Abstract
Rationale
Cognitive impairments are important determinants of functional outcome in psychosis, which are inadequately treated by antipsychotic medication. Modafinil is a wake-promoting drug that has been shown to improve attention, memory and executive function in the healthy population and in patients with schizophrenia.
Objectives
We aimed to establish modafinil’s role in the adjunctive treatment of cognitive impairments in the first episode of psychosis, a time when symptoms may be more malleable than at chronic stages of the disease.
Methods
Forty patients with a first episode of psychosis participated in a randomised, double-blind, placebo-controlled crossover design study assessing the effects of a single dose of 200 mg modafinil on measures of executive functioning, memory, learning, impulsivity and attention.
Results
Modafinil improved verbal working memory (d = 0.24, p = 0.04), spatial working memory errors (d = 0.30, p = 0.0004) and strategy use (d = 0.23, p = 0.03). It also reduced discrimination errors in a task testing impulsivity. Modafinil showed no effect on impulsivity measures, sustained attention, attentional set-shifting, learning or fluency.
Conclusions
Modafinil selectively enhances working memory in first episode psychosis patients, which could have downstream effects on patients’ social and occupational functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychotic disorders are debilitating mental illnesses characterised by disorganised thoughts and speech, hallucinations, delusions, and cognitive and emotional impairments. Cognitive impairments are common, often appearing before the onset of the psychotic syndrome (Salokangas and Mcglashan 2008) and contribute to poor functional outcomes (Green 2006). People with schizophrenia have abnormalities in attention, memory, verbal processes, impulsivity and executive functions, and deficits are present in first episode psychosis (FEP), often before a formal diagnosis of schizophrenia can be established (Addington et al. 2006). Abnormalities in working memory have been observed at all stages of the illness (Joyce et al. 2002; Pantelis et al. 2009), whereas cognitive flexibility seems to be progressive during the course of schizophrenia (Pantelis et al. 2009). Chronic schizophrenia and FEP patients also share deficits in inhibitory control (Enticott et al. 2008; Huddy et al. 2009) and verbal memory (Doughty and Done 2009; Leeson et al. 2009a).
Antipsychotics are the current pharmacological treatment for psychotic disorders. They show efficacy for positive symptoms, such as hallucination and delusions, but are less effective in treating patients’ cognitive impairments (Keefe et al. 2004; Purdon et al. 2000; Remington et al. 2010).
Modafinil is a central nervous system wake-promoting agent indicated for the treatment of excessive daytime sleepiness. Although its mechanisms are relatively unknown, human and animal research suggests that it directly or indirectly activates the dopaminergic (De Saint Hilaire et al. 2001; Volkow et al. 2009), glutamatergic (Ferraro et al. 1999), noradrenergic (De Saint Hilaire et al. 2001; Minzenberg et al. 2008) and serotonergic (De Saint Hilaire et al. 2001) systems in several regions of the brain, including the prefrontal cortex, hippocampus, hypothalamus and striatum, whereas it inhibits GABAergic pathways in the same cerebral regions (Ferraro et al. 1999). The hippocampus and its interplay with the prefrontal cortex control brain states associated with memory, and it is likely that modafinil improves this function by inducing changes in the neurotransmitter composition in these areas.
Modafinil has been shown to improve working memory in animals (Pierard et al. 2007), healthy humans (Turner et al. 2003) and individuals with neuropsychiatric disorders (Turner et al. 2004a, b). It has also shown enhancement in learning processes in animals (Beracochea et al. 2002) and improvement in attention, memory, inhibitory control and executive function in healthy (Turner et al. 2003) and sleep-deprived volunteers (Wesensten et al. 2005) and narcoleptic (Saletu et al. 2007) and ADHD (Turner et al. 2004a) patients. There are also studies that have failed to show any beneficial effect of the drug on cognitive function (Saletu et al. 1989; Smith et al. 2004); however, none of those show detrimental effects of modafinil on cognition. These contrasting results may in part be due to differences in study design, such as dosage, sample size, population and the cognitive domains assessed.
In chronic schizophrenia, modafinil has been shown to improve deficits in cognition as measured by tests of attention (Park et al. 2007), working memory (Hunter et al. 2006), verbal fluency and inhibitory control (Minzenberg et al. 2009). In a previous study of 20 patients in a crossover design (Turner et al. 2004b), we showed that a single 200-mg dose improved cognitive flexibility, increasing the number of patients who were able to achieve the crucial extra-dimensional stage of the intra-extra dimensional (IED) set-shifting task (Roberts et al. 1988), an attentional shift similar to that required in the Wisconsin Card Sorting Test. In the same study, modafinil also improved short-term and working memory and increased deliberation time during the Tower of London spatial planning task. Nevertheless, no studies have assessed the effect of modafinil at early stages of schizophrenia or other psychoses. The past decade has seen increasing focus on research and treatment in the early phases of psychotic illness as it is thought to be a critical period which is likely to have important implications for patients’ future health over the remainder of their life-course (Mcgorry et al. 2009; Thomas and Nandhra 2009). Conceptually, it is useful to study psychotic illness during this early phase where precise diagnostic is uncertain because the factors that cause psychosis may be different from those that lead to chronicity or that shape a particular diagnosis course.
Therefore, this study aimed to probe the mechanisms of modafinil’s action across cognitive domains and to assess whether it remediates cognitive impairments in FEP, which may be more tractable than those seen in chronic schizophrenia. Following our previous results in patients with chronic schizophrenia, we hypothesised that modafinil would improve measures of working memory via its action upon the dopamine and glutamatergic system in the prefrontal and hippocampal areas of the brain. Attentional set-shifting was also hypothesised to improve with modafinil’s administration, through mechanisms that may involve the noradrenergic system in the medial prefrontal cortex and the striatum (Kehagia et al. 2010). In addition, we hypothesised that FEP patients have problems of impulsivity that may not be as severe as in chronic schizophrenia and may be remediated by the administration of modafinil. We predicted that modafinil would enhance performance on measures of inhibitory control and reflection impulsivity. These are improved by modafinil in healthy individuals (Turner et al. 2003) but not in patients with chronic schizophrenia (Turner et al. 2004b). In addition, we assessed the effects of modafinil on sustained attention, learning and fluency in FEP patients. Patients have deficits in these cognitive domains and we hypothesised modafinil would be efficient in targeting these. Modafinil has not been administrated to assess efficacy in treating these cognitive impairments in chronic schizophrenia before. It may be of importance to try and remediate these deficits at early stages of the disease.
Methods and materials
Samples and procedures
The study consisted of a randomised double-blind, placebo-controlled, crossover design, with approximately half of the participants randomised to receive a single oral dose of 200 mg modafinil on the first session, followed by a single oral dose of a lactose placebo on the second session (M/P group) and the other half of the participants randomised to receive the placebo first, followed by modafinil (P/M group).
FEP patients were recruited from Cambridge Assessment and Management of Early Outcomes (CAMEO), the early intervention service for psychosis in Cambridgeshire and Peterborough—UK (www.cameo.nhs.uk). The inclusion criteria for CAMEO are that patients must be aged between 17 and 35 years, and suffering from first-episode psychosis according to Melbourne criteria (Mcgorry et al. 1996) in that they experience delusions, hallucinations or thought disorders, or a combination, and are help-seeking; subjects with sub-syndromal or at risk mental states were excluded. Participants were required to have a good level of English and were excluded for any history of head injury or major learning disability. Patients were assessed during periods of relatively stable symptoms. Most were taking atypical anti-psychotics (ten were on risperidone, nine on aripiprazole, eight on olanzapine, and three on quetiapine) and they were asked to continue their treatment for ethical and practical reasons.
Forty one patients were recruited for this study, according to calculations based on previous studies (Turner et al. 2003, 2004b) that predicted a power of 80% at α = 0.05 to detect an effect size (Cohen’s d) of 0.45, which seemed reasonable in terms of the clinical relevance of any such impairment. The study was approved by the Cambridgeshire 2 Research Ethics Committee (06/Q0108/276) and after complete description of the study, written informed consent was given by all participants prior to testing. One patient was excluded from the study because although he initially appeared to meet Melbourne criteria for FEP, it became clear that his symptoms were better characterised as at risk mental state (Yung et al. 2005) than as FEP.
Volunteers were tested at the Addenbrooke’s Centre for Clinical Investigation (ACCI) in Cambridge—UK. On both visits, a short medical interview by a psychiatrist assessed current symptoms and drug, alcohol and caffeine intake. Participants were given a tablet containing either 200 mg modafinil or placebo 2 h prior to a 2-hour neuropsychological tests battery. This dose of modafinil has its maximum concentration at around 2 to 4 h after administration and it has been well tolerated in previous studies. Pulse and blood pressure were monitored hourly.
Cognitive assessment
The Cambridge Neuropsychological Test Automated Battery (CANTAB) (www.camcog.com) and other computerised and pencil and paper tests were used. A motor screening was performed on the touch-screen computer before the cognitive tests and whenever possible, parallel versions of the cognitive tasks were used in order to limit practice effects. The majority of the tasks have been previously described in detail elsewhere and readers are directed to the cited references. Working memory was assessed with the CANTAB Spatial Working Memory (SWM) task (Owen et al. 1990) and the digit span test (Wechsler 1981). Impulsivity was assessed with the Stop Signal Task (SST) of motor inhibitory control (Aron et al. 2003) and the Information Sampling Test (IST), which assesses reflection impulsivity (Clark et al. 2006). Verbal and spatial learning were assessed with the Hopkins verbal learning task (HVLT) (Rasmusson et al. 1995) and the CANTAB Paired Associates Learning (PAL) (Robbins et al. 1997; Swainson et al. 2001) respectively. Attentional set-shifting was assessed using the IED set shifting task (Roberts et al. 1988) and sustained attention with the rapid visual information processing (RVIP) test (Park et al. 1994). Category fluency was also assessed (Marczinski and Kertesz 2006). A brief description of the key measures for each of the tasks is presented in Table 1. Studies with FEP patients reliably find group deficits in all these tests when compared with age- and IQ-matched controls.
Statistical analysis
In order to assess the effects of modafinil relative to placebo, we used repeated-measures analysis of variance (ANOVA) using a type III full factorial model. Drug condition was defined as a within-subject factor, and order modafinil was given in the paired trials (M/P or P/M) was defined as a between-subject factor. Normality and homogeneity of data distribution were confirmed using the Shapiro–Wilk and Levine tests, respectively. Where appropriate transformations did not result in normal distributions, the non-parametric Wilcoxon test was used.
Some results indicated a drug-by-order effect (see below). In these cases, the first and second visit data were analysed separately, post hoc, using parametric (multivariate or one-way) ANOVA or non-parametric Mann–Whitney U tests. Post hoc analysis of the order modafinil was administered was also performed using paired t tests. This allowed us to partition practice effects. Effects sizes were also calculated to compare different tests effects generated within the study and with results from other studies.
Bonferroni corrections for multiple comparisons were applied to comparisons showing statistically significant differences (Howell 1997). Data were analysed with SPSS software version 15 for Windows.
SWM errors and latency measures from SST and IST were normalised using a logarithmic transformation (log). Discrimination errors from the SST were normalised using a square root transformation (sqrt), and successful stops from the same task were normalised using a reflect sqrt.
Due to operational constraint during testing, SST data were available for only 35 patients and HVLT data for 33 patients.
Results
Demographic data, randomisation and adverse events
Randomisation of the sample was successful and resulted in no statistically significant differences in age, gender, ethnicity or premorbid IQ (Table 2). The average gap between sessions was 12 days.
Among participants, 28 had a diagnosis of schizophrenia, six of bipolar disorder, two of depressive psychosis, two of schizoaffective disorder and two of unspecified psychosis.
There were no serious adverse events after modafinil’s administration. There were three cases of mild adverse events: After placebo administration, one case of itchiness of the face was reported, and after modafinil administration, two participants reported not being able to sleep the night following the testing session.
Effect of modafinil on cognitive functions
Working memory
Spatial working memory
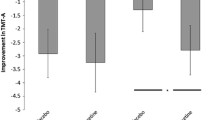
Participants made significantly fewer search errors on modafinil compared with placebo [F (1,31) = 15.55, p = 0.0004] (Fig. 1a). There was a drug by order interaction [F (1,31) = 17.62, p = 0.0002], but no main effect of order modafinil was administered on this measure. Post hoc analysis of the first and second session alone did not show any significant differences between placebo and modafinil administration. Post hoc analysis taking into account the order subjects received the drug revealed a statistically significant effect of the drug when it was administered on the second visit (t = 5.09, p = 0.0002; Fig. 1b). Modafinil also significantly improved strategy use [F (1,31) = 4.98, p = 0.03] (Fig. 1c), but this time, there were no significant drug by order interactions or order effects on this measure (Fig. 1d).
Spatial working memory results according to modafinil administration. a Modafinil significantly reduced spatial working memory (SWM) search errors in FEP patients [F (1,31) = 15.55, p = 0.0004]; b Post hoc analysis from subjects who received modafinil on the first visit or on the second visit showed that modafinil had a statistically significant effect on SWM search errors when administered on the second visit (t = 5.09, p = 0.0002); c Modafinil significantly reduced SWM strategy use scores [F (1,31) = 4.98, p = 0.03], which corresponds to an improvement in patients’ search strategy; d post hoc analysis of the order the drug was administered did not show any statistically significant effects of modafinil on SWM strategy use scores; *p ≤ 0.05 and **p ≤ 0.01. SWM spatial working memory. Error bars represent standard errors
Digit span
Modafinil significantly improved backward (z = −2.11, p = 0.04; Fig. 2a), but not forward digit span. Post hoc analyses did not show any statistically significant effect (Fig. 2b).
Digit backward results according to modafinil administration. a Modafinil statistically significantly increased digit backward scores in FEP patients (z = −2.11, p = 0.04); b post hoc analysis of the order the drug was administered did not show any statistically significant effects of modafinil on digit backwards scores; *p ≤ 0.05. Error bars represent standard errors
Impulsivity
Stop signal task
Modafinil had no effect on stop signal response time or the probability of successful inhibition. Modafinil significantly decreased discrimination errors [F (1,24) = 5.51, p = 0.03] (Fig. 3a). There was a drug by order effect for latencies on “Go” trials [F (1,24) = 5.02, p = 0.03], but post hoc analysis of the first visit alone, the second visit or the order modafinil was administered showed no effects of the drug on any of the SST outcome measures (Fig. 3b).
Stop signal task discrimination errors results according to modafinil administration. a Modafinil significantly reduced stop signal task (SST) discrimination errors [F (1,24) = 5.51, p = 0.03]; b post hoc analysis of the order the drug was administered did not show any statistically significant effects of modafinil on SST discrimination errors; *p ≤ 0.05. 1 SST stop signal task. Error bars represent standard errors
Information sampling test
Modafinil showed no significant effects on the probability of correct responding of the IST. It slowed response latencies at trend levels [F (1,37) = 3.51, p = 0.07]. This was not due to an effect of modafinil on motor latency, as response speed during the motor screening test was not affected by drug administration [F (1,37) = 0.45, p = 0.51]. There was a statistically significant drug by order effect on IST reaction time [F (1,37) = 47.39, p = 0.0004], but post hoc analysis of the first visit only failed to show any effect.
Executive functions, sustained attention and learning
Modafinil showed no main effects on IDED attention shifting, RVIP sustained attention, verbal fluency and verbal learning tasks, and no order by drug interaction effects. On the paired associates learning task, there was an effect of drug by order for PAL memory score [F (1,38) = 4.97, p = 0.03], but post hoc analysis did not show any drug effects. No other significant effects were found on this task.
A summary of the cognitive results is presented in Table 3. Means and standard deviations are reported for all the cognitive measures on placebo and on modafinil, as well as their corresponding effect sizes and significance p values. Bonferroni correction indicated a p of 0.002, limited to the reduction of errors in spatial working memory with modafinil.
When the data of the 28 patients with a diagnosis of schizophrenia were examined, the findings were generally the same as for the entire dataset, despite the reduced sample size. The improvement of between errors and strategy use for the spatial working memory task with modafinil’s administration remained statistically significant (F = 10.42, p = 0.004 and F = 4.25, p = 0.05 respectively). Digit backwards also remained significant (F = 6.15, p = 0.02), and digit forward reached trend levels (F = 3.13, p = 0.09). Improvements in discrimination errors of the stop signal task reached trend levels (F = 3.24, p = 0.09). The other tasks remained non-statistically significant.
Discussion
A single dose of modafinil improved cognitive performance in working memory, a core cognitive deficit in psychosis. Mild but consistent enhancement was found across a range of tests, which assess the same cognitive constructs, predominantly numeric and spatial working memory. The effect on numeric working memory is consistent with previous studies in ADHD (Turner et al. 2004a), chronic schizophrenia (Turner et al. 2004b) and healthy volunteers (Turner et al. 2003); hence, this effect is not specific to psychotic disorders. The effects found in spatial working memory may be specific to early psychosis (Turner et al. 2003, 2004a,b). For the first time, we have shown that a single dose of modafinil reduced search errors and improved strategy use in patients with FEP. These measures reflect the ability to update online search every time a new item is discovered in a determined place in space and to use spatial strategy to enhance this search.
Surprisingly, modafinil did not show any improvement in other measures of executive function, including the IED attentional set-shifting test. We had previously shown that chronic schizophrenia patients improved the number of extradimensional stages completed in this task. Instead, FEP patients had nearly optimal levels of success on placebo, with more than 80% of patients passing all the stages of the task, and modafinil administration did not change these levels (data not shown). We had found similar results in healthy volunteers (Turner et al. 2003), which suggest that significant decline in attentional set-shifting in schizophrenia probably arises from chronicity of the disease or from the antipsychotic medication. This further confirms the literature with regards to executive function being gradually impaired with the disorder, compared to working memory, for which impairments are shown at all stages of the disease (Pantelis et al. 2009). However, a recent study showed that attentional set-shifting remained stable over a period of 6 years in a different group of first episode psychosis patients (Leeson et al. 2009b). Longer longitudinal studies are therefore needed to analyse the evolution of the dysfunction, so it takes into account the first episode psychosis patients group, who had an average of 14 months of illness and the chronic schizophrenia group, who had an average of 17 years of illness (Turner et al. 2004b) in our different studies.
Working memory improvements did not appear to depend on increased sustained attention or improved inhibitory control, as modafinil showed no effect on the RVIP, and the key measures of SST and IST. Furthermore, although modafinil improved spatial working memory, it did not appear to have any effects on short-term learning as evidenced by the lack of effect of the drug on spatial and verbal learning. It was also observed that improvements in spatial working memory were larger and significant when modafinil was administered in the subjects’ second session, after the placebo session, i.e. when subjects had already experienced the task. This suggests the possibility of a synergistic effect between the drug and previous cognitive training at improving working memory.
The improvements induced by modafinil on working memory are likely to reflect modafinil’s action through the dopaminergic neurotransmission pathway (Robbins and Arnsten 2009). Working memory function is highly dependent on dopaminergic neurotransmission in the prefrontal cortex (Robbins 2005), which is dysregulated in schizophrenia (Constantinidis and Wang 2004). Modafinil might help to recruit more dopamine in the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex, two brain regions involved in the recruitment, maintenance and processing of memorised items (Ungerleider et al. 1998), hence improving working memory in FEP patients.
Working memory is a key pharmacological target for cognitive enhancement in schizophrenia. It is possible that it may be easier to improve these functions in FEP than it is to try to repair them in schizophrenia once the cognitive impairments are entrenched. Modafinil’s positive effect on working memory remained significant even after Bonferroni correction for multiple comparisons. Since working memory impairments in neuropsychiatric disorders are pervasive, these findings in FEP could have important implications for other neuropsychiatric disorders where problems of working memory have been identified, including schizophrenia and attention deficit hyperactivity disorder (Robbins and Arnsten 2009).
It would be of interest to replicate and extend the current study to determine whether the effect of modafinil on working memory is increased with concomitant cognitive training, as suggested by our data. This would enable modafinil to be acutely administered in combination with cognitive therapy, a tool commonly used to help patients with their impairments. Because of the selective and specific nature of improvements in this acute study, it cannot be assumed that modafinil would produce general benefits with chronic treatment. The use of armodafinil, the active enantiomer of modafinil, or higher doses of modafinil may improve performance on these cognitive domains, as modafinil appears to function in a dose-dependent manner (Wesensten et al. 2004) and that armodafinil has shown better efficacy compared to modafinil (Darwish et al. 2009). We believe that these areas merit further research.
This study showed for the first time that modafinil improved working memory, a core cognitive deficit in the first episode of psychosis, which remains substantially impaired in schizophrenia. Given the known associations between cognition and functional outcomes in schizophrenia, it is possible that the improvement in working memory induced by modafinil could have a significant beneficial effect on broader aspects of patients’ functioning, including functional outcome, quality of life and wellbeing. In this respect, pharmacological cognitive enhancement may be most beneficial if implemented early in the disorder, prior to chronic cognitive dysfunction and severe impacts on functioning and quality of life (Beddington et al. 2008; Sahakian et al. 2010).
References
Addington J, Chaves A, Addington D (2006) Diagnostic stability over one year in first-episode psychosis. Schizophr Res 86(1–3):71–75
Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6(2):115–116
Beddington J, Cooper CL, Field J, Goswami U, Huppert FA, Jenkins R, Jones HS, Kirkwood TB, Sahakian BJ, Thomas SM (2008) The mental wealth of nations. Nature 455(7216):1057–1060
Beracochea D, Celerier A, Borde N, Valleau M, Peres M, Pierard C (2002) Improvement of learning processes following chronic systemic administration of modafinil in mice. Pharmacol Biochem Behav 73(3):723–728
Clark L, Robbins TW, Ersche KD, Sahakian BJ (2006) Reflection impulsivity in current and former substance users. Biol Psychiatry 60(5):515–522
Constantinidis C, Wang XJ (2004) A neural circuit basis for spatial working memory. Neuroscientist 10(6):553–565
Darwish M, Kirby M, Hellriegel ET (2009) Comparison of steady-state plasma concentrations of armodafinil and modafinil late in the day following morning administration: post hoc analysis of two randomized, double-blind, placebo-controlled, multiple-dose studies in healthy male subjects. Clin Drug Investig 29(9):601–612
de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S (2001) Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. NeuroReport 12(16):3533–3537
Doughty OJ, Done DJ (2009) Is semantic memory impaired in schizophrenia? A systematic review and meta-analysis of 91 studies. Cogn Neuropsychiatry 14(6):473–509
Enticott PG, Ogloff JR, Bradshaw JL (2008) Response inhibition and impulsivity in schizophrenia. Psychiatry Res 157(1–3):251–254
Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K (1999) The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 20(4):346–356
Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67(Suppl 9):3–8, discussion 36–42
Howell D (1997) Statistical methods for psychology. International Thomson Publishing, Belmont
Huddy VC, Aron AR, Harrison M, Barnes TR, Robbins TW, Joyce EM (2009) Impaired conscious and preserved unconscious inhibitory processing in recent onset schizophrenia. Psychol Med 39(6):907–916
Hunter MD, Ganesan V, Wilkinson ID, Spence SA (2006) Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry 163(12):2184–2186
Joyce E, Hutton S, Mutsatsa S, Gibbins H, Webb E, Paul S, Robbins T, Barnes T (2002) Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br J Psychiatry Suppl 43:s38–s44
Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA (2004) Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry 161(6):985–995
Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20(2):199–204
Leeson VC, Robbins TW, Franklin C, Harrison M, Harrison I, Ron MA, Barnes TR, Joyce EM (2009a) Dissociation of long-term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol Med 39(11):1799–1808
Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM (2009b) Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66(6):586–593
Marczinski CA, Kertesz A (2006) Category and letter fluency in semantic dementia, primary progressive aphasia, and Alzheimer’s disease. Brain Lang 97(3):258–265
McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ (1996) EPPIC: an evolving system of early detection and optimal management. Schizophr Bull 22(2):305–326
McGorry PD, Nelson B, Amminger GP, Bechdolf A, Francey SM, Berger G, Riecher-Rossler A, Klosterkotter J, Ruhrmann S, Schultze-Lutter F, Nordentoft M, Hickie I, McGuire P, Berk M, Chen EY, Keshavan MS, Yung AR (2009) Intervention in individuals at ultra high risk for psychosis: a review and future directions. J Clin Psychiatry 70(9):1206–1212
Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS (2008) Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science 322(5908):1700–1702
Minzenberg M, Watrous AJ, Yoon JH, Nunez del Prado J, Ursu S, Ragland JD, Carter CS (2009) Modafinil effects on prefrontal cortex during cognitive control in schizophrenia: a pharmaco-fMRI study. International Congress on Schizophrenia Research, San Diego
Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990) Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28(10):1021–1034
Pantelis C, Wood SJ, Proffitt TM, Testa R, Mahony K, Brewer WJ, Buchanan JA, Velakoulis D, McGorry PD (2009) Attentional set-shifting ability in first-episode and established schizophrenia: relationship to working memory. Schizophr Res 112(1–3):104–113
Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ (1994) Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 33(3–4):575–588
Park I, Kim JJ, Seok JH, Chun JW, Son SJ, Park HJ, Lee JD (2007) The neural substrate of sustained attention to emotional stimuli and modafinil effect on attention in schizophrenia: a [15O] H2O PET study. International Congress of Schizophrenia Research, Colorado Springs, CO, USA, Schizophrenia Bulletin
Pierard C, Liscia P, Philippin JN, Mons N, Lafon T, Chauveau F, Van Beers P, Drouet I, Serra A, Jouanin JC, Beracochea D (2007) Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav 88(1):55–63
Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD (2000) Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry 57(3):249–258
Rasmusson DX, Bylsma FW, Brandt J (1995) Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol 10(1):21–26
Remington G, Foussias G, Agid O (2010) Progress in defining optimal treatment outcome in schizophrenia. CNS Drugs 24(1):9–20
Robbins TW (2005) Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol 493(1):140–146
Robbins TW, Arnsten AF (2009) The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32:267–287
Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K (1997) Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 134(1):95–106
Roberts AC, Robbins TW, Everitt BJ (1988) The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B 40(4):321–341
Sahakian BJ, Jones G, Levy R, Gray J, Warburton D (1989) The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry 154:797–800
Sahakian BJ, Malloch G, Kennard C (2010) A UK strategy for mental health and wellbeing. Lancet 375(9729):1854–1855
Saletu B, Frey R, Krupka M, Anderer P, Grunberger J, Barbanoj MJ (1989) Differential effects of the new central adrenergic agonist modafinil and d-amphetamine on sleep and early morning behaviour in elderlies. Arzneimittelforschung 39(10):1268–1273
Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J, Saletu B (2007) Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatr Res Neuroimaging 154(1):69–84
Salokangas RK, McGlashan TH (2008) Early detection and intervention of psychosis. A review. Nord J Psychiatr 62(2):92–105
Smith D, Pernet A, Rosenthal JM, Bingham EM, Reid H, Macdonald IA, Amiel SA (2004) The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia. Diabetologia 47(10):1704–1711
Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ (2001) Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord 12(4):265–280
Thomas SP, Nandhra HS (2009) Early intervention in psychosis: a retrospective analysis of clinical and social factors influencing duration of untreated psychosis. Prim Care Companion J Clin Psychiatry 11(5):212–214
Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ (2003) Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 165(3):260–269
Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ (2004a) Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 55(10):1031–1040
Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ (2004b) Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology 29(7):1363–1373
Ungerleider LG, Courtney SM, Haxby JV (1998) A neural system for human visual working memory. Proc Natl Acad Sci USA 95(3):883–890
Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K (2009) Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama 301(11):1148–1154
Wechsler D (1981) WAIS-R manual: Wechsler adult intelligence scale—revised. The Psychological Corporation, New York
Wesensten NJ, Belenky G, Thorne DR, Kautz MA, Balkin TJ (2004) Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med 75(6):520–525
Wesensten NJ, Killgore WD, Balkin TJ (2005) Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res 14(3):255–266
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J (2005) Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry 39(11–12):964–971
Acknowledgements
We would like to thank the patients for their participation in the present study and our financial support from the Stanley Medical Research Institute and the Pinsent Darwin Fund of Cambridge University. Prof. Jones has received research support from the Wellcome Trust, NIHR and GSK. Prof. Sahakian has received consulting fees from Cambridge Cognition Ltd., GSK, Novartis, Shire, Lilly, and Boehringer-Ingelheim and has received honoraria from the Journal of Psychological Medicine, Grand Rounds in Psychiatry and the International Conference on Cognitive Dysfunction in Schizophrenia and Mood Disorders. Dr. Barnett is an employee of Cambridge Cognition Ltd and a co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders) with Profs Jones and Sahakian. Dr. Soma and Dr. Scoriels reported no financial conflict of interests. The authors have full control of all primary data and agree to allow the journal to review this data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scoriels, L., Barnett, J.H., Soma, P.K. et al. Effects of modafinil on cognitive functions in first episode psychosis. Psychopharmacology 220, 249–258 (2012). https://doi.org/10.1007/s00213-011-2472-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2472-4