Abstract
Rationale and objectives
Somatostatin (SST) isoforms, SST 14 and SST 28, inhibit regulatory hormones in the periphery (e.g., growth hormone) and are widely distributed in the brain. In recent experiments, intracerebroventricular (ICV) SST produced anxiolytic-like effects in both behavioral and electrophysiological models. The sites of action of these anxiolytic effects in the brain, however, and the relative contributions of SST 14 and SST 28 to these effects are unknown.
Materials and methods
Anxiolytic effects were assessed in the plus-maze and shock-probe tests after (1) intra-amygdalar microinfusion of SST 14 (0.5 or 3 μg per hemisphere) or SST 28 (3 μg per hemisphere), (2) intra-septal microinfusion of SST 14 (0.5 or 1.5 μg per hemisphere) or SST 28 (1.5 μg per hemisphere), or (3) intra-striatal microinfusion of SST 14 (3 μg per hemisphere).
Results
Intra-amygdalar and intra-septal microinfusions of SST 14 and SST 28 produced robust anxiolytic-like effects in the behavioral tests, unlike intra-striatal microinfusions. The magnitude of the anxiolytic effects in the amygdala and septum were comparable to those found previously with ICV SST 14, ICV L-779976, an SST (sst2) receptor agonist, and ICV diazepam, a classical benzodiazepine anxiolytic.
Conclusions
SST receptors in the septum and amygdala are responsive to both SST 14 and SST 28, but not those in the striatum. Although no obvious differences in the anxiolytic-like effects of the isoforms were detected, quantitative or even qualitative differences in their specific anxiolytic effects may occur in different sub-regions of the septum and amygdala, as has been found for benzodiazepine anxiolytics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatostatin (SST) is a cyclic polypeptide, which exists in two biologically active isoforms: somatostatin 14 (SST 14) and somatostatin 28 (SST 28). SST 28 is synthesized from prosomatostatin, whereas SST 14 is synthesized from either prosomatostatin or by proteolytic conversion of SST 28. Peripherally, SST 14 and SST 28 inhibit the release of peptide hormones, including growth hormone from the pituitary, insulin and glucagon from the pancreas, and cholecystokinin from the gastrointestinal tract (Moller et al. 2003). While SST controls hormone function in peripheral tissues, numerous studies have shown that both SST 14 and SST 28 are distributed throughout the central nervous system, where they act as both neurotransmitters and neuromodulators (Cervia and Bagnoli 2007; Moller et al. 2003; Selmer et al. 2000).
Although it seems possible that SST 28 is merely a non-functional precursor of SST 14, SST 28 modulates similar hormonal functions, albeit with different potencies. For example, Mandarino et al. (1981) found that SST 28 suppressed insulin release with twice the potency of SST 14, whereas SST 14 suppressed glucagon release with six times the potency of SST 28. SST 14 and SST 28 bind at equally high levels in the cortex and in ‘limbic’ structures such as the septum, hippocampus, and amygdala (Leroux et al. 1985). SST is often co-localized with γ-amino butyric acid (GABA; Sur et al. 1994; Saha et al. 2002), an inhibitory neurotransmitter that has long been implicated in anxiety (Tallman and Gallager 1985). Five different G-protein linked SST receptors (sst1–sst5) have been cloned, all of which are expressed in the brain. It is these subtypes that presumably mediate the biological effects of SST 14 and SST 28 (Cervia and Bagnoli 2007; Moller et al. 2003).
SST 14 and SST 28 can act as neurotransmitters or neuromodulators in the brain, modifying neuronal excitability. For example, Meis et al. (2005) found that SST 14 induced a G-protein-receptor linked inward rectifying K+ (GIRK) current within the lateral amygdala, dampening cell excitability. Wang et al. (1989) found that SST 14 increased voltage-dependent K+ currents in cortical neurons, while SST 28 decreased K+ currents in the same neurons. These effects of SST 14 and SST 28 on K+ currents showed no cross-desensitization, suggesting actions at different SST receptor subtypes (Wang et al. 1989). Karschin (1995) showed that stimulation of the sst1 receptor in oligodendrocytes also decreased GIRKs. Finally, Kreienkamp et al. (1997) found that the EC50 values for the activation of inhibitory GIRK currents by SST 14 and SST 28 could differ by as much as an order of magnitude, depending on their action at SST receptor subtypes (sst1-5). Thus, in addition to differences in the magnitude of the hormonal effects of SST 14 and SST 28, their effects on neuronal excitability can also differ dramatically (Kreienkamp et al. 1997; Meis et al. 2005).
At a behavioral level, SST 14 and SST 28 have been implicated in locomotion (Semenova et al. 2010), analgesia (Williams et al. 1987), epilepsy (Vécsei et al. 1990), spatial memory (Dutar et al. 2002), and emotion (Engin et al. 2008). Engin et al. found that intracerebroventricular (ICV) microinfusions of either SST 14 or a selective sst2 agonist produced significant reductions in rat ‘anxiety’ in the elevated plus maze (Engin et al. 2008; Engin and Treit 2009). In contrast, no significant changes in affective behavior were found following microinfusions of selective agonists of sst1, 3, 4, and 5 (Engin and Treit 2009). ICV SST 14 also suppressed hippocampal theta activity, an effect common to all known anxiolytic drugs (e.g., benzodiazepines, 5-HT1A agonists, and SSRIs; McNaughton et al. 2007).
Whether or not brain SST 28 is also involved in anxiety is unknown, as are the sites of action in the brain where SST produces its anxiolytic-like effects. In addition, it would be useful to demonstrate anxiolytic effects in more than one model. Accordingly, the purpose of experiment 1 was to compare the effects of intra-amygdalar microinfusions of the SST 14 and SST 28 in two animal models, the elevated plus-maze and shock-probe burying test. The amygdala was chosen as the target site for three reasons. First, the involvement of the amygdala in the regulation of fear and anxiety is well documented (e.g.,LeDoux 2000; Pesold and Treit 1995). Second, SST in some amygdalar neurons is both co-localized and co-released with GABA (Batten et al. 2002). GABA, through the GABAA receptor and an allosteric binding site for benzodiazepines, modulates both experimental anxiety in animals and clinical anxiety in humans (Treit et al. 2010). Third, SST receptor gene expression in the amygdala is increased by predatory stress (Nanda et al. 2008).
The septum also has high levels of both SST and GABA, and has been independently implicated in anxiety (e.g., Degroot and Treit 2004; Gray and McNaughton 2000; Pesold and Treit 1994; 1996; Shin et al. 2009; Treit and Menard 1997; 2000). Thus, it seemed likely that SST microinfusions into the septum would also produce anxiolysis. Thus, the purpose of experiment 2 was to corroborate the findings in experiment 1 in another limbic structure implicated in anxiety, extending our neuroanatomical understanding of SST's anxiolytic effects. The purpose of experiment 3 was to assess the effects of a low dose of SST 14 in the amygdala and the septum, and experiment 4 addressed the site specificity of the anxiolytic effects of SST 14 in the amygdala and septum by infusing it into nearby areas of the striatum. The striatum also synthesizes SST, and expresses various SST receptor sub-types, including sst2 (Santis et al. 2009), previously implicated by Engin et al. in anxiety (Engin et al., 2008; Engin and Treit, 2009).
Materials and methods
Subjects
Subjects were 98 male, Sprague–Dawley rats, weighing 200–300 g at the time of surgery. Rats were individually housed in polycarbonate cages for the duration of the experiment and maintained on a 12:12 h light/dark cycle (lights on at 0600 hours). Food and water were available ad libitum. The treatment of all animals was in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals and the Canadian Council on Animal Care. Power analyses were carried out before the experiments to minimize the number of animals used, and all possible measures to minimize suffering and stress were taken during the experiments. Just prior to surgery, the rats were assigned to surgery conditions (amygdala, septum, striatum).
Surgery
Rats were anesthetized with isofluorane (5% induction, 1.5% maintenance in 67% N2O, and 33% O2; Halocarbon Product Corp. River Edge NJ, USA), injected with atropine sulfate (0.1 mg/0.2 mL i.p.; Bimeda-MTC, Cambridge, Ontario, Canada) and Marcaine (1.5 mg/0.3 mL s.c. just under the cranial skin; Hospira, Quebec, Canada), and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Following hydration with 0.9% saline (3 cc, i.p.), an incision was made to expose skull. The subjects were then bilaterally implanted with stainless-steel 22-gauge guide cannulae (Plastics One, Roanoke, VA, USA) targeting the amygdala (AP, −2.5; ML, −4.2; DV, −6.6), the septum (AP, 0.7 mm; ML, −2.6; DV, −4.2; angled 22° towards the midline) or the striatum (AP, +0.5; ML +/− 3.0; DV −5.6). The cannulae were lowered to within 0.5 mm of their intended targets and secured to the skull with three jewelers' screws and cranioplastic cement. A dummy cannula was inserted into each guide cannula in order to keep the cannula tract clear. The surgery area was treated with 2.5 mg carprofen (Rimadyl©, Pfizer; 2.5 mg/0.5 mL s.c. on the head). Following the surgery, the subjects were placed in a warm environment until they regained consciousness. Rats were then allowed to recover for at least 5 days in their home cages before the start of behavioral testing.
Infusion procedure
Seventy-five rats from the amygdalar and septal surgical groups were randomly assigned to receive SST 14, SST 28, or vehicle prior to behavioral testing. Both SST 14 (Sigma, St. Louis, MO, USA) and SST 28 (AnaSpec, Fremont, CA, USA) were dissolved in a 5% DMSO vehicle at concentrations of 3 μg/μL (1.5 μg per hemisphere) and 6 μg/μL (3.0 μg per hemisphere). Smaller groups of amygdalar (n = 7) and septal-implanted (n = 8) rats received 1 μg/μL of SST 14 (0.5 μg per hemisphere) or vehicle before testing, while a striate-implanted group (n = 8) received 6 μg/μL of SST 14 (3.0 μg per hemisphere) or vehicle before testing. The drugs were infused bilaterally (0.5, 1.5, or 3.0 μg/hemisphere) via an infusion pump (Harvard Apparatus 22, MA, USA) at a rate of 1 μL/min for 30 s per hemisphere. SST 14, SST 28, and vehicle solutions were infused through 26-gauge stainless-steel internal cannulae, attached to a 10-μl Hamilton syringe by polyethylene tubing. The internal infusion cannulae extended 0.5 mm below the ventral tip of the guide cannulae. Drug flow was confirmed by displacement of a bubble inside the polyethylene tubing. The internal infusion cannulae were left in place for 40 s after the end of the infusion period to allow for diffusion.
Behavioral testing
The behavioral procedures were the same as those described previously (for details see Treit 1985; Treit et al. 1993; Treit et al. 2010; Treit and Menard 1997; Treit and Pinel 2005). The experimenter handled each of the rats for 5 min, checking the cannulae tracts for blockage and habituating the rats to the infusion procedures, on each of the four consecutive days prior testing. All behavioral testing occurred in a quiet testing room between 0900 and 1700 hours and was recorded on videotape. Testing started 10 min after the end of infusion procedure, as described in previous studies (e.g., Engin et al. 2008). The subjects were assigned to the same drug treatment groups for both behavioral tests. The plus-maze test occurred first, followed by the shock-probe test 7 days later.
Elevated plus maze
The maze was a plus-shaped apparatus with an open roof, consisting of two 50 × 10 cm open arms, and two 50 × 10 × 50 cm enclosed arms, and elevated at a height of 50 cm. All testing was conducted between 0900 and 1700 hours in a quiet and dimly illuminated room. Each animal was tested for 5 min. Four variables were measured: (1) time spent in the open arms; (2) time spent in the closed arms; (3) number of entries into the open arms; and (4) number of entries into the closed arms. A rat was considered to have entered or spent time in an arm only when all four paws were in the respective arm. The time spent in the open arms and the number of open-arm entries were expressed as a percentage of total arm activity (open-arm time/open-arm time + closed-arm time) × 100, and total arm entries (open-arm entries/open-arm entries + closed-arm entries) × 100, respectively. A higher percentage of open-arm time or open-arm entries are taken as measures of anxiety-reduction (anxiolysis). In addition, the total of all arms entered, as well as the total of closed arms entered, were used as indexes of general activity (Hogg, 1996; Pellow and File 1986; Treit et al. 1993).
Shock-probe burying
Three days after the elevated plus-maze test, the rats began habituations for the shock-probe burying test. The 40 × 30 × 40 cm Plexiglas shock-probe chamber contained woodchip bedding material distributed evenly on the floor of the chamber. Rats were habituated individually in the shock-probe chamber, without the probe in place, for 30 min on each of the four consecutive days before the test. On the test day, rats were placed individually on the floor of the chamber, which now had an electrified probe (6.5 cm long and 0.5 cm in diameter) protruding from one of the walls, 2 cm above the bedding material. Each time the rats came into contact with the probe, they received a shock (2 mA). Current was generated with an AC shocker (H13-15 precision regulated shocker, Colbourn Instruments, Allentown, PA, USA). The 15-min test period began when the first shock was received, and the probe remained electrified throughout the testing period. During this period, the following measures were taken: (1) total amount of time spent spraying bedding material towards or on top of the shock probe with rapid, alternating pushing movements of the forepaws (i.e., burying behavior); (2) number of shocks received due to contact with the probe; (3) amount of time spent immobile (e.g., rest, sleep); and (4) reactivity to shock, which was measured on a four-point scale: (1) flinch involving only head or forepaw, (2) whole body flinch, with or without slow ambulation away from the probe, (3) whole body flinch, and/or jumping, followed by immediate ambulation away from the probe, and (4) whole body flinch and jump (all four paws in the air), followed by immediate and rapid ambulation (i.e., running) to the opposite end of the chamber. An average reactivity score was computed for each animal by summing up reactivity scores for all the shocks taken and dividing this by total number of shocks taken. Total amount of time spent burying the probe was taken as a measure of anxiety, with reduced burying indicating anxiolysis. The number of contact-induced probe shocks was also used as a measure of anxiety, with increased contacts indicating reduced anxiety. Time spent immobile (e.g., resting on the floor of the chamber) was an inverse index of general activity. Finally, mean shock reactivity was used as a measure of pain sensitivity. All testing took place between 0900 and 1700 hours. The bedding material was cleaned between animals and smoothed to an even thickness before the next animal was tested (Treit and Pinel, 2005).
Histology
Following behavioral testing, rats were euthanized with an overdose of sodium pentobarbital (Nembutal) and perfused intracardially with 0.9% (w/v) saline followed by 4% (v/v) formaldehyde. Post-fixation, the brains were removed from the skull and placed in a 4% formaldehyde solution for at least 48 h. The brains were then frozen with dry ice and cut into 60-μm sections with a sliding microtome (model 860, American Optical Company, Buffalo, NY, USA). Every second section was collected and mounted onto a microscope slide and later stained with thionin. The behavioral data from animals with either one or both cannulae outside of the target area were excluded from the behavioral analysis.
Statistical analyses
The results from the elevated plus-maze test and shock-probe test were expressed as means and standard errors of the mean (S.E.M). Behavioral measures from both tests were analyzed with planned comparisons (Keppel and Zedeck 1989; ANOVA; α = 0.05)
Results
Histology
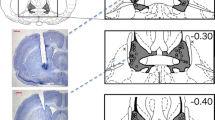
Figure 1 shows the approximate infusion sites of rats with amygdalar cannulae included in the behavioral analysis of experiment 1. Most of the cannulae tips were bilaterally clustered in the medial and lateral divisions of the central amygdala, as well as the basolateral amygdala. Four out of 36 rats, however, were excluded from the main behavioral analyses as a result of misplaced cannulae (indicated by the black squares in Fig. 1). Their data were later compared to that of the included control rats in a small, post hoc analysis of site specificity. Figure 2 shows the approximate infusion sites of rats with septal cannulae included in the behavioral analysis of experiment 2. All 39 rats in this surgical group had cannulae tips bilaterally centered in the intermediate and dorsolateral regions of the septum. Three rats in experiment 2 fell off the plus maze so their data was excluded from that analysis. The same three rats were tested in the shock probe without incident, and their data were included in that analysis. Figure 3 shows the approximate infusion sites of rats with striatal cannulae included in an a priori analysis of site specificity in experiment 4. All placements were bilaterally centered in striatum.
Schematic diagram of coronal sections of the rat brain illustrating the approximate locations (black circles) of amygdalar infusion sites in experiment 1. The black squares illustrate misplaced cannulae in this experiment. The atlas plates are adapted from Paxinos and Watson (1986). Below is a representative photomicrograph of amygdalar cannulae sites
Schematic diagram of coronal sections of the rat brain illustrating the approximate locations (black circles) of septal infusion sites in experiment 2. There were no misplaced cannulae in experiment 2. The atlas plates are adapted from Paxinos and Watson (1986). Below is a representative photomicrograph of septal cannulae sites
Schematic diagram of coronal sections of the rat brain illustrating the approximate locations (black circles) of striatal infusion sites in experiment 4. There were no misplaced cannulae. The atlas plates are adapted from Paxinos and Watson (1986). Below is a representative photomicrograph of striatal cannulae sites
Experiment 1: intra-amygdalar infusions
Elevated plus maze
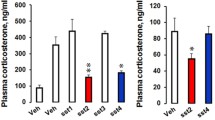
As can be seen in Fig. 4, both SST 14 and SST 28 produced robust anxiolytic-like effects in the elevated plus maze. Planned comparisons showed that both the SST 14 group (F(1,30) = 4.21; p = 0.044) and the SST 28 group (F(1,30) = 7.52; p = 0.001) had significantly higher percentages of open-arm entries, compared to the vehicle control group. Likewise, both the SST 14 (F(1,30) = 5.37; p = 0.012) and SST 28 (F(1,30) = 8.14; p = 0.001) groups spent a significantly higher percentage of time in the open arms than the vehicle control group. Neither the percentage of open-arm entries nor the percentage of time spent in the open arms differed significantly between the SST 14 and SST 28 groups (% open-arm entries (F(1,30) = 3.31; p > 0.11);% open-arm time (F 1,30) = 2.77; p > 0.17). There were no differences between the groups on either measure of general activity (closed-arm entries: F(1,30) = 2.10; p > 0.30; total arm entries F(1,30) = 1.92; p > 0.46; see Table 1). When the four rats with misplaced cannulae (data not shown) were directly compared to the original vehicle control group (see Fig. 4), there were no significant differences in the percentage of open-arm entries (F(1, 13) = 0.59; p > 0.5) or percentage of open-arm time (F(1,13) = 0.25; p > 0.5), suggesting some degree of site specificity in the anxiolytic effects of SST in the amygdala.
Open-arm activity following intra-amygdalar microinfusions of vehicle, 3 μg bilateral of SST 14, or 3 μg bilateral of SST 28 in experiment 1. Black bars represent mean (±SEM) percentage of open-arm entries; white bars represent mean (±SEM) percentage of open-arm time. *Significantly different from the vehicle control group (p < 0.05). †Significantly different from the vehicle control group (p < 0.01)
Shock-probe test
Figure 5 shows that SST 14 and SST 28 produced clear anxiolytic-like effects in the shock-probe burying test. Planned comparisons revealed a significant suppression of burying behavior in both the SST 14 group (F(1,30) = 5.09; p = 0.016) and SST 28 group (F(1,30) = 4.65; p = 0.027) compared to the vehicle control group. A direct comparison of the effect of SST 14 and SST 28 in the shock-probe test was not significant (F(1,30) = 0.44; p > 0.87). Measures of general activity (F(1,30) = 0.38; p > 0.85), number of probe contacts (F(1,30) = 0.62; p > 0.76), and reactivity to shock (F(1,30) = 1.35; p > 0.51) did not differ between groups (Table 2). There was no significant difference in the duration of burying behavior between the four rats with misplaced cannulae (data not shown) and the vehicle infused controls (F(1,13) = 4.16; p > 0.06)
Experiment 2: intra-septal infusions
Elevated plus maze
Similar to the findings of experiment 1, Fig. 6 shows that intra-septal infusion of either SST 14 or SST 28 produced unambiguous anxiolytic-like effects in the elevated plus maze. Planned comparisons showed that the percentages of open-arm entries for the SST 14 group (F(1,33) = 6.44; p = 0.01) and the SST 28 group (F(1,33) = 9.61; p = 0.004) were significantly higher than the vehicle control group, as were the percentages of open-arm time [SST 14: F(1,33) = 7.29; p = 0.011; SST 28: F(1,33) = 14.13; p = 0.001]. Neither measure of anxiolysis differed significantly between the SST 14 and SST 28 groups (% open-arm entries): F(1,33) = 0.38; p > 0.50;% open-arm time: (F(1,33) = 2.38; p > 0.24). There were no significant between-group differences in closed entries (F(1,33) = 0.51; p > 0.80), or total arm entries (F(1,33) = 0.49; p > 0.81; Table 3).
Open-arm activity following intra-septal microinfusions of vehicle 1.5 μg bilateral of SST 14, or 1.5 μg bilateral of SST 28 in experiment 2. Black bars represent mean (±SEM) percentage of open-arm entries; white bars represent mean (±SEM) percentage of open-arm time. *Significantly different from the vehicle control group (p < 0.05). †Significantly different from the vehicle control group (p < 0.01)
Shock-probe test
The anxiolytic effects of intra-amygdalar SST 14 or SST 28 in the shock probe burying test in experiment 1 were replicated by intra-septal infusions of the same compounds in experiment 2 (see Fig. 7). Here, a significant suppression of burying behavior occurred in both groups compared to control [SST 14: F(1,36) = 7.95; p = 0.008; SST 28: F(1,36) = 6.47; p = 0.01]. Defensive burying did not differ significantly in rats given SST 14 compared to rats given SST 28: (F(1,36) = 0.28; p > 0.89). Measures of resting behavior (F(1,36) = 1.96; p > 0.33), number of probe contacts (F(1,36) = 1.99; p > 0.33), and reactivity to shock (F(1,36) = 2.08; p > 0.31) did not differ significantly from control (Table 4).
Experiment 3: intra-amygdalar infusions II: low dose
Table 5 shows the mean (±SEM) differences in open-arm activity and burying behavior in rats given intra-amygdalar infusions of vehicle or a low dose of SST 14 (0.5 μg bilateral). Neither the percentage of open-arm entries (F(1,5) = 3.37; p > 0.5) nor the percentage of open-arm time (F(1,5) = 2.59; p > 0.5) were significantly different between the groups. Importantly, the direction of the mean differences in the plus maze is opposite to that of an anxiolytic effect. The mean difference in the duration of burying behavior seen in Table 5 was also not significant (F(1,5) = 1.18; p > 0.5). Taken together with the positive results of experiment 1, experiment 3 provides preliminary evidence that intra-amygdalar doses of SST 14 below 1.5 μg per hemisphere may not be sufficient to reliably produce anxiolytic effects in the plus-maze and shock-probe tests.
Experiment 3: intra-septal infusions II: low dose
Table 6 shows mean (±SEM) differences between the low dose (0.5 μg per hemisphere) SST 14 groups and the vehicle control groups in the plus-maze and the shock-probe burying tests. None of these differences was statistically significant [% entries:(F(1,6) = 0.26; p > 0.5);% time:[(F(1,6) = 0.1; p > 0.5)]; duration of burying: (F(1,6) = 0.93; p > 0.5)]. In the shock-probe burying test, the mean differences are opposite to an anxiolytic effect (Table 6). Combined with the null effects of intra-amygdalar microinfusions of a 0.5 μg bilateral dose of SST 14, described above, the null effects of the same microinfusion regimen in the septum suggest that doses higher than 0.5 μg per hemisphere may be necessary to produce reliable anxiolytic effects in the plus-maze and shock-probe tests.
Experiment 4: intra-striatal infusions: high dose
The striatum synthesizes SST and expresses SST receptor sub-types (e.g., sst2; Santis et al. 2009). Be this as it may, neither the percent open-arm entries (F(1,6) = 1.10; p > 0.3), percent open-arm time (F(1,6) = 1.31; p > 0.3), nor the duration of burying behavior (F(1,6) = 2.57; p > 0.5) differed significantly between the SST 14 group (3.0 μg per hemisphere) and the vehicle control group (Table 7). These findings add evidence of site specificity, since the same dose of SST 14 microinfused into the amygdala or septum in experiments 1 and 2 produced reliable anxiolytic effects in both behavioral tests.
Discussion
SST 14 and SST 28 both had potent anxiolytic-like effects in the elevated plus-maze and shock-probe burying tests after microinfusions into two brain areas consistently implicated in anxiety: the amygdala and the septum. The increase in open-arm activity produced by intra-amygdalar or intra-septal microinfusion of SST 14 or SST 28 was comparable to that previously observed following ICV infusions of SST 14, L-779976, (a synthetic sst2 agonist), and diazepam, a classical anxiolytic drug (see Engin et al. 2008; Engin and Treit 2009). The anxiolytic effects of SST appeared to be site specific, since they did not occur in the striatum, an area that similarly expresses the sst2 subtype. Finally, the results show that the shock-probe burying test is also sensitive to the anxiolytic effects of SST agonists, providing converging evidence of their anxiolytic effects.
The anxiolytic-like effects of SST 14 and SST 28 are not readily explained by non-specific effects on general activity. Neither intra-amygdalar nor intra-septal SST 14 or SST 28 significantly changed measures of general activity in either test (see Tables 1–4). It is possible that anxiolytic-like effects in the shock-probe burying test were confounded by prior drug experience in the first test (elevated plus maze). However, this would have required the development of drug tolerance (or sensitization) after a single, microgram dose of SST, and the maintenance of this drug-induced change across the 7-day interval between the first test and the second. Nor does it seem plausible that 5% DSMO, the vehicle used to dissolve SST, could have produced neurotoxic effects that somehow led to the anxiolytic-like effects found in the present experiments for three reasons. First, all drug conditions were compared to surgically equivalent DSMO vehicle control groups. Second, doses of DSMO used for medical treatments (e.g., 2–6 g/kg/20 days; Brien et al. 2008), which have sometimes been associated with adverse side effects, are over five orders of magnitude greater than those used here. Third, high doses of DMSO administered to adult rats for long durations have produced little or no evidence of neurotoxic effects (Authier et al. 2002).
The site specificity of the anxiolytic effects of SST found in the amygdala and septum was tested by microinfusing an effective dose (3.0 μg/hemisphere) of SST 14 into the striatum in an area approximately 2.5 mm posterior to the emergence of central and basolateral sub-regions of the amygdala and 2.0 mm lateral to the septum (Paxinos and Watson 1986). The absence of an anxiolytic effect of this dose of SST 14 in the striatum, which also contains SST receptors, is consistent with the view that the anxiolytic effects previously observed in amygdala and septum were site-specific.
More problematic is the lack of difference in the anxiolytic effects of SST 14 and SST 28. Based on their relative potency differences in the periphery (e.g., Mandarino et al. 1981) and their varied effects on potassium channel currents in the brain (e.g., Karschin 1995 Kreienkamp et al. 1997; Wang et al. 1989, 2000), we expected some differences in their anxiolytic effects. Higher doses of SST 14 and SST 28 might have revealed these differences, but pilot studies showed doses higher than 3 μg/per hemisphere (e.g., 8–12 μg) produced adverse side effects (e.g., sedation, ataxia). On the other hand, we found that a lower dose (0.5 μg bilateral) of SST was not anxiolytic in the plus-maze or the shock-probe tests (experiment 3). Further study of their dose effects in the plus-maze and shock-probe tests is warranted.
Another possibility is that metabolic factors obscured differences in the anxiolytic effects of the two isoforms. SST 14 is produced through two biosynthetic pathways: direct enzymatic processing of prosomatostatin and/or the proteolytic conversion of SST 28 to SST 14 (Patel, 1999; Zingg and Patel 1982). Both conversion pathways are rapid in cell preparations (direct, 4 min; indirect, 8 min). Therefore, given the 10-min interval between microinfusion and behavioral testing in these experiments, enough time had passed for SST 28 to have been completely converted into SST 14 before the tests began, explaining the similarity of anxiolytic effects. The relative importance of these biosynthetic pathways, however, is highly dependent on the brain area under examination (Patel 1999; Robbins and Reichlin 1983; Zingg and Patel 1982). Directly quantifying the conversion kinetics of the two pathways in the amygdala and septum would be less feasible than two other approaches. The simplest is to reduce the time between microinjection and behavioral testing to 3 min, thus reducing the possibility of conversion of SST 28 to SST 14. The second is to combine microinfusion of SST 28 with hexa-d-arginine, a known inhibitor of PACE-4, the enzyme that converts SST 28 into SST 14 (Hall et al. 2007; Schindler et al. 1996). Anxiolytic effects that survive a reduction of the injection-test interval and/or enzyme inhibition could then be attributed to SST 28.
SST 14 and SST 28 have similar affinities for each of the five SST receptor subtypes, with the exception of sst5, where SST 28 has a 10 to 50-fold greater affinity than SST 14 (Olias et al. 2004; Patel 1999). However, only an sst2 receptor agonist reduced anxiety in previous experiments; agonists of sst1, sst3, sst4, and sst5 were not effective (Engin and Treit 2009). Given the similar affinities of SST 14 and SST 28 for the sst2 sub-type and the similar densities of sst2 receptors in the amygdala and septum (Bassant et al. 2005; Breder et al. 1992), commonalities in their anxiolytic effects might be expected. Nevertheless, previous experiments have found qualitative differences in the anxiolytic effects of GABAA receptor agonists (e.g., midazolam) microinfused into the septum and the amygdala as a function of the specific sub-regions into which they were infused (e.g., Pesold and Treit 1995; 1996). Thus, it seems possible—given overlap in the functions and distributions of SST and GABAA receptors in these regions—that similar sub-regional differences in the anxiolytic SST might also be discovered.
Synthetic analogues of SST have been developed that already play a useful role in the treatment of a number of medical disorders, including tumor cell proliferation (e.g., de Jong et al. 1999; Hofland et al. 1992). As well, there is emerging evidence that SST could play a role in CNS and PNS disorders, such as epilepsy, pain, and perhaps dementia (Chrubasik and Ziegler 1996; Pinter et al. 2006). In view of the present results, psychiatric disorders such as anxiety might be another class of therapeutic targets for SST and its analogues (Weckbecker et al. 2003).
References
Authier et al (2002) Behavioural assessment of dimethylsulfoxide neurotoxicity in rats. Toxicology Letters 132(2):117–121
Bassant M, Simon A, Poindessous-Jazat F, Csaba Z, Epelbaum J, Dournaud P (2005) Medial septal GABAergic neurons express the somatostatin sst2a receptor: functional consequences on unit firing and hippocampal theta. J Neurosci 25:2032–2041
Batten TFC, Gamboa-Esteves FO, Saha S (2002) Evidence for peptide co-transmission in retrograde and anterograde-labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci 98:28–32
Breder CD, Yamada Y, Yasuda K, Seino S, Saper CB, Bell GI (1992) Differential expression of somatostatin receptor subtypes in brain. J Neurosci 12:3920–3934
Brien S, Prescott P, Bashir N, Lewith H, Lewith G (2008) Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis Cartilage 16:1277–1288
Cervia D, Bagnoli P (2007) An update on somatostatin receptor signalling in native systems and new insights on their pathophysiology. Pharmacol Ther 116:322–341
Chrubasik S, Ziegler R (1996) Does the somatostatin analogue pctreotide have a role in pain relief? Pain Clin 8:369–375
De Jong M, Breeman WAP, Bernard HF, Kooij PPM, Slooter GD, Van Eijck CHJ, Kwekkeboom DJ, Valkema R, Macke HR, Krenning EP (1999) Therapy of neuroendocrine tumors with radiolabeled somatostatin-analogues. Q J Nucl Med 43:356–366
Degroot A, Treit D (2004) Anxiety is functionally segregated within the septo-hippocampal system. Brain Res 1001:60–71
Dutar P, Vaillend C, Viollet C, Billard JM, Potier B, Carlo AS, Ungerer A, Epelbaum J (2002) Spatial learning and synaptic hippocampal plasticity in type 2 somatostatin receptor knock-out mice. Neuroscience 112:455–466
Engin E, Treit D (2009) Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl) 206:281–289
Engin E, Stellbrink J, Treit D (2008) Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157:666–676
Gray JA, McNaughton N (2000) The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system, 2nd edn. Oxford University Press, Oxford
Hall T et al (2007) A high performance liquid chromatography assay for monitoring proprotein convertase activity. J Chromatogr A 1148:46–54
Hofland LJ, van Koetsveld PM, Wouters N, Waaijers M, Reubi JC, Lamberts SW (1992) Dissociation of antiproliferative and antihormonal effects of the somatostatin analog octreotide on 7315b pituitary tumor cells. Endocrinology 131:571–577
Hogg S (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54:21–30
Karschin A (1995) Molecular single cell analysis intensifies somatostatin type 1 (sst1) receptors to block inward rectifying K+ channels in rat brain oligodendrocytes. Neuroreport 7:121–124
Keppel G, Zedeck S (1989) Data analysis for research design: analysis of variance and multiple regression/correlation approaches. W. H. Freeman, New York
Kreienkamp HJ, Hönck HH, Richter D (1997) Coupling of rat somatostatin receptor subtypes to a G-protein gated inwardly rectifying potassium channel (GIRK1). FEBS Lett 419:92–94
LeDoux J (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Leroux P, Quirion R, Pelletier G (1985) Localization and characterization of brain somatostatin receptors as studied with somatostatin-14 and somatostatin-28 receptor radioautography. Brain Res 347:74–84
Mandarino L, Stenner D, Blanchard W, Nissen S, Gerich J, Ling N, Brazeau P, Bohlen P, Esch F, Guillemin R (1981) Nature 291:76–77
McNaughton N, Kocsis B, Hajos M (2007) Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav Pharmacol 18:329–346
Meis S, Sosulina L, Schulz S, Höllt V, Pape HC (2005) Mechanisms of somatostatin-evoked responses in neurons of the rat lateral amygdala. Eur J Neurosci 21:755–762
Moller LN, Stidsen CE, Hartmann B, Holst JJ (2003) Somatostatin receptors. Biochim Biophys Acta 1616:1–84
Nanda SA, Qi C, Roseboom PH, Kalin NH (2008) Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes Brain Behav 7:639–648
Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W (2004) Regulation and function of somatostatin receptors. J Neurochem 89:1057–1091
Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, New York
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Pesold C, Treit D (1994) The septum and amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res 638:295–301
Pesold C, Treit D (1995) The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res 671:213–221
Pesold C, Treit D (1996) The neuroanatomical specificity of the anxiolytic effects of intra-septal infusions of midazolam. Brain Res 710:161–168
Pinter E, Helyes Z, Szolcsanyi J (2006) Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112:440–456
Robbins RJ, Reichlin S (1983) Somatostatin biosynthesis by cerebral cortical cells in monolayer culture. Endocrinol 113:574–581
Saha S, Henderson Z, Batten TF (2002) Somatostatin immunoreactivity in axon terminals in rat nucleus tractus solitarii arising from central nucleus of amygdala: coexistence with GABA and postsynaptic expression of sst2A receptor. J Chem Neuroanat 24:1–13
Santis S, Kastellakis A, Kotzamani D, Pitarokoili K, Kokona D, Thermos K (2009) Somatostatin increases rat locomotor activity by activating sst2 and sst4 receptors in the striatum and via glutamatergic involvement. Naunyn-Schmiedeberg's Arch Pharmacol 379:181–189
Schindler M, Humphrey PPA, Emson PC (1996) Somatostatin receptors in the central nervous system. Prog Neurobiol 50:9–47
Selmer IG, Schindler M, Allen JP, Humphrey PPA, Emson PC (2000) Advances in understanding neuronal somatostatin receptors. Regul Peptide 90:1–18
Semenova S, Hoyer D, Geyer MA, Markou A (2010) Somatostatin-28 modulates prepulse inhibition of the acoustic startle response, reward processes and spontaneous locomotor activity in rats. Neuropeptides 44:421–429
Shin J, Gireesh G, Kim S et al (2009) Phospholipase c β4 in the medial septum controls cholinergic theta oscillations and anxiety behaviors. J Neurosci 29:15375–15385
Sur C, Korn H, Triller A (1994) Colocalization of somatostatin with GABA or glutamate in distinct afferent terminals presynaptic to the Mauthner cell. J Neurosci 14:576–589
Tallman JF, Gallager DW (1985) The GABA-ergic system: a locus of benzodiazepine action. Annu Rev Neurosci 8:21–44
Treit D (1985) Animal models for the study of anti-anxiety agents: a review. Neurosci Biobehav Rev 9:203–222
Treit D, Menard J (1997) Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behav Neurosci 111:653–658
Treit D, Menard J (2000) The septum and anxiety. In: Numan R (ed) The behavioral neuroscience of the septal region. Springer, New York, pp 210–233
Treit D, Pinel JPJ (2005) Defensive burying. In: Wishaw IQ, Kold B (eds) The behavior of the laboratory rat. Oxford University Press, New York, pp 353–362
Treit D, Menard J, Royan C (1993) Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav 44:463–469
Treit D, Engin E, McEown K (2010) Animal models of anxiety and anxiolytic drug action. In: Stein MB, Steckler T (eds) Behavioral neurobiology of anxiety and its treatment. Springer, New York, pp 121–160
Vécsei L, Widerlöv E, Alling C, Zsigó J, Pávó I, Penke B (1990) Somatostatin28(15–28), but not somatostatin28(1–12), affects central monoaminergic neurotransmission in rats. Neuropeptides 16:181–186
Wang HL, Bogen C, Reisine T, Dichter M (1989) Somatostatin-14 and somatostatin-28 induce opposite effects on potassium currents in rat neocortical neurons. Proc Natl Acad Sci USA 86:9616–9620
Wang HL, Dichter M, Reisine T (2000) Lack of cross-desensitization of somatostatin-14 and somatostatin-28 receptors coupled to potassium channels in rat neocortical neurons. Mol Pharmacol 38:357–361
Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C (2003) Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Dis 2:999–1017
Williams G, Ball JA, Lawson RA, Joplin GF, Bloom SR, Maskill MR (1987) Analgesic effect of somatostatin analogue (octreotide) in headache associated with pituitary tumours. Br Med J (Clin Res Ed) 295:247–248
Zingg HH, Patel YC (1982) Processing of synthetic somatostatin-28 and a related endogenous rat hypothalamic somatostatin-like molecule by hypothalamic enzymes. Life Sci 30:525–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeung, M., Engin, E. & Treit, D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology 216, 557–567 (2011). https://doi.org/10.1007/s00213-011-2248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2248-x