Abstract
Rationale
Neonatal maternal separation (MS) has been used to model the effects of early life stress in rodents. MS alters behavioral responses to a variety of abused drugs, but few studies have examined its effects on methamphetamine sensitivity.
Objectives
We sought to determine the effects of MS on locomotor and stereotyped responses to low-to-moderate doses of methamphetamine in male and female adolescent rats.
Methods
Male and female rat pups were subjected to 3 h per day of MS on postnatal days (PN) 2–14 or a brief handling control procedure during the same period. During adolescence (approximately PN 40), all rats were tested for locomotor activity and stereotyped behavior in response to acute methamphetamine administration (0, 1.0, or 3.0 mg/kg, s.c.).
Results
MS rats of both sexes exhibited increased locomotor activity in a novel environment, relative to handled controls. MS increased the locomotor response to methamphetamine (METH), and this effect occurred at different doses for male (3.0 mg/kg) and female (1.0 mg/kg) rats. MS also increased stereotyped behavior in response to METH (1.0 mg/kg) in both sexes.
Conclusions
MS enhances the locomotor response to METH in a dose- and sex-dependent manner. These results suggest that individuals with a history of early life stress may be particularly vulnerable to the psychostimulant effects of METH, even at relatively low doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As many as 46% of U.S. adults report adverse childhood experiences, such as abuse, neglect, or family dysfunction (Dube et al. 2010). Such stressful early life events can have lasting negative effects on physical and mental health. In particular, children who are abused or neglected are at increased risk for developing substance use disorders (Dube et al. 2010; Sinha 2008). This increased vulnerability is presumed to reflect complex interactions between biological, psychological, and social risk factors, all of which are influenced by early adverse experience. These complexities, coupled with high attrition rates in clinical studies of substance abusers, have made it difficult to isolate biological mechanisms that contribute to increased rates of substance abuse in this high-risk population.
Animal models have shed considerable light on the long-term consequences of early life stress for brain development and behavior. The most extensively studied rodent model of early life stress is neonatal maternal separation (MS), in which rat pups are removed from the dam’s nest for a few hours each day during the first 2 weeks of postnatal life. From postnatal days (PN) 4–14, activation of the hypothalamic–pituitary–adrenal (HPA) axis of the neonatal rat is inhibited by the presence and maternal behavior of the dam (Levine 2001; Stanton et al. 1987).
Thus, this period of development has been called the stress hyporesponsive period (SHRP) (Rosenfeld et al. 1992; Sapolsky and Meaney 1986). During prolonged maternal separation, pups exhibit significant increases in plasma corticosterone and reduction of corticotrophin-releasing factor expression in the median eminence (Pihoker et al. 1993), reflecting a loss of maternal stress buffering. It is believed that abnormal activation of the HPA axis during the SHRP leads to persistent neuroadaptations in stress-responsive circuits, which contribute to later behavioral alterations. As adults, MS rats exhibit exaggerated neuroendocrine stress responses (Plotsky and Meaney 1993) and increased anxiety-like behavior (Daniels et al. 2004; Huot et al. 2001; Troakes and Ingram 2009).
The effects of MS on behavioral and neurochemical responses to a variety of abused drugs have also been studied, and many of these studies have focused on psychostimulants. MS animals show enhanced locomotor responses to low-dose cocaine and develop a sensitized locomotor response to d-amphetamine under mildly stressful conditions which do not sensitize handled or nonhandled controls (Brake et al. 2004). Similarly, Marin and Planeta (2004) observed increased sensitivity to the locomotor-activating effects to cocaine in adolescent MS rats, relative to nonhandled controls. However, other studies have found no difference between MS and control rats in behavioral responses to d-amphetamine (Hensleigh et al. 2011; Marmendal et al. 2004; Muhammad and Kolb 2011). These divergent findings are likely due to methodological differences in the MS procedure, such as duration, frequency, and developmental timing of separation, as well as the age at which behavioral testing occurred.
Though MS has been reported to alter the locomotor and reinforcing effects of cocaine and d-amphetamine, surprisingly, little has been published about the effects of MS on behavioral responses to methamphetamine (METH). A recent report indicated that MS does not alter the rewarding effects of METH in rats, as measured by conditioned place preference (Faure et al. 2009). However, this study did not quantify the acute locomotor or stereotypy-inducing effects of METH. To our knowledge, this is the only published study that has examined the effects of maternal separation on METH-induced behaviors. The goal of the present study is to characterize the effects of maternal separation on acute METH-induced locomotor activation and stereotyped behaviors in male and female adolescent rats at low-to-moderate doses.
Materials and methods
Subjects
Long–Evans rats (N = 62) were bred in the University of Nevada Las Vegas animal colony. All animals were housed in a temperature- and humidity-controlled room, on a 12:12 light cycle (lights on 0600), with ad libitum access to water and standard rodent chow. Breeding pairs were placed in breeding cages in the evening, and tray papers beneath the cages were checked each morning for semen plugs. Upon discovery of a plug, females were housed individually in standard polypropylene tub cages until parturition. The day of birth was designated postnatal day 0 (PN 0). On PN 1, pups were counted and sexed, and litters were culled to achieve litter sizes of 8–12 pups with approximately equal sex ratios.
Apparatus
Locomotor activity chambers were 45 × 45 × 45-cm open-top, clear Plexiglas boxes housed inside sound-attenuating cabinets. Each chamber was equipped with a fan, for ventilation and noise masking, a 15-W light bulb, a video camera, and a 16 × 16 photobeam grid placed 2.5 cm above the floor. Locomotor data were collected by the photobeam grid and processed on a Dell computer equipped with Motor Monitor software (Kinder Scientific, Poway, CA). Digital video recordings of each testing session were made and archived on the same Dell computer using VistaPro software (Lorex, Ontario, Canada).
Drugs
(+)-Methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline at two concentrations: 1.0 and 3.0 mg/ml. Concentrations are expressed as salt. Injections were given subcutaneously in a volume of 1.0 ml/kg body weight.
Procedures
All procedures were approved by the University of Nevada Las Vegas Institutional Animal Care and Use Committee and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research 1996).
Maternal separation
On PN 1, each litter was assigned to either the MS or control condition, and experimental manipulations began on PN 2. MS pups were removed from the dam’s home cage, weighed, given an identification mark with a nontoxic pen, and isolated from littermates in individual containers (Ziploc® 10-oz storage containers without lids) for 3 h per day on PN 2–14. Pups were maintained at nest temperature (30–33°C) by placing containers on an electric heating pad. Control pups were removed from the dam’s home cage, weighed, marked for identification, and immediately returned to the dam. Control pups were handled only to the extent necessary to weigh and mark them and did not receive any stroking or anogenital stimulation. This brief handling procedure resulted in separation from the dam for approximately 15 min per day. All litters underwent twice-weekly cage changes throughout the study. After PN 14, all litters were left undisturbed, except for cage changes, until PN 21, when they were weaned and housed in same-sex groups of two to three rats per cage.
Methamphetamine testing
Behavioral testing took place during the period of development corresponding to adolescence. On approximately PN 40 (range, PN 40–43), rats were tested for behavioral responses to acute methamphetamine treatment. On the testing day, rats were transported from the colony to a testing room and allowed to habituate to the testing room in the home cage for 30 min. Each rat was then placed individually in a locomotor activity chamber for 30 min to assess the behavioral response to a novel environment and to habituate to the activity chamber. After the 30-min exposure to the novel activity chamber, each rat was injected with 0.9 % saline (s.c., 1.0 ml/kg) and placed back in the activity chamber for an additional 30 min.
Finally, each rat was injected with saline or one of two doses of methamphetamine (1.0 or 3.0 mg/kg, s.c.; N = 5–7 per group) and placed back in the activity chamber for 2 h. During each session (novelty, saline, and drug), locomotor activity was recorded by the photobeam array, and digital video recordings were made for later assessment of stereotyped behaviors. Each dose group was comprised of rats from at least three different litters.
Data analysis
Because litter effects are of particular concern in maternal separation studies, such effects were controlled methodologically and statistically. First, animals from each litter were distributed evenly among dose groups. Second, all animals from a particular litter that were assigned to the same dose/sex group were treated as a single data point, as suggested by Zorrilla (1997). That is, all male rats treated with 1.0 mg/kg METH from a single litter were considered as a single subject, and the averages of their locomotor activity or stereotypy scores were used for analyses. Locomotor activity was expressed as distance traveled in centimeters. Stereotyped behaviors were scored by an observer blind to treatment condition using the method of Creese and Iversen (1974). Briefly, video recordings were divided into 10-min segments. Behavior during the first minute of each segment was rated on a 0–6 scale: 0 = asleep or lying down, 1 = predominantly slow locomotor activity with nonstereotyped rearing and sniffing, 2 = predominantly rapid locomotor activity with bursts of stereotyped rearing and sniffing, 3 = predominantly stereotyped rearing and sniffing with some locomotor activity, 4 = stereotyped rearing and sniffing maintained in a small area of the enclosure, 5 = stereotyped behavior maintained in a small area with oral stereotypies (licking or gnawing), and 6 = assumption of awkward or bizarre posture. All raters were trained on the same set of sample videos and achieved an inter-rater reliability of at least 0.90 before scoring experimental videos. Total distance traveled was analyzed by three-way ANOVA (condition × sex × dose). Distance traveled per 10-min interval after METH injection was analyzed by mixed model ANOVA, with time as the within-subjects factor and condition, sex, and dose as between-subjects factors. Stereotypy data were analyzed using the Aligned Rank Transform (Wobbrock et al. 2011). This approach allows for factorial analysis of nonparametric data, including interactions between factors, by first applying a transform that aligns data for each effect, then ranking the data points. The aligned rank data are then subjected to a factorial ANOVA. Significant interaction effects were followed up with simple effects analysis, and significant dose effects were followed by Fisher’s least significant difference post hoc tests. Significance was set at α = 0.05. Locomotor activity data during the novel/habituation session were lost for four control animals (two males and two females) due to a computer malfunction. One animal was excluded from the stereotypy analyses due to poor video image quality.
Results
Rearing condition did not affect body weight (Table 1). All pups gained weight across the study period, as evidenced by a significant effect of time (F 14, 112 = 1,158.48, p < 0.001). There was also a significant effect of sex (F 1, 8 = 29.43, p = 0.001), due to a larger weight gain in male pups than in females between weaning (PN 21) and testing (PN 40–43) (significant time × sex interaction F 14, 112 = 26.14, p < 0.001). Simple effects analysis revealed a sex difference only on the testing day (p < 0.01).
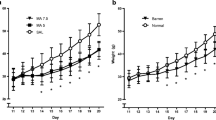
For the novelty test (Fig. 1), there was a significant effect of condition, with MS rats exhibiting greater activity than controls (F 1, 11 = 7.71, p = 0.02). The effect of sex was also significant, with females exhibiting higher activity than males (F 1, 11 = 6.56, p = 0.03). The interaction between condition and sex was not significant. There were no significant condition or sex effects on the locomotor response to a saline injection (data not shown).
METH dose-dependently increased locomotor activity (main effect of dose F 2, 32 = 88.30, p < 0.001; Fig. 2). There were also significant effects of rearing condition (F 1, 32 = 7.80, p = 0.01) and of sex (F 1, 32 = 16.07, p = 0.001) for the locomotor response to METH. The sex × dose interaction was also significant (F 2, 32 = 4.17, p = 0.03). Females treated with 1.0 or 3.0 mg/kg METH were more active than males treated with the same dose (p < 0.05), but the sex difference was not significant for saline. The condition × sex and condition × dose interactions were not significant. There was, however, a significant three-way interaction between condition, sex, and dose (F 2, 32 = 5.64, p = 0.01). That is, MS females were more active in response to 1.0 mg/kg METH than were same-sex controls (p < 0.01), whereas MS and control males did not differ at this dose. At 3.0 mg/kg, MS males were more active than controls (p < 0.05), whereas MS and control females did not differ.
Locomotor activity after acute methamphetamine administration. Bars represent mean ± SEM of distance traveled (cm) for 2 hours after METH injection. *p < 0.05 vs. same dose, same sex control (condition effect); **p < 0.01 vs. same dose, same sex control, #p < 0.05 vs. same condition, same dose males (sex effect), §p < 0.05 vs. same condition, same sex saline (dose effect)
The time course of the locomotor response to METH was also analyzed. The main effect of time was significant (F 11, 231 = 23.36, p < 0.001), as evidenced by an initial increase (intervals 1–3), followed by a decrease (intervals 4–12) in distance traveled (Fig. 3). There was also a significant interaction between time and dose (F 22, 231 = 7.14, p < 0.001), such that animals treated with 3.0 mg/kg METH maintained locomotor activity above baseline for a longer period than did those treated with 1.0 mg/kg or saline. There were no significant interactions between time and condition or time and sex.
METH also dose-dependently increased expression of stereotyped behaviors (main effect of dose F 2, 31 = 79.26, p < 0.001; Fig. 4). Fisher’s LSD post hoc test confirmed that both doses of METH differed significantly from saline, as well as from each other (all comparisons p < 0.01). There was a significant effect of condition (F 1, 31 = 24.30, p < 0.001), with MS rats generally exhibiting more intense stereotypy than controls. There was also a significant interaction between condition and dose (F 2, 31 = 9.84, p = 0.001), such that MS rats treated with 1.0 mg/kg METH had higher stereotypy than did controls treated with the same dose (p < 0.01). Stereotypy scores did not differ between MS and control rats treated with 3.0 mg/kg METH. Unlike locomotor activity, there was no sex difference in the stereotypy-inducing effects of METH.
Discussion
Numerous studies have indicated that repeated neonatal maternal separation alters acute behavioral responses to cocaine and d-amphetamine. However, the effects of MS on sensitivity to METH have remained largely unknown. The results of the present study demonstrate that adolescent MS rats are more sensitive than are controls to both the locomotor-activating and stereotypy-inducing effects of METH. The effect of MS was both sex and dose dependent. MS enhanced the locomotor response to METH in female rats only at a low dose (1.0 mg/kg) and in males only at a moderate dose (3.0 mg/kg). The lack of an MS effect at the high dose in female rats may be due to a ceiling effect, and evaluation of additional doses of METH would be necessary to confirm this explanation. We did not observe any group differences in the time course of METH-induced locomotor activity, suggesting that the effects of MS are not due to pharmacokinetic alterations. MS increased the stereotyped response to low-dose, but not moderate-dose METH, in both sexes. Again, further enhancement of stereotyped behavior may not have been observable at the moderate dose due to a ceiling effect, and testing additional doses may shed light on this issue. In general, however, these results suggest that repeated maternal separation enhances both locomotor and stereotyped responses to METH at relatively low doses.
In contrast to the present findings, other studies that have examined the effects of MS on METH-induced behaviors have not found increased METH responsiveness in MS animals. Faure and colleagues (2009) found no difference in the magnitude or duration of METH-conditioned place preference (1.0 mg/kg), nor any difference in the acute locomotor effects of apomorphine between male rats separated for 3 h on PN 2–14 and nonseparated controls. However, MS animals with repeated METH exposure exhibited gradual reduction in apomorphine-induced locomotor activity over three test sessions. This result could be interpreted either as development of tolerance to the locomotor-activating effects of apomorphine or sensitization of its stereotypy-inducing effects, which would compete with locomotor activity. However, stereotyped behaviors were not quantified in that study nor were the locomotor-activating effects of acute or repeated METH. Therefore, it is difficult to make direct comparisons with the present findings.
Though there are few published reports on the effects of MS on METH responsiveness, there are several studies on the effects of MS on cocaine sensitivity. Our results are in line with those of Brake and colleagues (2004) and Marin and Planeta (2004), who found that adult and adolescent male MS rats were more sensitive to the locomotor-activating effects of low-to-moderate doses (10–20 mg/kg s.c. or i.p.) of cocaine. Though these two studies differed in the duration and timing of the MS procedure and in the type of control groups employed, the effects on cocaine sensitivity were consistent. In contrast, female MS (15 min or 3 h/day) and animal facility-reared (AFR) rats did not differ in the acute locomotor response to i.v. cocaine (1.0 mg/kg) (Li et al. 2003). However, in the same study, AFR rats exhibited more robust sensitization to repeated i.v. cocaine administration. Planeta and Marin (2002) found similar acute and sensitizing effects of cocaine (10.0 mg/kg, s.c.) in periadolescent MS (3 h/day) and nonhandled (equivalent to AFR) rats. Thus, the effects of MS on locomotor responsiveness to cocaine may depend on the cocaine dose, route and pattern of administration, as well as the age and sex of the animals.
The effects of MS on behavioral responses to d-amphetamine have also been investigated by several studies. Matthews and colleagues (1996) found that adult, female rats subjected to ten sessions of MS from PN 5–20 were less sensitive to the unconditioned locomotor-activating effects of low-dose d-amphetamine (0.5 mg/kg) than were handled controls. This decrease in locomotor activity was not accounted for by an increase in stereotyped behavior in MS females nor were there any effects of MS on locomotor activity at higher amphetamine doses.
A study of adult, male rats found no difference in amphetamine-stimulated locomotor activity (1.0 mg/kg) between MS (4 h/day on PN 1–15) and handled controls (Marmendal et al. 2004). Similarly, a recent study in our lab demonstrated no effect of MS (3 h/day from PN 2–8) on locomotor or stereotyped responses to d-amphetamine across a range of doses (0.5–5.0 mg/kg) in adult rats of either sex (Hensleigh et al. 2011). Muhammad and Kolb (2011) also found no differences between control rats and those maternally separated on PN 3–21 in their acute locomotor response to d-amphetamine (1.0 mg/kg) in adulthood nor in development or expression of amphetamine sensitization. Thus, most studies have found no effect of maternal separation on d-amphetamine-induced locomotor activity or stereotypy. One key difference between the study that did (Matthews et al. 1996) and those that did not find an effect of MS on d-amphetamine sensitivity was that, where a significant MS effect was observed, separation took place on randomly selected days, rather than every day. Taken together, these findings suggest that unpredictable early life stress may be more effective at engaging neuroadaptations that result in altered behavioral responses to d-amphetamine.
As discussed above, most studies suggest that repeated MS has no effect on or decreases adult locomotor responses to d-amphetamine. Given that d-amphetamine and METH are thought to have nearly identical mechanisms of action, our results are somewhat surprising. The most likely explanation for this discrepancy is that most of these studies tested the effects of d-amphetamine in adult, rather than adolescent rats. Indeed, other studies have suggested that adolescent rats are less sensitive to the psychomotor stimulant effects of d-amphetamine than are adults (Bolanos et al. 1998). Therefore, it is possible that MS-induced increases in amphetamine response are more easily detected in adolescent rats. However, it should also be noted that adolescent rats have shown increased locomotor activity and dopamine efflux in response to dopamine transporter inhibitors (e.g., methylphenidate), relative to adults (Walker et al. 2010). Thus, adolescence is a time of altered sensitivity of psychostimulant drugs, possibly due to incomplete development of monoamine transporter expression. It will, therefore, be important to more thoroughly characterize the effects of MS on d-amphetamine responses in adolescent rats and on METH responses in adult rats.
There are also some pharmacological differences between METH and d-amphetamine that could account for the divergent effects of MS on sensitivity to the locomotor-activating effects of these drugs. Though it is widely regarded as a more potent psychomotor stimulant, METH is actually less effective than d-amphetamine at increasing locomotor activity at equivalent doses (Shoblock et al. 2003). Thus, it is possible that the enhancing effects of MS on locomotor activation by d-amphetamine are masked by ceiling effects and may only be detectable at very low doses (i.e., <0.5 mg/kg). There are also subtle differences in the neurochemical effects of d-amphetamine and METH. Though both drugs promote dopamine release in the nucleus accumbens, only d-amphetamine increases dopamine release in the prefrontal cortex. Conversely, METH, but not d-amphetamine, increases extracellular glutamate concentrations in the prefrontal cortex. Furthermore, d-amphetamine, but not METH, increases extracellular glutamate concentrations in the nucleus accumbens (Shoblock et al. 2003). Perhaps the glutamate-releasing effects of d-amphetamine in the nucleus accumbens compensate for any changes produced by MS in dopaminergic function, resulting in equivalent locomotor responses in MS and control rats. Alternatively, MS may trigger alterations in glutamatergic inputs to the prefrontal cortex that render separated rats more sensitive to the effects of METH, but not d-amphetamine.
A number of neuroadaptations have been observed in MS animals, which may account for increased sensitivity to the psychomotor stimulant effects of METH. These include changes in tissue monoamine content and metabolism. Matthews et al. (2001) found that adult MS rats (6 h/day on ten occasions from PN 5–20) had increased tissue dopamine (DA) content in both the dorsal and ventral striatum, as well as evidence of decreased DA turnover in the prefrontal cortex. A more recent study, however, found no changes in tissue dopamine in adult female rats previously exposed to long MS (3 h twice per day on PN 1–13). In that study, MS females had higher basal tissue concentrations of 5-HIAA and HVA in the nucleus accumbens relative to AFR controls, suggesting increased serotonergic activity in and increased extracellular metabolism of DA (Arborelius and Eklund 2007). Changes in evoked dopamine release, as well as transporter and receptor expression, have also been found in MS rats. Rats subjected to MS (3 h/day) on PN 2–14 exhibit reduced dopamine transporter density in the striatum and nucleus accumbens, as well as increased extracellular dopamine in the nucleus accumbens in response to tail-pinch, relative to briefly separated controls (Brake et al. 2004; Meaney et al. 2002). Another study found enhanced accumbens dopamine release in MS rats after d-amphetamine administration (Hall et al. 1999). Ploj and colleagues (2003) found elevated D2 dopamine receptor binding in the ventral tegmentum of handled rats, relative to those subjected to long MS (6 h/day) and AFR controls. The same study found reduced D1 dopamine receptor binding in the nucleus accumbens of long MS rats, relative to handled controls, after chronic ethanol consumption. However, increased D1 receptor binding in the nucleus accumbens has been observed in drug-naïve MS rats, relative to AFR controls (Moffett et al. 2007). Taken together, these findings suggest that MS rats may have reduced dopamine reuptake capacity and alterations in both presynaptic and postsynaptic dopamine receptor expression that contribute to enhanced dopaminergic signaling and consequent hypersensitivity to psychostimulants.
The present results demonstrate that, at moderate doses (1.0 mg/kg), the effects of MS on METH-induced locomotor activity are more pronounced in females. Other studies have also demonstrated that female rats are more sensitive to the long-term effects of maternal separation than are males (Desbonnet et al. 2008; Matthews et al. 1999; Matthews et al. 2001). Furthermore, our findings are consistent with the wealth of evidence that female rats are more sensitive to the locomotor-activating effects of psychostimulants than are males (Festa et al. 2004; Milesi-Halle et al. 2005; Schindler et al. 2005). Among human users, women begin taking METH earlier and show stronger dependence than men (Dluzen and Liu 2008) and may escalate their use of METH more rapidly than men (Brecht et al. 2004). Our results suggest that, by enhancing the already increased sensitivity of females, early life stress could be particularly detrimental to women in terms of risk for METH abuse and dependence.
A strong correlation exists between early life stress and risk for developing substance use disorders (Dube et al. 2010; Sinha 2008). With regard to METH, a history of childhood adverse events predicts earlier onset and increased severity of METH dependence (Messina et al. 2008).
The present results support clinical findings and suggest that early life stress may enhance the psychomotor stimulant effects of METH during adolescence, the developmental stage during which drug use is most often initiated. Furthermore, our results suggest that the effects of early life stress on low-dose METH sensitivity may be more pronounced in females. Future research should address the effects of early life stress on the reinforcing effects of METH, as well as the underlying neuroadaptations responsible for the MS-induced increase in METH sensitivity. A better understanding of these neural mechanisms could provide a framework for the development of interventions to decrease substance abuse risk among individuals with early adverse experiences.
References
Arborelius L, Eklund MB (2007) Both long and brief maternal separation produce persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience 145(2):738–750
Bolanos CA, Glatt SJ, Jackson D (1998) Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res 111(1):25–33
Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19(7):1863–1874
Brecht ML, O'Brien A, von Mayrhauser C, Anglin MD (2004) Methamphetamine use behaviors and gender differences. Addict Behav 29(1):89–106
Creese I, Iversen SD (1974) The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia 39(4):345–357
Daniels WM, Pietersen CY, Carstens ME, Stein DJ (2004) Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis 19(1):3–14
Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG (2008) Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci 26(3–4):259–268
Dluzen DE, Liu B (2008) Gender differences in methamphetamine use and responses: a review. Gend Med 5(1):24–35
Dube SR, Cook M, Edwards VJ (2010) Health-related outcomes of adverse childhood experiences in Texas, 2002. Prev Chron Dis 7(3):1–9
Faure J, Stein DJ, Daniels W (2009) Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metab Brain Dis 24(4):541–559
Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46(5):672–687
Hall FS, Wilkinson LS, Humby T, Robbins TW (1999) Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 32(1):37–43
Hensleigh E, Smedley L, Pritchard LM (2011) Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Dev Psychobiol 53(2):132–140. doi:10.1002/dev.20499
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 158(4):366–373
Institute for Laboratory Animal Research (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington D.C
Levine S (2001) Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol Behav 73(3):255–260
Li Y, Robinson TE, Bhatnagar S (2003) Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res 960(1–2):42–47
Marin MT, Planeta CS (2004) Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res 1013(1):83–90
Marmendal M, Roman E, Eriksson CJ, Nylander I, Fahlke C (2004) Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol 45(3):140–152
Matthews K, Hall FS, Wilkinson LS, Robbins TW (1996) Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology (Berl) 126(1):75–84
Matthews K, Robbins TW, Everitt BJ, Caine SB (1999) Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 141(2):123–134
Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW (2001) Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse 40(1):1–10
Meaney MJ, Brake W, Gratton A (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27(1–2):127–138
Messina N, Marinelli-Casey P, Hillhouse M, Rawson R, Hunter J, Ang A (2008) Childhood adverse events and methamphetamine use among men and women. J Psychoact Drugs S5:399–409
Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM (2005) Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol 209(3):203–213
Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73(3):321–330
Muhammad A, Kolb B (2011) Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res 223(1):7–16
Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB (1993) Maternal separation in neonatal rats elicits activation of the hypothalamic–pituitary–adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology 18(7):485–493
Planeta CS, Marin MT (2002) Effect of cocaine on periadolescent rats with or without early maternal separation. Braz J Med Biol Res 35(11):1367–1371
Ploj K, Roman E, Nylander I (2003) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neurosci 121(3):787–799
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 18(3):195–200
Rosenfeld P, Wetmore JB, Levine S (1992) Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav 52(4):787–791
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396(1):64–76
Schindler CW, Bross JG, Thorndike EB (2005) Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol 442(3):231–235
Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD (2003) Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 165(4):359–369
Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci 1141:105–130
Stanton ME, Wallstrom J, Levine S (1987) Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol 20(2):131–145
Troakes C, Ingram CD (2009) Anxiety behaviour of the male rat on the elevated plus maze: associated regional increase in c-fos mRNA expression and modulation by early maternal separation. Stress 12(4):362–369
Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM (2010) Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther 335(1):124–132
Wobbrock JO, Findlater L, Gergle D, Higgins JJ (2011) The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. Conf Hum Factors Comput Syst-Proc 143–146
Zorrilla EP (1997) Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 30(2):141–150
Acknowledgments
This work was supported by grants from the National Center for Research Resources (5P20RR016464-11) and the National Institute of General Medical Sciences (8 P20 GM103440-11) of the National Institutes of Health. We would like to thank Kelly AbuAli and Alan Jager for their assistance with editing and scoring behavioral videos. The experiments described herein comply with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pritchard, L.M., Hensleigh, E. & Lynch, S. Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology 223, 27–35 (2012). https://doi.org/10.1007/s00213-012-2679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2679-z