Abstract
Rationale
There is extensive evidence that alcoholism and impulsivity are related, but the direction of causality is unclear.
Objectives
The aim of the present investigation was to study the effects of chronic ethanol treatment and withdrawal in measures of attention and impulse control in the five-choice serial reaction time task (5CSRTT) in mice.
Materials and methods
C57BL/6J mice were trained in the 5CSRTT and then tested in a variable inter-trial interval (vITI) session, which promotes the emergence of premature responses, a measure of poor inhibitory control. Following chronic ethanol treatment, mice were tested in additional vITI sessions—in experiment 1, at 1, 7 and 14 days post-withdrawal, and in experiment 2, at 14, 28, 42 and 56 days post-withdrawal.
Results
Control animals showed a reduction in premature responding with experience of the vITI schedule. Compared to controls, previous ethanol treatment did not affect attention or impulsivity on first experience of the vITI procedure. Ethanol-treated animals showed sustained increased premature responding over sessions. This effect of ethanol treatment was not apparent in experiment 2, in which first exposure to the vITI schedule was delayed for 2 weeks following ethanol treatment.
Conclusions
Chronic ethanol treatment impaired the ability to learn to modify behaviour in order to gain access to reinforcement more frequently. This effect was related to the time since withdrawal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug and ethanol abuse are often associated with loss of control of drug-taking behaviour, attributed to impaired function of cortico-striatal neural circuitries. (Jentsch and Taylor 1999; Kalivas and Volkow 2005). For instance, in young adult social drinkers, impaired executive function correlates with both the frequency of drinking to “get high” and “get drunk” (Deckel et al. 1995) and the severity of drinking consequences (Giancola et al. 1996). An important question in addiction research is whether such impaired function is a predisposing trait (Kollins 2003) or whether it can result from drug taking. Many studies have suggested that prefrontal dysfunction is a predisposing factor to heavy drinking, while it is generally accepted that acute ethanol ingestion may lead to increased impulsivity (Critchlow 1986; Noel et al. 2007), and thus to disruptive and antisocial behaviours such as dangerous driving, aggression and unprotected sex, as well as further drug abuse (Cherpitel 1993; 1999; Ericksen and Trocki 1994). Whether there are long-term effects of chronic misuse of ethanol on impulsive behaviours that may thus contribute to loss of control over future alcohol consumption is less clear.
Alcohol itself is known to have long-term effects on prefrontal cortex function (Moselhy et al. 2001; Tarter et al. 2004), and studies of alcoholic patients who have undergone multiple detoxifications suggest that previous experience of detoxification is associated with prefrontal cortex dysfunction and impulsivity (Duka et al. 2003). Alcohol dependence does not appear to be necessary for such effects, since social drinkers showing a binge pattern of consumption are impaired in a number of tasks requiring suppression of a prepotent action (Townshend and Duka 2005). Increased impulsivity is not always deleterious, and the same study found binge drinkers to be faster on the visual search matching task, a task from the CANTAB test battery that allows a separation between choice and movement time. Binge drinkers showed faster movement time, rather than thinking time, suggestive of a motor impulsivity. Such impulsivity is associated with altered functioning of prefrontal–subcortical circuits, particularly the orbitofrontal circuit (Spinella 2004).
The term impulsivity is not a unitary construct and can be defined in several ways. For example, impulsivity may refer to the tendency to act without thinking, on the urge of the moment, the inability to delay gratification, distractibility, or the difficulty in the inhibition of incorrect or inappropriate responses (Dougherty et al. 2008; Evenden 1999). Different aspects of impulsivity are mediated by different neurobiological and neurochemical substrates (Dalley et al. 2008). In rats, ethanol intoxication decreases performance in sustained attention or vigilance tasks, such as in a two-choice serial reaction time task (Givens 1997; Givens and McMahon 1997) and in a visual signal detection task (Rezvani and Levin 2003), indicating that these are promising animal models for assessing ethanol effects on attentional performance. However, in a similar task, the five-choice serial reaction time task (5CSRTT; (Carli et al. 1983; Robbins 2002)), it has been reported that, in rats, ethanol did not affect the accuracy of responding, but acted mainly by slowing the speed or rate of responding at high doses (1.2–1.6 g/kg) while showing no effect in any parameter at low doses (0.4–0.8 g/kg; Bizarro et al. 2003). In the 5CSRTT, animals are required to wait until the illumination of a light at a particular location indicates the location of a correct response. Premature responding provides a measure of impulsivity (Robbins 2002).
Like Bizarro et al., we found no effects of ethanol on impulsive responding in well-trained mice (Oliver et al. 2009). Nevertheless, we found effects of ethanol in two mouse strains in the 5CSRTT when well-trained mice were exposed to occasional sessions in which the inter-trial interval (ITI) was extended from 5 to 7 s. In the present experiment, we used a further modification of the task, introducing occasional test sessions in which the ITI was varied within the session at 2, 5, 10 and 15 s, to provide a parametric study of the effects of changes in ITI. Using this modification, we studied the consequences of exposing mice to chronic treatment with ethanol, and its withdrawal, on performance of a modified version of this task.
Materials and methods
Subjects
C57BL/6J (C57, n = 30) male mice were purchased from Charles River (UK) and weighed 20–25 g at the beginning of the experiments. They were housed in groups of two or three per cage on a 12-h light/dark cycle (lights off at 7 p.m.), at a temperature of 19–21°C and 50% humidity. Mice were food restricted to reduce their body weights to 90% of their free-feeding weight and were kept under food restriction until the end of the study, except where stated. Water was available ad libitum. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation [Animal (Scientific Procedures) Act, 1986] following ethical approval.
Test apparatus
Five-choice serial reaction time task
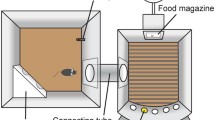
The test apparatus consisted of eight mouse operant chambers (Med Associates Inc., St. Albans, Vermont, USA). Each chamber was housed in a sound-attenuating cabinet, with a ventilator fan providing a constant background noise. The left wall of the chamber contained five apertures, each with an infrared detector at the mouth to detect nose-poke responses. Apertures were illuminated by a yellow stimulus light located at the rear of each one. The right wall of the chamber contained a receptacle hole in the centre with an access opening where the liquid reinforcer was delivered. A 0.01 ml of a 30% (v/v) condensed milk solution was used as a reinforcer and was delivered by means of a dipper. Head entries into the magazine were recorded by an infrared photocell beam crossing the entrance of the receptacle hole, which could be illuminated by a yellow stimulus light inside it. Above the food magazine, and at the top of the wall, a house light was located. The presentation of stimuli and the recording of the responses were controlled by a Smart Control Package (8 in/16 out) with an additional interface by MED-PC for Windows (Med Associates Inc., St. Albans, Vermont, USA).
Behavioural procedures
Habituation to the test boxes and mode of reinforcement
During the initial three sessions, the animals were placed in the boxes for 30 min, and the liquid reward was available in the magazine on a continuous schedule. The animal had only to nose-poke in the magazine to receive the condensed milk solution that was available for 10 s, after which the dipper was refilled and available again. All lights remained on throughout the session. Magazine head entries and number of reinforcers earned were recorded.
In a second stage of habituation, for a maximum of ten 30-min sessions, animals were placed in the 5CSRTT boxes. During these sessions, every light within the five nose-poke apertures was illuminated. A nose-poke into any aperture resulted in illumination of the food magazine, signalling availability of the reinforcer. After 10 s, the magazine light went off, and the animal was again able to nose-poke in one of the response holes to earn a reinforcer. Trials initiated and number of reinforcers earned were recorded. When the animal had earned at least 50 reinforcers in two consecutive sessions, then it started the training in the 5CSRTT.
5CSRTT training
The session started with the illumination of the house light and an automatic delivery of the reinforcer accompanied by the illumination of the food magazine, which signalled reward availability. When the animal nose-poked into the magazine to obtain the reward (dipper on for 3 s), the first trial was initiated. After a fixed delay (inter-trial interval, ITI), one of the stimulus lights in the holes was illuminated for a brief time, and the animal was required to nose-poke within a certain period of time (limited hold, LH) into the correct hole in order to obtain the reinforcer. After a correct response, the animal was required to nose-poke into the magazine to collect the reward and to initiate the next trial. An incorrect response occurred when the animal made a response in a different hole from the one that had been illuminated. An error of omission occurred when the animal had failed to respond into any of the holes after the completion of the LH. Any response into the holes during the inter-trial interval (the period during which the stimulus light had not yet been presented) was registered as a premature response. Incorrect, omitted and premature responses were all followed by a time out period (TO), during which the lights were extinguished for 5 s. Responses made into the holes during this period restarted the TO. After a TO period, the next trial was restarted by a nose-poke into the magazine. Perseverative responses (further responses into the holes after a response) were registered but had no programmed consequences.
At the beginning of training, the stimulus duration was set to 30 s and the ITI was set to 2 s, but these parameters were subsequently adjusted according to the individual performance of each animal. When the animal produced two consecutive sessions achieving the performance criteria (shown in Table 1), the stimulus duration was reduced in the following pattern: 30, 20, 10, 5, 2.5, 1.8, and the LH and the ITI were set at 5 s, following Oliver et al. (2009). Testing was carried out daily (5–6 days a week), and the session lasted for 100 trials or 30 min, whichever came first. On achieving criterion at stage six, animals were stabilised at this level for a further seven sessions.
5CSRTT testing phase
In experiment 1, following training to criterion, animals (n = 14) were exposed to a single session immediately prior to first alcohol exposure, in which the ITI was varied pseudorandomly within the session, with values of 2, 5, 10 and 15 s (variable ITI session; vITI), but with the stimulus duration remaining 1.8 s. The session lasted 45 min or 100 trials, whichever occurred first. Identical sessions were run on days 1, 7 and 14 following cessation of ethanol exposure. Performance within the task was maintained during ethanol treatment by 30-min training sessions using the fixed 5-s ITI.
In experiment 2, following training under baseline conditions, as described in experiment 1 both prior, during and following ethanol exposure, animals (n = 16) were exposed to four variable ITI sessions. These occurred at days 14, 28, 42 and 56 post-cessation of ethanol treatment.
Ethanol treatment
Mice were allocated to two groups in each experiment, ethanol-treated (ETH) and control (CTR) groups (experiment 1, group n = 7; experiment 2, group n = 8). The groups were approximately matched for levels of accurate and premature responding on the two baseline sessions prior to treatment. An attempt was made to match animals on all variables, but this was not possible. The animals in the ethanol group received a nutritionally complete liquid diet (Dyets, Bethlehem, PA, USA) containing 3.5% ethanol as their only food source. Fresh diet was provided daily at approximately 1000 hours. The controls were fed with a calorifically matched control diet, restricted to approximately the average weight consumed by the ethanol-treated group the previous day. In each experiment, there was no initial difference in the body weight between the two groups and the pair feeding system maintained body weights at a comparable level. Each group was fed its respective diet for 24 consecutive days. All animals were given fresh diet daily. Ethanol consumption was calculated as grammes of ethanol consumed per kilogramme body weight. A subgroup of mice was sacrificed 2 h following replacement of diet on day 7 of ethanol treatment and trunk blood collected for estimation of blood ethanol levels using the Analox (London, UK) AM1 Alcohol analyser.
On the final day, animals were withdrawn from the liquid diet at 0830 hours and remained in their home cages with ad libitum access to water but no food for 8 h. Following this withdrawal period, the animals were allowed restricted access to rodent chow as above, during subsequent testing.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 16.0).
The variables considered in the analysis of the 5CSRTT were:
-
Total trials: total correct responses + total incorrect + total omissions.
-
Accuracy: \( {\text{correct responses}}/\left( {{\text{correct responses}} + {\text{total incorrect responses}}} \right) \times {1}00 \).
-
Percentage of omissions: \( {\text{total omissions}}/\left( {{\text{correct responses}} + {\text{incorrect responses}} + {\text{omissions}}} \right) \times {1}00 \).
-
Percentage of premature responding: \( {\text{premature responses}}/\left( {{\text{correct responses}} + {\text{incorrect responses}} + {\text{omissions}} + {\text{premature responses}}} \right) \times {1}00 \).
-
Correct latency: latency to nose-poke into the correct hole after the onset of the stimulus/stimuli.
-
Magazine latency: latency to collect the reward after a correct response (s).
-
Perseverative responses: total number of responses made into the holes after a correct response and before the collection of the reward.
Two-way repeated measures ANOVA, with treatment as the between-subjects factor and stage of training as the within-subjects factor, was used for the analysis of each variable of the 5CSRTT training.
For the vITI probe sessions, three-way ANOVA was applied, with treatment as the between-subjects factor and day of testing and ITI as within-subjects factor.
One-way ANOVAs and two-tailed t tests were used for post hoc analysis. For within-subjects ANOVA, where sphericity assumptions were violated, the Greenhouse–Geisser correction was applied. A p < 0.05 was required for results to be considered statistically significant.
Results
Experiment 1
Ethanol intake
ETH animals consumed an average of 16.77 ± 0.55 g of liquid diet per day. This equated to an average daily ethanol intake of 32.88 ± 0.99 g/kg. A subgroup of mice sacrificed at 7 days showed intakes of 32.99 ± 0.87 g/kg and blood alcohol levels of 280.6 ± 25.5 mg/dl.
5CSRTT training
Mice acquired stable baseline performance in the fixed ITI version of the 5CSRTT in 44.5 ± 0.99 sessions. There were no differences between experimental groups in any variable during the various stages of the training phase, with the exception of percentage of omission errors made (stage × treatment interaction, F(5,60) = 2.46, p < 0.05), with mice allocated to the CTR group (but not yet treated) showing more omissions at stages 5 and 6. By the final baseline session before ethanol treatment, there was no difference between the percentage of omissions performed between the two groups (t = 1.34, df = 9, n.s.).
Effects of variable ITI session on performance prior to treatment with alcohol
No difference in the performance of accurate responses was found under the vITI conditions, as illustrated in Fig. 1. Varying the ITI led to differences in the number of omitted responses (effect of ITI; F(3,36) = 7.49, p < 0.001), with all animals showing a higher percentage of omission errors at the 2-s ITI. Percentage of premature responding significantly increased in both groups at longer ITIs (effect of ITI; F(3,36) = 77.74, p < 0.001).
Performance of mice in the control group (open circles) and ethanol group (filled circles) on first testing in a novel vITI task prior to treatment with ethanol. Graphs show the mean and SEM of a accuracy of responding, b percentage omissions, c percentage premature, d latency to make a correct response, e latency to collect the reward and f perseverative responses. Bars represent SEMs
Latency to make a correct response decreased at longer ITIs (effect of ITI; F(3,36) = 6.71, p < 0.001) whereas the latency to collect the earned reinforcer from the magazine increased at longer ITIs (effect of ITI; F(3,36) = 3.85, p < 0.05). The number of perseverative responses emitted also decreased at longer ITIs (effect of ITI; F(3, 36) = 5.51, p < 0.05, ε = 0.43). There were no group differences found in any of the measures.
Effects of chronic alcohol treatment on performance during variable ITI sessions
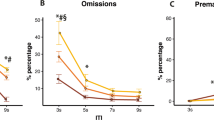
Figure 2 illustrates the effects of chronic ethanol treatment on performance during the three post-withdrawal variable ITI sessions. Accuracy improved with time from withdrawal (effect of session; F(2,72) = 5.01, p < 0.05), and whilst ethanol-treated animals tended to perform less accurately across sessions, this difference did not reach significance (effect of treatment; F(1,12) = 3.47, n.s.).
Effects of chronic ethanol treatment on accuracy of responding, percentage omissions and percentage prematures at 1, 7 and 14 days post-withdrawal in mice in the control group (open circles) and ethanol group (filled circles). Premature responding decreased at all ITI durations as experience of variable ITI sessions increased (session × ITI interaction; F(6, 72) = 9.18, p < 0.001, ε = 0.41). The rate of reduction varied across treatment groups (session × treatment interaction; F(2, 24) = 4.12, p < 0.05, ε = 0.67). Specifically, session by treatment interactions were found at the 10- and 15-s ITIs (10 s, F(2,24) = 3.64, p < 0.05; 15 s, F(2,24) = 4.42, p < 0.05), with ethanol-treated animals showing sustained high levels of premature responding across sessions in comparison to a reduction in premature responding by control animals
Three-way ANOVA of percentage omission errors indicated a three-way interaction between session, ITI and treatment group (F(6, 72) = 2.93, p < 0.05, ε = 0.49). Two-way ANOVA of each session revealed that the interaction was mainly attributable to variations in omissions associated with treatment and ITI in the session 7 days following withdrawal from ethanol (ITI × treatment group interaction; F(3,36) = 2.95, p < 0.05). Ethanol-treated animals made a significantly lower percentage of omission errors at the 2- and 10-s ITIs (2 s, t = 2.83, df = 12, p < 0.05; 10 s, t = 3.57, df = 12, p < 0.05) than control animals.
The pattern of premature responding across ITI varied with both treatment and session (session × ITI × treatment interaction; F(6, 72) = 3.33, p < 0.05, ε = 0.41). Premature responding decreased at all ITI durations as experience of variable ITI sessions increased (session × ITI interaction; F(6, 72) = 9.18, p < 0.001, ε = 0.41). This reduction in premature responding over session differed across treatment groups (session × treatment interaction; F(2, 24) = 4.12, p < 0.05, ε = 0.67). Specifically, session by treatment interactions were found at the 10- and 15-s ITIs (10 s, F(2,24) = 3.64, p < 0.05; 15 s, F(2,24) = 4.42, p < 0.05), with ethanol-treated animals showing sustained high levels of premature responding across sessions in comparison to a reduction in premature responding by control animals.
A strong tendency for ethanol-treated subjects to be quicker in making correct responses was evident but this failed to reach significance (effect of treatment; F(1,12) = 4.24, n.s.). Ethanol treatment did not affect the latency to collect earned rewards nor the number of perseverative responses made.
Experiment 2
Ethanol intake
ETH animals consumed an average of 17.67 ± 0.49 g of liquid diet per day. This equated to an average daily ethanol intake of 27.57 ± 0.78 g/kg.
5CSRTT training
There were no differences in accuracy or premature responding between experimental groups across the stages of the training phase.
Percentage omissions increased across the training phase (effect of stage; F(5, 70) = 8.68, p < 0.001) to a greater extent in those animals that went on to receive control diet (stage × group interaction; F(5, 70) = 3.38, p < 0.05). The same group of mice reduced magazine latency and perseverative responding more steeply (stage × group interaction; F(5,70) = 2.55, p < 0.05; F(5, 70) = 6.34, p < 0.001, ε = 0.58) than animals subsequently treated with ethanol.
Effects of repeated withdrawal on performance during variable ITI sessions
Accuracy improved over subsequent post-treatment vITI sessions (effect of session; F(3, 42) = 14.77, p < 0.001, ε = 0.51), as indicated in Fig. 3. Percentage of omission errors varied across sessions, with a higher incidence of omitted responding at the 2-s ITI (session × ITI interaction; F(9,126) = 10.05, p < 0.001), demonstrated in Fig. 4. No group differences were found in the pattern of responding on either of those two measures.
Figure 5 illustrates the pattern of premature responding in the vITI sessions. Similar to experiment 1, the introduction of longer ITIs led to a higher percentage of premature responding (effect of ITI; F(3, 42) = 77.26, p < 0.001, ε = 0.42), though this pattern was not the same in every session (session × ITI interaction; F(9, 126) = 7.36, p < 0.001, ε = 0.26). The level of premature responding was reduced, session upon session (effect of session; F(3, 42) = 15.70, p < 0.001), equally across treatment groups (session × treatment interaction; F(3, 42) = 0.75, n.s.). Latency to make a correct response was slightly higher under the 2-s ITI (effect of ITI; F(3, 42) = 5.92, p < 0.05, ε = 0.56), though this did not vary across the treatment groups.
Discussion
This study explored the effects of 24 days of ethanol administration giving rise to substantial intakes (ca. 30 g/kg/day) and high blood alcohol levels (ca. 280 mg/dl) resulting in behavioural signs of intoxication, and withdrawal, on performance in a modified version of the 5CSRTT. After extensive training using baseline task parameters, introduction of a probe trial, wherein time spent waiting for stimulus light illumination (inter-trial interval; ITI) was varied randomly, led to a pronounced increase in premature responding by all animals at the longer ITIs. With repeated exposure to such sessions, mice in the control group reduced their premature responding, thus gaining access to the condensed milk reinforcer more frequently. In experiment 1, animals treated with, and recently withdrawn from, ethanol reduced their premature responding over sessions significantly more slowly, so that they remained impulsive even when the control animals had adapted their behaviour to the vITI schedule.
The second experiment was carried out in order to determine whether the apparent deficit in adapting to the novel schedule following chronic ethanol treatment in experiment 1 was enduring. In this experiment, mice were first exposed to the vITI schedule 14 days following withdrawal. As in experiment 1, control animals reduced their impulsive responding with increased experience of the vITI probe trials, but in contrast to experiment 1, the ethanol-treated animals showed the same reduction. Deficiencies in adapting to the vITI induced by chronic ethanol were therefore not apparent, suggesting that the effects of chronic ethanol treatment were not long lasting.
It is notable that, apart from the initial post-withdrawal slowness in adapting to the vITI schedule, chronic ethanol treatment had little effect on performance of the 5CSRTT. Thus, there was no evidence in altered attentional ability, as assessed by accuracy of responding, even on the first day of withdrawal (experiment 1, 2nd vITI session; Fig. 2). Neither were there changes in percentage of omissions or latency to collect earned rewards. Thus, there was no evidence of altered motivation.
The variable schedule, considerably less predictable than baseline sessions, required the mouse to monitor its readiness to respond consistently and continuously over the session in order to gain access to the reinforcer as often as possible. The common initial inability to inhibit inappropriate responding when waiting time was increased and varied appears inherent to the demands of the task. Animals had acquired the expectation from extensive training under baseline parameters that they were required to wait for 5 s for the stimulus light to illuminate. The introduction of a variable time of stimulus onset within these sessions introduced an increased demand upon attentional capacity since the animal could no longer rely on automatic processing (i.e. internal timing) to determine when to attend to the signal. One interpretation of the pattern of results found in the present study is that, whilst the altered difficulty of the task initially induced increased impulsive responding in all subjects, control animals learned to adapt to the new requirements of the task and to inhibit their impulsive behaviour, and thus reduced the number of premature responses made. The results of experiment 1 suggest that animals that had been chronically administered with alcohol were less able to adjust their behaviour to meet the requirements of the vITI task. However, whether the failure of the ethanol-treated mice to adapt to the vITI task reflects an effect of the treatment on impulsivity (the mice were unable to withhold responses at longer ITIs) or an impaired ability to learn the new requirements of the task following introduction of the vITI is not clear.
In both training and probe (i.e. vITI) 5CSRTT sessions, failed trials (incorrect, omission, premature) are punished by a time out period, 5 s of darkness, which signals to the animal that the opportunity to gain access to reward in that specific trial has been lost. The offset of the time out (TO) period indicates that the opportunity is available once again. Over the course of training, in order to meet criteria for progression, animals needed to learn and integrate the significance of the TO into their behavioural response. Indeed, other researchers have found that use of training schemes lacking TO periods leads to significantly slower task acquisition (Bari et al. 2008). Thus, one suggested mechanism is that chronic ethanol treatment and withdrawal may have led to a decrease in sensitivity to TO punishment and/or absence of reinforcement. This may have resulted in a failure to integrate all salient information and to perform with greater success, relying instead on previously learnt stimulus–response associations. We have previously reported impairments both in aversive learning (Stephens et al. 2001) and in a negative patterning task (Borlikova et al. 2006) in rats subjected to a similar ethanol treatment regimen that could in principle reflect such a deficit in integrating novel requirements into a behavioural strategy previously successful in obtaining reward.
In this context, it is of interest that rats with lesions disconnecting nucleus accumbens core from medial PFC show impaired control of impulsive action in the 5CSRTT compared to sham-operated animals following punished failures to perform accurately, but not following correct responses (Christakou et al. 2004); such lesions thus disrupt a circuitry especially necessary for the modulation of performance by affective outcome. Conceivably, prolonged alcohol exposure could contribute to disruption of such pathways.
Alcohol withdrawal is associated with increased glutamatergic transmission that may give rise to inappropriate synaptic plasticity or to neurotoxicity (Stephens and Duka 2008) either of which may interfere with subsequent learning. In the case of both the amygdala and the hippocampus, regions which are implicated in associative learning (Everitt et al. 2003), rats chronically treated with ethanol in a fashion similar to the present experiment showed reduced capacity for long-term potentiation (LTP), consistent with synaptic saturation arising from previous withdrawal experience (Stephens et al. 2005). There is some evidence to suggest that such physiological deficits may not be permanent and that capacity to support LTP returns following a period of abstinence (Roberto et al. 2002). Although speculative, taken together, these studies may support an account explaining the temporary nature of the learning deficits found in the present study.
In conclusion, the results of the present study demonstrated that chronic treatment with alcohol had no effect on attentional performance in the 5CSRTT. While the treatment resulted in increased impulsive behaviour in experiment 1, whether this impaired ability to withhold responding reflects impulsivity or a failure to learn the new contingencies of the task remains unclear. Introduction of the vITI altered the demands of the task, necessitating behavioural flexibility, which led to increased impulsive responding in all animals. Chronic ethanol treatment appeared to lead to a learning deficit that resulted in a failure to modify behaviour, a perseveration of previously learned but, in the context of the probe task, less successful behavioural strategies. In accordance with studies of human alcoholics (Loeber et al. 2009), the deficit appeared to be related to length of abstinence, with normal functioning being observed after a longer break from alcohol. The present results thus suggest that while alcohol exposure and withdrawal may result in impulsive behaviour, with limited exposure of the kind studied here, this effect may not persist following several weeks of abstinence. On the other hand, it is well known that alcoholic patients may show impulsive behaviour that seems to be exacerbated by long-term abuse and withdrawal (e.g. (Loeber et al. 2009)) so that a more extensive period of alcohol exposure and withdrawal may produce longer-lasting effects.
References
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3:759–767
Bizarro L, Patel S, Stolerman IP (2003) Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend 72:287–295
Borlikova GG, Elbers NA, Stephens DN (2006) Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: evidence for effect on frontal cortical but not hippocampal function? Eur J Neurosci 24:205–216
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Cherpitel CJ (1993) Alcohol and injuries: a review of international emergency room studies. Addiction 88:923–937
Cherpitel CJ (1999) Substance use, injury, and risk-taking dispositions in the general population. Alcohol Clin Exp Res 23:121–126
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical–ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24:773–780
Critchlow B (1986) The powers of John Barleycorn. Beliefs about the effects of alcohol on social behavior. Am Psychol 41:751–764
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260
Deckel AW, Bauer L, Hesselbrock V (1995) Anterior brain dysfunctioning as a risk factor in alcoholic behaviors. Addiction 90:1323–1334
Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW (2008) A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend 96:111–120
Duka T, Townshend JM, Collier K, Stephens DN (2003) Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res 27:1563–1572
Ericksen KP, Trocki KF (1994) Sex, alcohol and sexually transmitted diseases: a national survey. Fam Plann Perspect 26:257–263
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361
Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW (2003) Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann NY Acad Sci 985:233–250
Giancola PR, Zeichner A, Yarnell JE, Dickson KE (1996) Relation between executive cognitive functioning and the adverse consequences of alcohol use in social drinkers. Alcohol Clin Exp Res 20:1094–1098
Givens B (1997) Effect of ethanol on sustained attention in rats. Psychopharmacology (Berl) 129:135–140
Givens B, McMahon K (1997) Effects of ethanol on nonspatial working memory and attention in rats. Behav Neurosci 111:275–282
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413
Kollins SH (2003) Delay discounting is associated with substance use in college students. Addict Behav 28:1167–1173
Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K (2009) Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol 44:372–381
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36:357–368
Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P (2007) Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (Berl) 192:291–8
Oliver YP, Ripley TL, Stephens DN (2009) Effect of ethanol on impulsivity in two strains of mice: similarities to diazepam and ketamine. Psychopharmacology 204:679–692
Rezvani AH, Levin ED (2003) Nicotine–alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav 76:75–83
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Roberto M, Nelson TE, Ur CL, Gruol DL (2002) Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol 87:2385–2397
Spinella M (2004) Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci 114:95–104
Stephens DN, Duka T (2008) Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Phil Trans R Soc Lond B: Biol Sci 363:3169–3179
Stephens DN, Brown G, Duka T, Ripley TL (2001) Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci 14:2023–2031
Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T (2005) Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry 58:392–400
Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M (2004) Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend 73:121–132
Townshend JM, Duka T (2005) Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res 29:317–325
Acknowledgements
This work was carried out with the support of UK Medical Research Council Programme Grant G0400568. YPO was funded by the IMAGEN consortium that receives research funding from the European Community’s Sixth Framework Programme (LSHM-CT-2007-037286). This paper reflects only the authors’ views and the Community is not liable for any use that may be made of the information contained therein. We thank T. Ripley for providing support with programming and data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, S.E., Peña-Oliver, Y. & Stephens, D.N. Learning not to be impulsive: disruption by experience of alcohol withdrawal. Psychopharmacology 217, 433–442 (2011). https://doi.org/10.1007/s00213-011-2298-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2298-0