Abstract
Rationale
Preclinical data suggest a possible role for 5-HT6 receptors in depression and anxiety. However, the results of pharmacological studies are equivocal since both blockade and stimulation of 5-HT6 receptors may evoke antidepressant- and anxiolytic-like effects.
Objectives
In the present study, the effects of the 5-HT6 receptor agonist EMD 386088, administered intrahippocampally (i.hp.) to rats, were assessed in behavioral tests commonly used for evaluating antidepressant- and anxiolytic-like activities.
Results
EMD 386088 (10 and 20 μg, i.hp.) exerted a significant antidepressant-like effect as revealed by decreased duration of rats' immobility in the forced swim test. This effect was blocked by systemic administration of the selective 5-HT6 receptor antagonist SB-399885. Additionally, the anxiolytic-like activity was demonstrated in the Vogel conflict and elevated plus maze tests, as EMD 386088 reduced the number of punished responding (5–20 μg, i.hp.) and increased the percentage of open arm entries (10 and 20 μg, i.hp.). The tested 5-HT6 agonist (5–20 μg, i.hp.) affected neither distance traveled in the open field test nor motor coordination assessed in the rotarod test.
Conclusions
The results of the present study demonstrate that the 5-HT6 agonist produces antidepressant- and anxiolytic-like effects and that the hippocampus could be one of the brain regions involved in this action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide body of evidence suggests that the 5-hydroxytryptamine 6, serotonin 6 (5-HT6) receptor, one of the most recently identified members of the serotonin receptor family, may be a potential target for developing new therapies against psychiatric disorders. Preclinical data provide support for the use of serotonin 5-HT6 receptor antagonists as promising medications for treating cognitive dysfunctions associated with Alzheimer's disease and schizophrenia (Fone 2008). Additionally, 5-HT6 ligands may represent a new approach to the management of obesity (Heal et al. 2008). However, while the majority of 5-HT6 research has focused on the pro-cognitive and appetite-suppressing effects, the role of these receptors in depression and anxiety has also been postulated (Wesolowska 2010).
The potential involvement of 5-HT6 receptors in depression has been suggested by Yau et al. (1997), who demonstrated that the pharmacological blockade of the endogenous corticosterone synthesis upregulated 5-HT6 receptor expression in the rat hippocampus. Since corticosterone synthesis blockers (e.g., metyrapone) exert an antidepressant-like activity (Healy et al. 1999), it may be speculated that 5-HT6 receptors are involved in their effects. Furthermore, the blockade of 5-HT6 receptor expression using an antisense oligonucleotide produced an anxiogenic response in the social interaction and elevated plus maze tests (Otano et al. 1999; Hamon et al. 1999); however, 5-HT6 receptor knockout mice did not display an anxious phenotype in the elevated zero maze (Bonasera et al. 2006). A recent development of selective agonists and antagonists of 5-HT6 receptors allowed for further evaluation of the role of this receptor subtype in animal models of anxiety and depression. However, the results of pharmacological studies are equivocal and somewhat controversial. Using the forced swim and tail suspension tests, the antidepressant-like activity of both agonists and antagonists of 5-HT6 receptor has been demonstrated (Svenningsson et al. 2007; Wesolowska et al. 2007; Wesolowska and Nikiforuk 2007; Hirano et al. 2009). Similarly, both blockade and stimulation of 5-HT6 receptors exerted anxiolytic-like effects. However, different models of anxiety have been used for detecting this action, i.e., the Vogel conflict test and the elevated plus maze test were employed in the antagonist studies (Wesolowska et al. 2007; Wesolowska and Nikiforuk 2007), whereas an anxiolytic effect of the agonist was demonstrated in the defensive burying and novelty-induced hypophagia tests (Carr et al. 2011)
The hippocampus is implicated in the pathophysiology of anxiety and depression and, as demonstrated by intracerebral infusion studies, in mediating the effects of antidepressant and anxiolytic drugs (Campbell and Macqueen 2004; Engin and Treit 2007). Consistent with the high expression of 5-HT6 mRNA in the hippocampus (Monsma et al. 1993; Ruat et al. 1993), our previous study has clearly suggested that this brain region is involved in the antidepressant- and anxiolytic-like activities of the selective 5-HT6 receptor antagonist SB-258585 (Wesolowska et al. 2007). Since recent literature data indicate that the stimulation of 5-HT6 receptors may also result in antidepressant and anxiolytic effects (Svenningsson et al. 2007; Carr et al. 2011), we aimed to elucidate whether the hippocampus could be involved in these effects.
Therefore, our present study was designed to examine whether the 5-HT6 receptor agonist EMD 386088 would exert antidepressant- and anxiolytic-like effects in preclinical animal models after intrahippocampal administration. EMD 386088 is a high-affinity (IC50 = 7.4 nM) full agonist at 5-HT6 receptors (Mattsson et al. 2005), and its behavioral effects have been previously demonstrated in animal tasks assessing cognitive functions (Meneses et al. 2008; Kendall et al. 2011). In the present experiment, the effects of EMD 386088 administration into the CA1 region of the dorsal hippocampus of rats have been investigated in preclinical tests previously used for revealing the antidepressant- and anti-anxiety-like activities of 5-HT6 receptor antagonists after systemic (Wesolowska and Nikiforuk 2007) and intrahippocampal (Wesolowska et al. 2007) administrations, i.e., in the forced swim, Vogel conflict, and elevated plus maze tests. The compound's impact on the rat’s activity and motor coordination was assessed in the open field and rotarod tests, respectively. Additionally, a separate experiment was conducted to confirm that the antidepressant-like action of EMD 386088 is mediated specifically by the 5-HT6 receptors. In this experiment, we assessed the ability of systemic administration of an inactive dose of the selective antagonist of 5-HT6 receptors SB-399885 (Hirst et al. 2006) to block the anti-immobility effect of EMD 386088.
Materials and methods
Animals
Male Sprague Dawley rats (Charles River, Germany) weighing 250–280 g on arrival were used in this study. The animals were kept in the temperature- (21 ± 1°C) and humidity-controlled (40–50%) colony room under 12:12-h light/dark cycle (lights on at 0600 hours). They were housed individually in the standard animal cages (40 × 27 × 15 cm) with ad libitum access to food and water. The experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Ethics Committee for Animal Experiments, Institute of Pharmacology.
Experimental procedures
All the experiments were conducted during the light phase of the light/dark cycle. The forced swim and Vogel conflict tests were performed in two separate groups of animals. The third group of rats was subjected to a battery of tests including open field, elevated plus maze, and rotarod tests (Popik et al. 2006).
Surgical procedures
The rats were anesthetized with an intramuscular injection of ketamine (100 mg/kg) and xylazine (65 mg/kg) in 0.9% NaCl. A socket with two stainless-steel guide cannulae (0.4 mm outer diameter, 0.3 mm inner diameter, 8.0 mm long) was implanted stereotaxically 2 mm above the CA1 region of the dorsal hippocampus (A, 5.2 mm; L, 2.0 mm; and H, 7.3 mm from the interaural line) (Paxinos and Watson 1986) and fixed to the skull with stainless-steel screws and a dental acrylic cement. Seven days later, the rats were subjected to behavioral testing.
Forced swim test
Rats were placed individually in a 10-L glass cylinder (40 cm height, 18 cm diameter) filled to a height of 30 cm with water at 25°C, which was changed after every rat. Two swim sessions were conducted: a 15-min pretest followed 24 h later by a 5-min test. EMD 386088 or a vehicle was administered into the hippocampus intrahippocampally (i.hp.) 10 min before the test. The total time spent immobile as well as the times the rats spent on climbing and swimming was recorded during the 5-min test session (Detke et al. 1995). Immobility was characterized as floating in the water with minimal movements required to keep the head above water. The swimming behavior entailed active swimming motions, e.g., moving around in the cylinder. An upward-directed movement of the forepaws along the wall of the cylinder was scored as a climbing behavior.
Vogel conflict test
The procedure was adopted from Vogel et al. (1971) with slight modifications (Popik et al. 2006). On the first day of the experiment, water-deprived (for approximately 16 h) rats were adapted to the test chamber (30 × 32 × 26 cm) for 10 min. The animals were allowed to explore the chamber freely and drink water from the bottle spout. Afterwards, the rats were returned to their home cages and were given 30 min access to the water followed by a 24-h water deprivation period. The adaptation session and water deprivation protocols were repeated on the second day of the experiment. On the third day, rats were placed in the test chamber 10 min after i.hp. drug administration and were given free access to the drinking tube. After 20 licks, a 2-s, 0.2-mA, two-phase shock was delivered to the metal drinking tube, and it was repeated after every 20 licks. Each session lasted for 3 min from the delivery of the first shock, and the number of punished responses was recorded.
Elevated plus maze test
The apparatus, made of Plexiglas and elevated to the height of 50 cm, consisted of two open arms (40 × 12 cm) and two closed arms (40 × 12 × 20 cm) placed 90o to each other and extending from a central platform (12 × 12 cm). The experiments were conducted under a low-intensity light (30 lx). The test was initiated by placing a rat on the central platform of the maze, facing an open arm. Testing lasted for 5 min, and the number of open- and closed-arm entries was recorded using the Any-maze® tracking system. The percentage of open-arm entries (total number of entries/number of open-arm entries × 100) served as the measure of anxiety. Additionally, the total number of arm entries and the distance traveled were used as a measure of locomotor activity.
Open field test
Rats were placed in the corner of a dimly lit (40 lx) open field made of black plywood (66 × 56 × 30 cm), and the distance traveled was measured using the Any-maze® tracking system during a 3-min test session. After each measurement, the floor was cleaned and dried.
Rotarod test
The impact of treatment on motor coordination was assessed using the rotarod apparatus (ENV-577; MED Associates, St. Albans, VT). Rats were placed on a rod rotating at 6 rpm. Those animals that did not fall off the apparatus within 2 min were considered to have normal balance and coordination.
Histological analysis
On the completion of each experiment, the location of infusion was verified visually. All the animals were killed on the final test day, and their brains were removed and stored in 10% formalin solution. To identify the position of cannula tracks, the frozen brains were cut up in the coronal plane using a Cryo-cut. Only data from the rats in which the cannulae were located bilaterally in the intended structure were included in the results (Fig. 1).
Drugs
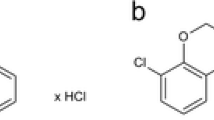
EMD 386088 (5-chloro-2-methyl-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-indole hydrochloride; Tocris Cookson, Bristol, UK) was dissolved in 5% dimethylsulfoxide. SB-99885 (N-(3,5-dichloro-2-methoxyphenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide; Ascent Scientific, Bristol, UK) was dissolved in distilled water. EMD 386088 and its vehicle (5% dimethylsulfoxide) were administered (i.hp.), bilaterally in a volume of 0.5 μl/site (i.e., a dose expressed in microgram per 1 μl per rat) 10 min before each test. Intrahippocampal injections were made using Hamilton microsyringes connected to two stainless-steel needles (0.3 mm outer diameter) via polyethylene tubing. The injection needles were inserted 2 mm below the tip of the guide cannula (i.e., at the level of the CA1 region of the dorsal hippocampus). Solutions were administered bilaterally for 60 s. The injection needle remained in place for another 60 s before it was removed and replaced with a stylet. SB-99885 was administered intraperitoneally (i.p.), in a volume of 1 ml/kg of body weight, 30 min prior to the FST. The inactive FST dose of SB-399885 was chosen on the basis of our preliminary experiments (unpublished results) and previous literature data (Wesolowska et al. 2007; Hirano et al. 2009).
Data analysis
All the data are presented as mean ± SEM. The statistical significance of EMD 386088 effects was evaluated using one-way ANOVAs. In the antagonism experiment, data were analyzed with a two-way ANOVA with EMD 386088 treatment (0 and 10 μg) and SB-399885 treatment (0 and 1 mg/kg) as the between-subject factors. Post hoc comparisons were performed using Newman–Keuls test. The alpha value was set at the p < 0.05 level. The data fulfilled the criteria of normal distribution. Statistical analyses were performed with the use of Statistica 7.0 for Windows.
Results
Forced swim test
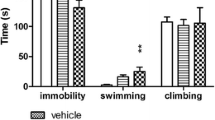
As demonstrated in Fig. 2, EMD 386088 administered i.hp. at a dose of 10 and 20 μg significantly decreased the immobility (ANOVA: F(3,34) = 26.05, p < 0.001) and increased the swimming times (ANOVA: F(3,34) = 13.10, p < 0.001) of rats in the forced swim test. There was no significant drug effect on the duration of climbing behavior (ANOVA: F(3,34) = 1.69; not significant, NS).
Effects of EMD 386088 in the forced swim test in rats. EMD 386088 and its vehicle were administered i.hp. for 10 min before the test. Data represent the mean ± SEM of the total duration of immobility, swimming, and climbing during the 5-min test session. The number of animals in experimental groups was 8–10. ***p < 0.001 vs. vehicle-treated group, Newman–Keuls post hoc test
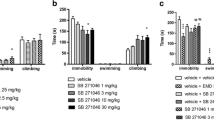
The selective antagonist of 5-HT6 receptors, SB-399885 (Fig. 3), blocked the reduction of immobility (ANOVA: interaction: F(1,32) = 17,638, p < 0.001) and the increase in swimming behavior (ANOVA: interaction: F(1,32) = 5,78, p < 0.05) produced by EMD 386088. There was no effect of the combined drug administration on climbing behavior (ANOVA: interaction: F(1,32) = 0.01, NS).
Effect of SB-399885 on the EMD 386088-induced anti-immobility action in the forced swim test in rats. SB-399885 (1 mg/kg, i.p.) and EMD 386088 (10 μg, i.hp) were administered 30 and 10 min before the test, respectively. Data represent the mean ± SEM of the total duration of immobility, swimming, and climbing during the 5-min test session. The number of animals in the experimental groups was 8–10. **p < 0.01, ***p < 0.001 vs. vehicle + vehicle-treated group, ### p < 0.001 vs. vehicle + EMD 386088-treated group; Newman–Keuls post hoc test
Vogel conflict test
EMD 386088 (5–20 μg) significantly increased the number of accepted shocks during 3-min experimental sessions in the Vogel test (Fig. 4; ANOVA: F(3,30) = 6.36, p < 0.01).
Effects of EMD 386088 in the Vogel conflict test in rats. EMD 386088 and its vehicle were administered i.hp. for 10 min before the test. Results represent the means ± SEM of the number of shocks accepted. The number of animals in the experimental groups was 7–10. *p < 0.05, **p < 0.01 vs. vehicle-treated group; Newman–Keuls post hoc test
Elevated plus maze test
As shown in Table 1, EMD 386088 (10–20 μg) exhibited an anxiolytic-like activity, reflected by an increase in the percentage of open-arm entries (F(3,35) = 3.94, p < 0.05). There was no effect of drug treatment on the total number of arm entries and distance traveled in the elevated plus maze apparatus (ANOVAs: F(3,35) = 1.47, NS, and F(3,35) = 1.58, NS, respectively).
Open field test and rotarod test
There was no significant effect of EMD 386088 administration on the distance traveled in the open field apparatus (Table 1; ANOVA: F(3,35) = 2.36, NS). Moreover, the drug administered up to a dose of 20 μg i.hp. did not disturb the motor coordination of rats in the rotarod test (data not shown).
Discussion
The results of the present study demonstrate that the 5-HT6 receptor agonist EMD 386088, administered intrahippocampally to rats, produced antidepressant- and anxiolytic-like effects. Specifically, EMD 386088 exerted an antidepressant-like activity as revealed by shortening of the immobility time in the forced swim test. This effect was fully blocked by the selective 5-HT6 receptor antagonist SB-399885 administered at an inactive dose. An anti-immobility effect appeared to be specific, since EMD 386088 did not affect the rats' activity measured in the open field apparatus. Moreover, EMD 386088 significantly and dose-dependently increased the number of shocks accepted in the Vogel conflict test. The anti-anxiety action of EMD 386088 has also been demonstrated in the elevated plus maze test, since the tested compound increased the percentage of open-arm visits. Since the EMD 386088 compound affected neither the total number of arm visits nor the distance traveled in the apparatus, its anxiolytic-like action cannot be explained by competing behaviors, such as the enhancement of locomotor activity.
The present demonstration of the antidepressant-like effect of EMD 386088 following i.hp. administration is in agreement with literature data showing the anti-immobility action of systemic administration of another 5-HT6 agonist in the mouse tail suspension test (Svenningsson et al. 2007) and in the rat forced swim test (Carr et al. 2011). Interestingly, in line with the results of Carr et al. (2011), EMD 386088 also specifically increased swimming behavior in the modified FST in rats. This pattern of rats' responding has been regarded as indicative of an enhanced serotonergic transmission and attributed to selective serotonin reuptake inhibitors (SSRIs) action (Detke and Lucki 1996). Therefore, it may be suggested that the activation of 5-HT6 receptors is implicated in the behavioral response to SSRIs in the forced swim test. The potential involvement of 5-HT6 receptor stimulation in the antidepressant-like action of SSRIs is also supported by biochemical data. Svenningsson et al. (2007) has demonstrated that the 5-HT6 receptor agonist 2-ethyl-5-methoxy-N,N-dimethyltryptamine increased the phosphorylation state at Thr34-DARPP-32 (a dopamine- and cAMP-regulated phosphoprotein). This effect, observed also after fluoxetine administration, has been involved in a fluoxetine-mediated decrease in immobility (Svenningsson et al. 2002). Moreover, similar to the chronic antidepressant treatment, a direct 5-HT6 receptor activation resulted in a rapid rise in the hippocampal brain-derived neurotrophic factor mRNA expression (de Foubert et al. 2007). Therefore, it seems possible that the activation of 5-HT6 receptors might participate in the behavioral and biochemical outcome of SSRIs and that the hippocampus might be one of the brain regions involved in this action.
The anti-anxiety action of EMD 389088 in the Vogel conflict and elevated plus maze tests corroborates the results of Carr et al. (2011). These authors demonstrated that the 5-HT6 receptor activation produced an anxiolytic-like action in the defensive burying and novelty-induced hypophagia tests. Literature data suggest that the gamma-aminobutyric acid (GABA) system is involved in the modulation of anxiety-like behavior by 5-HT6 receptor agonists. Consistent with the localization of 5-HT6 receptors on GABAergic interneurons (Woolley et al. 2004), the activation of 5-HT6 receptors enhanced GABA transmission in the hippocampus as revealed by in vivo microdialysis (Schechter et al. 2008) and electrophysiological (West et al. 2009) techniques. Since positive modulators of GABA receptors as well as agents enhancing GABAergic tone have been widely used to exert anxiolytic effects, it is possible that an elevated GABA release underlies the anti-anxiety action of 5-HT6 receptor agonists. In addition to facilitation of GABA-mediated inhibitory transmission, a 5-HT6 agonist also decreased stimulated glutamate release (Schechter et al. 2008). This effect may also contribute to the effectiveness of 5-HT6 agonists especially in the stress-related conditions associated with enhanced excitatory glutamatergic transmission.
It should be noted that 5-HT6 antagonists may also exert antidepressant- and anxiolytic-like activities in preclinical animal models (Wesolowska et al. 2007; Hirano et al. 2009). It is likely, however, that different neurochemical mechanisms underlie the similar effects of agonists and antagonists of 5-HT6 receptors. Microdialysis studies suggest that in contrast to the 5-HT6 receptor agonist, the antagonist may increase dopamine and noradrenaline concentrations (Lacroix et al. 2004). The involvement of these neurotransmitters in the anti-immobility action of 5-HT6 antagonist has been supported by a study demonstrating that a selective 5-HT6 receptor antagonist enhanced the anti-immobility action of the noradrenaline reuptake inhibitor (desipramine) and the dopamine reuptake inhibitor (bupropion) in the forced swim test (Wesolowska and Nikiforuk 2008). The antidepressant-like action of a 5-HT6 antagonist was further attributed to the action at dopamine D1 and D2 receptors as well as α2-adrenoceptor (Wesolowska 2007). In contrast to dopaminergic and noradrenergic neurotransmission, the 5-HT system does not appear to be involved in the antidepressant-like effect of the 5-HT6 antagonist as revealed in the study using 5-HT-depleted animals (Wesolowska 2007). On the other hand, the 5-HT6 receptor antagonist has been demonstrated to counteract the anti-immobility action of fluoxetine in the tail suspension test in mice (Svenningsson et al. 2007). This result together with the above-cited data related to the 5-HT6 agonist-induced antidepressant-like action points to the role of 5-HT6 receptor stimulation in the behavioral outcome of SSRIs.
In contrast to 5-HT6 agonists, the anxiolytic action of 5-HT6 antagonists cannot be simply ascribed to the restoration of balance between inhibitory GABAergic and excitatory glutamatergic transmission. In fact, the blockade of 5-HT6 receptor counteracted the increase in extracellular GABA concentration induced by 5-HT6 agonists (Schechter et al. 2008) and increased glutamate levels in the frontal cortex and dorsal hippocampus (Dawson et al. 2001). Though the 5-HT6 blockade-induced increase in acetylcholine release has been previously suggested to participate in anxiolytic-like effects (Wesolowska and Nikiforuk 2007), the precise mechanism of the 5-HT6 antagonist anti-anxiety action remains unknown. Nevertheless, a similar action of an agonist and antagonist of 5-HT6 receptors is not restricted to animal models of depression and anxiety. For instance, both stimulation and blockade of 5-HT6 receptors may exert pro-cognitive actions (Burnham et al. 2010; Kendall et al. 2011, but see also Meneses et al. 2008; Loiseau et al. 2008). Similarly, the anti-obesity actions have been demonstrated after administration of both agonists and antagonists of these receptors (reviewed in Heal et al. 2008).
However, while different mechanisms may be responsible for the effects of 5-HT6 agonists and antagonists, the hippocampus appears to be involved in behavioral effects of both 5-HT6 receptor stimulation (present study) and blockade (Wesolowska et al. 2007). The hippocampus may mediate the effects of compounds with diverse mechanisms of action in preclinical models of anxiety and depression. For example, the reduced immobility in the forced swim test in rats has been observed after intrahippocampal administration of tricyclic antidepressants, imipramine (Przegalinski et al. 1997), and desipramine (Kostowski 1985); 5-HT7 receptor antagonists (Wesolowska et al. 2006); ionotropic glutamate receptor antagonist (Padovan and Guimaraes 2004); as well as neurotrophic factors (Shirayama et al. 2002). Moreover, the hippocampus mediates the effects of benzodiazepines or a direct GABAA receptor agonist (Menard and Treit 2001; Rezayat et al. 2005), serotonin receptor (i.e., 5-HT1A and 5-HT7) ligands (Menard and Treit 1998; Wesolowska et al. 2006), an acetylcholinesterase inhibitor (Degroot and Treit 2002), ionotropic or metabotropic glutamate receptor ligands (Padovan et al. 2000; Palucha et al. 2004), and neurosteroids (Bitran et al. 1999) in various models of anxiety, including the conflict drinking test and the elevated plus maze test.
In conclusion, the present study demonstrates that the hippocampus is involved in antidepressant- and anxiolytic-like effects of EMD 386088, a 5-HT6 receptor agonist.
References
Bitran D, Dugan M, Renda P, Ellis R, Foley M (1999) Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res 850:217–224
Bonasera SJ, Chu HM, Brennan TJ, Tecott LH (2006) A null mutation of the serotonin 6 receptor alters acute responses to ethanol. Neuropsychopharmacology 31:1801–1813
Burnham KE, Baxter MG, Bainton JR, Southam E, Dawson LA, Bannerman DM, Sharp T (2010) Activation of 5-HT(6) receptors facilitates attentional set shifting. Psychopharmacology (Berl) 208:13–21
Campbell S, Macqueen G (2004) The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29:417–426
Carr GV, Schechter LE, Lucki I (2011) Antidepressant and anxiolytic effects of selective 5-HT(6) receptor agonists in rats. Psychopharmacology (Berl) 213:499–507
Dawson LA, Nguyen HQ, Li P (2001) The 5-HT(6) receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology 25:662–668
de Foubert G, O'Neill MJ, Zetterstrom TS (2007) Acute onset by 5-HT(6)-receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein mRNA expression. Neuroscience 147:778–785
Degroot A, Treit D (2002) Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res 949:60–70
Detke MJ, Lucki I (1996) Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73:43–46
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121:66–72
Engin E, Treit D (2007) The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol 18:365–374
Fone KC (2008) An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology 55:1015–1022
Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Delrio J, Verge D (1999) Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21:S68–S76
Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H (2008) Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther 117:207–231
Healy DG, Harkin A, Cryan JF, Kelly JP, Leonard BE (1999) Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacology (Berl) 145:303–308
Hirano K, Piers TM, Searle KL, Miller ND, Rutter AR, Chapman PF (2009) Procognitive 5-HT6 antagonists in the rat forced swimming test: potential therapeutic utility in mood disorders associated with Alzheimer's disease. Life Sci 84:558–562
Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, Heidbreder CA, Atkins AR, Lacroix LP, Dawson LA, Foley AG, Regan CM, Upton N (2006) SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol 553:109–119
Kendall I, Slotten HA, Codony X, Burgueno J, Pauwels PJ, Vela JM, Fone KC (2011) E-6801, a 5-HT(6) receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology (Berl) 213:413–430
Kostowski W (1985) Possible relationship of the locus coeruleus–hippocampal noradrenergic neurons to depression and mode of action of antidepressant drugs. Pol J Pharmacol Pharm 37:727–743
Lacroix LP, Dawson LA, Hagan JJ, Heidbreder CA (2004) 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 51:158–164
Loiseau F, Dekeyne A, Millan MJ (2008) Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology (Berl) 196:93–104
Mattsson C, Sonesson C, Sandahl A, Greiner HE, Gassen M, Plaschke J, Leibrock J, Bottcher H (2005) 2-Alkyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as novel 5-HT6 receptor agonists. Bioorg Med Chem Lett 15:4230–4234
Menard J, Treit D (1998) The septum and the hippocampus differentially mediate anxiolytic effects of R(+)-8-OH-DPAT. Behav Pharmacol 9:93–101
Menard J, Treit D (2001) The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal L-glutamate. Brain Res 888:163–166
Meneses A, Perez-Garcia G, Liy-Salmeron G, Flores-Galvez D, Castillo C, Castillo E (2008) The effects of the 5-HT(6) receptor agonist EMD and the 5-HT(7) receptor agonist AS19 on memory formation. Behav Brain Res 195:112–119
Monsma FJ Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR (1993) Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43:320–327
Otano A, Frechilla D, Cobreros A, Cruz-Orive LM, Insausti A, Insausti R, Hamon M, Del Rio J (1999) Anxiogenic-like effects and reduced stereological counting of immunolabelled 5-hydroxytryptamine6 receptors in rat nucleus accumbens by antisense oligonucleotides. Neuroscience 92:1001–1009
Padovan CM, Delbel EA, Guimaraes FS (2000) Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: influence of restraint stress. Pharmacol Biochem Behav 67:325–330
Padovan CM, Guimaraes AR (2004) Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats. Pharmacol Biochem Behav 77:15–19
Palucha A, Tatarczynska E, Branski P, Szewczyk B, Wieronska JM, Klak K, Chojnacka-Wojcik E, Nowak G, Pilc A (2004) Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology 46:151–159
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, New York
Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, Chen Z, Russek SJ, Gibbs TT, Farb DH, Skolnick P, Lippa AS, Basile A (2006) The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl-(pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha-1 subunit-containing GABAA receptors. J Pharmacol Exp Ther 319:1244–1252
Przegalinski E, Tatarczynska E, Derenwesolek A, Chojnackawojcik E (1997) Antidepressant-like effects of a partial agonist at strychnine- insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology 36:31–37
Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B (2005) Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol Behav 84:775–782
Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC (1993) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193:268–276
Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, Brandt MR, Dawson LA, Cole D, Bernotas R, Robichaud A, Rosenzweig-Lipson S, Beyer CE (2008) Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology 33:1323–1335
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P (2002) DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA 99:3188–3193
Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P (2007) Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci 27:4201–4209
Vogel JR, Beer B, Clody DE (1971) A simple and reliable conflict procedure for testing anti- anxiety agents. Psychopharmacologia 21:1–7
Wesolowska A (2007) Study into a possible mechanism responsible for the antidepressant-like activity of the selective 5-HT6 receptor antagonist SB-399885 in rats. Pharmacol Rep 59:664–671
Wesolowska A (2010) Potential role of the 5-HT6 receptor in depression and anxiety: an overview of preclinical data. Pharmacol Rep 62:564–577
Wesolowska A, Nikiforuk A (2007) Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology 52:1274–1283
Wesolowska A, Nikiforuk A (2008) The selective 5-HT(6) receptor antagonist SB-399885 enhances anti-immobility action of antidepressants in rats. Eur J Pharmacol 582:88–93
Wesolowska A, Nikiforuk A, Stachowicz K (2006) Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol 553:185–190
Wesolowska A, Nikiforuk A, Stachowicz K (2007) Anxiolytic- and antidepressant-like effects produced by the selective 5-HT6 receptor antagonist SB-258585 after its intrahippocampal administration to rats. Behav Pharmacol 18:439–446
West PJ, Marcy VR, Marino MJ, Schaffhauser H (2009) Activation of the 5-HT(6) receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in rat hippocampus. Neuroscience 164:692–701
Woolley ML, Marsden CA, Fone KC (2004) 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3:59–79
Yau JL, Noble J, Widdowson J, Seckl JR (1997) Impact of adrenalectomy on 5-HT6 and 5-HT7 receptor gene expression in the rat hippocampus. Brain Res Mol Brain Res 45:182–186
Acknowledgments
This study was supported by the statutory activity funds from the Institute of Pharmacology, Polish Academy of Sciences (Kraków, Poland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikiforuk, A., Kos, T. & Wesołowska, A. The 5-HT6 receptor agonist EMD 386088 produces antidepressant and anxiolytic effects in rats after intrahippocampal administration. Psychopharmacology 217, 411–418 (2011). https://doi.org/10.1007/s00213-011-2297-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2297-1