2006; Smith and Berridge 2007; Stratford et al. 1999). Muscimol, in contrast, has been reported to decrease intake of saccharin and water (Shimura et al. 2006). Interestingly, there was an increase in food intake by rats receiving DAMGO or DAMGO and bicuculline during the 90-min session. Enhanced intake of food may have resulted from the administration of DAMGO, since injections of DAMGO into the posterior and central VP have been found to stimulate eating (Smith and Berridge 2005, 2007). The reduction in water intake by the rats injected with baclofen or bicuculline and baclofen in one experiment during the 90-min session is difficult to explain, since neither bicuculline nor baclofen could modify water intake when given alone in different doses in other experiments. In line with the latter findings, microinjections of bicuculline did not modify intake of water in an earlier study (Shimura et al. 2006).
Bilateral injection sites in the present study were located in the central and posterior parts of the VP. These areas were responsive for all the GABA and μ-opioid receptor agonists and antagonists excluding saclofen. The results are for the most part in line with earlier brain mapping studies suggesting functional differences within the VP in the mediation of reward and motivation (Smith and Berridge 2007; Smith et al. 2009). According to these studies, blockade of pallidal GABAA receptors increases “wanting” throughout the VP, while posterior VP μ-opioid neurotransmission encodes hedonic reward “liking”.
In summary, administration of the GABAA receptor agonist muscimol into the VP decreased ethanol intake dose dependently, while administration of the GABAA receptor antagonist bicuculline had an opposite effect. The GABAB receptor agonist baclofen also suppressed intake of ethanol, but the GABAB receptor antagonist saclofen failed to modify it. Animals coadministered with bicuculline and baclofen consumed ethanol significantly less than those treated with bicuculline alone. Coadministration of bicuculline with the μ-opioid receptor agonist DAMGO could antagonize the DAMGO-induced suppression of ethanol intake, but enhanced consumption of ethanol was seen after coadministration of bicuculline and the μ-opioid receptor antagonist CTOP. These results along with the earlier work on the involvement of opioidergic mechanism of the VP in ethanol intake (Kemppainen et al. 2012) demonstrate that both GABAergic and opioidergic mechanisms can regulate ethanol consumption in the VP and that the GABAergic striatopallidal circuitry is a major mechanism in the mediation of ethanol reward and possibly contributes to the development of alcohol dependence.
References
Austin MC, Kalivas PW (1990) Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther 252:1370–1377
Bardo MT (1998) Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol 12:37–67
Bourdelais A, Kalivas PW (1990) Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res 516:132–136
Brebner K, Phelan R, Roberts D (2000) Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav 66:857–862
Caille S, Parsons LH (2004) Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci 20:593–596
Chrobak JJ, Napier TC (1993) Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm Gen Sect 93:123–143
Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL (2000) Ability of baclofen in reducing alcohol intake and withdrawal severity: I—preclinical evidence. Alcohol Clin Exp Res 24:58–66
Colombo G, Vacca G, Serra S, Brunetti G, Carai M, Gessa G (2003) Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology 167:221–224
Cousins MS, Roberts DC, de Wit H (2002) GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend 65:209–220
Echo JA, Lamonte N, Ackerman TF, Bodnar RJ (2002) Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental area region of rats. Physiol Behav 76:107–16
Eriksson K (1968) Genetic selection for voluntary alcohol consumption in the albino rat. Science 159:739–741
Gong W, Neill D, Justice JB Jr (1996) Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res 707:64–74
Heilig M, Egli M (2006) Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111:855–876
Hubner C, Koob G (1990) The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res 508:20–29
June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC et al (2003) The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology 28:2124–2137
Kandov Y, Israel Y, Kest A, Dostova I, Verasammy J, Bernal SY, Kasselman L, Bodnar RJ (2006) GABA receptor subtype antagonists in the nucleus accumbens shell and ventral tegmental area differentially alter feeding responses induced by deprivation, glucoprivation and lipoprivation in rats. Brain Res 1082:86–97
Kemppainen H, Raivio N, Nurmi H, Kiianmaa K (2010) GABA and glutamate overflow in the VTA and ventral pallidum of alcohol-preferring AA and alcohol-avoiding ANA rats after ethanol. Alcohol Alcohol 45:111–118
Kemppainen H, Raivio N, Suo-Yrjo V, Kiianmaa K (2012) Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin Exp Res 36:286–293
Kiianmaa K, Andersson K, Fuxe K (1979) On the role of ascending dopamine systems in the control of voluntary ethanol intake and ethanol intoxication. Pharmacol Biochem Behav 10:603–608
Kiianmaa K, Nurmi M, Nykänen I, Sinclair JD (1995) Effect of ethanol on extracellular dopamine in the nucleus accumbens of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol Biochem Behav 52:29–34
Koistinen M, Tuomainen P, Hyytia P, Kiianmaa K (2001) Naltrexone suppresses ethanol intake in 6-hydroxydopamine-treated rats. Alcohol Clin Exp Res 25:1605–1612
Koob G (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184
Koob GF (2004) A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol 68:1515–1525
Koob GF, Sanna PP, Bloom FE (1998) Neuroscience of addiction. Neuron 21:467–476
Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564
Li X, Li J, Peng XQ, Spiller K, Gardner EL, Xi ZX (2009) Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: involvement of a ventral pallidal GABAergic mechanism. Neuropsychopharmacology 34:1783–1796
Moore M, Boehm S (2009) Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6 J mice. Behav Neurosci 123:555–63
Napier TC, Mitrovic I (1999) Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci 877:176–201
Nurmi M, Sinclair JD, Kiianmaa K (1999) Brain ethanol levels after voluntary ethanol drinking in AA and Wistar rats. Alcohol 19:113–118
Olive MF, Maidment NT (1998) Opioid regulation of pallidal enkephalin release: bimodal effects of locally administered mu and delta opioid agonists in freely moving rats. J Pharmacol Exp Ther 285:1310–131
Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT (1997) Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci 17:7471–7479
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Rassnick S, Stinus L, Koob GF (1993) The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res 623:16–24
Roberts D, Andrews M, Vickers G (1996) Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15:417–423
Robledo P, Koob GF (1993) Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res 55:159–166
Shimura T, Imaoka H, Yamamoto T (2006) Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci 23:1596–1604
Shoaib M, Swanner L, Beyer C, Goldberg S, Schindler C (1998) The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol 9:195–206
Shoemaker WJ, Vavrousek-Jakuba E, Arons CD, Kwok FC (2002) The acquisition and maintenance of voluntary ethanol consumption in the rat: effects of dopaminergic lesions and naloxone. Behav Brain Res 137:139–148
Smith KS, Berridge KC (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. Neuroscience 25:8637–8649
Smith KS, Berridge KC (2007) Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Neuroscience 27:1594–1605
Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196:155–67
Sommer W, Hyytiä P, Kiianmaa K (2006) The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol 11:289–309
Stratford T, Kelley A (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17:4434–4440
Stratford T, Kelley A, Simansky K (1999) Blockade of GABA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825:199–203
Tang XC, McFarland K, Cagle S, Kalivas PW (2005) Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci 25:4512–4520
Ticku M (1980) The effects of acute and chronic ethanol administration and its withdrawal on gamma-aminobutyric acid receptor binding in rat brain. Br J Pharmacol 70:403–410
Ticku M, Lowrimore P, Lehoullier P (1986) Ethanol enhances GABA-induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull 17:123–126
Tsuji M, Nakagawa Y, Ishibashi Y et al (1996) Activation of ventral tegmental GABAB receptors inhibits morphine-induced place preference in rats. Eur J Pharmacol 313:169–173
Vlachou S, Markou A (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371
Wise RA (1998) Drug-activation of brain reward pathways. Drug Alcohol Depend 51:13–22
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240
Xi ZX, Stein EA (1999) Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther 290:1369–1374
Yoon SS, Kim JA, Lee BH, Choi KH, Shim I, Choi SH, Hwang M, Yang CH (2009) Role for GABA agonists in the nucleus accumbens in regulating morphine self-administration. Neurosci Lett 462:289–293
Zahm DS, Zaborszky L, Alones VE, Heimer L (1985) Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res 325:317–321
Acknowledgments
This paper was supported by grants from the Academy of Finland and the Finnish Foundation for Alcohol Studies. We thank Ms. Leena Tanner-Väisänen for her technical assistance.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemppainen, H., Raivio, N. & Kiianmaa, K. Role for ventral pallidal GABAergic mechanisms in the regulation of ethanol self-administration. Psychopharmacology 223, 211–221 (2012). https://doi.org/10.1007/s00213-012-2709-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2709-x