Abstract
Rationale
Pramipexole and other direct dopamine agonist medications have been implicated in the development of impulsive behavior such as pathological gambling among those taking the drug to control symptoms of Parkinson’s disease or restless leg syndrome. Few laboratory studies examining pramipexole’s effects on gambling-like behavior have been conducted.
Objectives
The present study used a rodent model approximating some aspects of human gambling to examine within-subject effects of acute pramipexole (0.03, 0.1, 0.18, and 0.3 mg/kg) on rat’s choices to earn food reinforcement by completing variable-ratio (VR; i.e., gambling-like) or fixed-ratio (FR) response requirements.
Results
In a condition in which the VR alternative was rarely selected, all but the lowest dose of pramipexole significantly increased choice of the VR alternative (an average of 15% above saline). The same doses did not affect choice significantly in a control condition designed to evaluate the involvement of nonspecific drug effects. Pramipexole increased latencies to initiate trials (+9.12 s) and to begin response runs on forced-choice trials (VR = +0.21 s; FR = +0.88 s), but did not affect measures of response perseveration (conditional probabilities of “staying”).
Conclusions
The findings are consistent with clinical reports linking pramipexole to the expression of increased gambling in humans. Results are discussed in the context of neurobehavioral evidence suggesting that dopamine agonists increase sensitivity to reward delay and disrupt appropriate feedback from negative outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pramipexole (PPX) is a dopamine (DA) D2/D3 selective receptor agonist commonly prescribed as part of dopamine replacement therapy for Parkinson’s disease (PD). PPX has higher selective affinity for the D3 receptor subtype (7.8-fold over D2; Kvernmo et al. 2006) which is expressed predominantly in the limbic areas of the brain (Sokoloff et al. 1990). Limbic areas are thought to mediate aspects of addictions to drugs and gambling (e.g., Lader 2008). Several clinical reports indicate that when some patients with PD are treated with D2/D3 agonists, like PPX, they develop impulse control disorders (ICDs) such as pathological gambling (Molina et al. 2000; Driver-Dunckley et al. 2003; Dodd et al. 2005; Grosset et al. 2006), compulsive shopping (Giladi et al. 2007), hypersexuality (Giovannoni et al. 2000; Klos et al. 2005; McKeon et al. 2007; Munhoz et al. 2009), and compulsive eating (Nirenberg and Waters 2006). A recent laboratory study reported that PD patients who developed an ICD (pathological gambling or compulsive shopping) prior to the study and after taking PPX or ropinorole made significantly fewer impulsive choices on an Experiential Discounting Task when use of these medications was suspended (Voon et al. 2010). Choices of PD patients without an ICD were unaffected by the DA agonists.
PPX is also used in the treatment of restless leg syndrome and fibromyalgia and, as among PD patients, there have been clinical reports of increased impulsive behavior, including pathological gambling (Driver-Dunckley et al. 2007; Holman 2009; Cornelius et al. 2010). Given these reports, and because some research has exploited the antidepressant effects of PPX in humans (Corrigan et al. 2000; Zarate et al. 2004) and an animal model (Breuer et al. 2009), it is important to conduct controlled laboratory studies of the effects of PPX on gambling and impulsive decision making among those without PD.
To that end, four laboratory experiments have examined the relation between PPX and activities that may be of relevance to gambling. In a study by Riba et al. (2008), healthy participants wagered 5 or 25 cents on probabilistic wins that matched the amount they bet. Acute PPX (0.5 mg) significantly increased 25-cent wagers immediately following 25-cent wagers that resulted in unexpected “boost” wins of 50 cents. No other gambling activity was significantly affected by PPX. In another experiment, Hamidovic et al. (2008) found no significant effect of acute PPX (0.25 or 0.5 mg) on eight healthy participants’ impulsive or risky choices in delay and probability discounting tasks. However, a non-significant trend toward more impulsive choice was apparent in the delay discounting task. Consistent with this trend, Madden et al. (2010) reported a significant increase in impulsive choice in outbred rats given pre-session injections of PPX at doses of 0.1, 0.18, and 0.3 mg/kg. However, in a second experiment in the same report, PPX did not significantly increase impulsive choice when the delay to the larger reward increased within-sessions (rather than between-conditions as in the first experiment).
The present study was designed to explore the effects of PPX on preference for gambling-like schedules of food reinforcement in rats. To this end, a choice preparation modeled two aspects of the human gambling milieu: the unpredictable occurrence of gambling wins and the net loss of income associated with gambling (Grant et al. 2010). The former was modeled by a variable-ratio (VR) schedule approximating the unpredictable number of responses preceding a win. This was contrasted with a non-gambling alternative where food was delivered on a predictable, fixed-ratio (FR) schedule. The net loss of income associated with human gambling was modeled by employing two procedures. First, the average number of responses required to obtain food from the gambling-like alternative (i.e., the VR schedule) was always higher than the FR value. Second, sessions were completed in a closed economy (i.e., long-duration sessions in which rats’ choices determined their daily food intake; see Hursh 1984) and ended when the rat had expended its daily budget which corresponded to a finite number of lever-press responses that could be allocated to the VR and FR alternatives. Given a finite budget and the higher price of food on the gambling-like alternative, choosing to gamble decreased food intake (i.e., income).
Rats completed two conditions in which the effects of PPX (0.03, 0.1, 0.18, 0.3 mg/kg) on choice were investigated. One condition established a baseline preference for the non-gambling (FR) alternative. From this baseline, we could determine if PPX increased choice of the VR alternative. A baseline preference for the gambling-like (VR) alternative was established in the other condition by manipulating the FR value. From this baseline, we could assess nonspecific effects of PPX on choice.
Method
Subjects
Seven experimentally naïve male Wistar rats were obtained from Charles River (Raleigh, NC, USA). Rats were 14 weeks old at the start of the experiment and were housed individually with the exception of two that were housed together due to space limitations. A 12 h/12 h light/dark cycle was programmed in the colony room and water was available ad libitum in the home cages and the experimental chambers. Daily rations of food were determined by choices in the experimental chambers (i.e., no supplemental food was provided by the experimenter). At no time did any rat’s weight fall below pre-experiment ad libitum weight. Animal use was in accordance with the Institutional Animal Care and Use Committee of the University of Kansas.
Apparatus and materials
Sessions took place within standard operant conditioning chambers (24.1 cm × 30.5 cm × 21.0 cm; Med Associates, Inc., St. Albans, VT, USA). Centered on the front wall of each chamber and positioned 1 cm above the floor grid was a pellet receptacle (3 cm × 4 cm) into which a pellet dispenser (H14-23R; Coulbourn Instruments, Allentown, PA, USA) could deliver nutritional grain-based rat pellets (45 mg; Bio-Serv, Frenchtown, NJ, USA). Above the receptacle (10 cm) was a non-retractable lever with retractable levers to the left and right (spaced 11 cm apart). A 28-V DC cue light was positioned 6 cm above each lever. A house light was centered 19 cm above the floor on the rear wall. Each chamber was equipped with a white-noise speaker and was situated within a sound-attenuating box (ENV-018MD; Med Associates). All experimental events were programmed using MED-PC™ IV software and were executed via a PC in an adjacent room.
Procedure
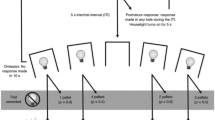
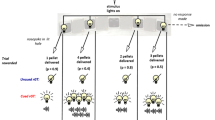
Prior to the first experimental session, reliable responding on all three levers was established using an autoshaping procedure (Brown and Jenkins 1968). Experimental sessions began with a series of four 21-trial blocks. The first 16 trials in each block were forced-choice trials, and the remaining five were free-choice trials. On forced-choice trials, pressing the center lever once extinguished the cue light above the center lever and lit the cue light above one of the two side levers which was inserted into the chamber; eight of the 16 forced-choice trials were completed on the left lever (random sequence). The levers to which the VR and FR alternatives were assigned were counterbalanced across rats. The gambling-like lever arranged a VR schedule of reinforcement, with ratio values of 1, 33, 67, and 99 (VR-50). The VR requirement in each trial was determined randomly with replacement. If the non-gambling lever was inserted on a forced-choice trial, an FR schedule was in effect; the FR value depended upon the baseline condition (see below).
To enhance discrimination between the contingencies arranged on the two levers, for four of the rats the cue light above the lever associated with the VR flashed once every 0.5 s, while the cue light above the lever associated with the FR was constantly illuminated. For the other three rats, this stimulus pairing was reversed. Once a response requirement was completed, the cue and house lights were extinguished, the side lever retracted, and three food pellets were delivered, each accompanied by a 0.5-s flash of light in the pellet receptacle. The next trial was initiated immediately after the last pellet was delivered (i.e., the cue light above the center lever was illuminated).
The five remaining trials within each 21-trial block were free-choice trials. With three exceptions, free-choice trials were the same as forced-choice trials. First, after the rat pressed the center lever, the cue lights above both side levers were lit (one flashing). Second, to allow adequate exposure to the stimuli, the rat was then required to emit four additional responses on the center lever before the center cue light was extinguished and both side levers were inserted into the chamber. Third, a single response on a side lever retracted the other lever and extinguished its cue light for the remainder of the trial. After four of these 21-trial blocks (84 trials), the remainder of the session was composed of free-choice trials.
Low-gambling baseline condition
Four rats (randomly assigned) completed this condition first. On free-choice trials, rats chose between a VR-50 and an FR-5. If the gambling-like VR alternative was selected on >20% of the free-choice trials for two consecutive sessions, the FR requirement was decreased until percent VR choice was ≤20% (see Table 1). Adjustments to the FR value were made in increments of one or two responses (depending on how far outside the target range was choice). One rat (R2) chose the VR alternative on >20% of the free-choice trials even at FR-2; so for this rat, FR-2 served as the non-gambling alternative.
After ten consecutive sessions in which VR choice was ≤20%, the pre-session drug-administration regimen was initiated (see below). At the conclusion of the condition, rats that completed the low-gambling condition first completed the high-gambling condition next, and vice versa.
High-gambling baseline condition
In this condition, rats chose between a VR-50 and an FR-40. All rats selected the VR alternative on ≥80% of the free-choice trials for ten consecutive sessions with no adjustments to the FR value. Once the stability criterion was met, the pre-session drug-administration regimen was initiated in the next session.
Response budget
Side-lever responses emitted in free-choice trials were subtracted from a daily response budget. The response budget was set so that 121 free-choice trials could be completed if the rat chose the FR alternative on every free-choice trial. For example, if the FR requirement was 40, then the budget was set at 4,840 responses (i.e., \( {121} \times {4}0 = {4,84}0 \)). When the response budget was exhausted, the session ended—all lights in the chamber were extinguished and side levers were retracted. If the response budget had not been expended within 4 h, the session ended. Because the average VR value (50) was always higher than that arranged on the FR lever, free choices allocated to the VR alternative decreased the number of free-choice trials and daily income. Responses emitted in forced-choice trials were not counted against the budget.
Drug administration
PPX hydrochloride (N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride) was synthesized and provided by Drs. Shaomeng Wang and Jianyong Chen (University of Michigan, Ann Arbor, MI, USA). PPX was dissolved in physiological saline. Four dosages of PPX (0.03, 0.1, 0.18, 0.3 mg/kg) or saline vehicle were administered subcutaneously 10 min prior to the session at a volume of 1.0 ml/kg. Our dose range was selected because it corresponds to doses known to activate D3-mediated behavior in rats (Collins et al. 2007, 2009).
Once a stable low- or high-gambling baseline was established, an initial saline test was administered. PPX was then administered in a descending dose order. Each saline or drug administration was separated by at least four no-injection sessions (median = 4; range = 4–22 days). The no-injection sessions continued until choice returned to the baseline range for four consecutive sessions. In all, the dosing sequence was repeated three times in each baseline condition, each time separated by a saline test.
Data analysis
The primary dependent measure was percent choice of the VR alternative. Data from low- and high-gambling baseline conditions were analyzed using separate two-way repeated-measures analysis of variance (ANOVA; SPSS 16.0, SPSS Inc., Chicago, IL, USA) with “dose” (saline, 0.03, 0.1, 0.18, 0.3) and “series” (first, second, and third) as within-subject factors. One rat (Bl4) failed to complete any free-choice trials in his third exposure to the 0.3 mg/kg dose in the low-gambling baseline condition. This single piece of missing data was replaced by the across-subjects mean percent VR choice at that dose–series–condition combination.
Because of the potential motor-impairing effects of PPX in rats (Lagos et al. 1998), median response latencies on all three levers (center, FR, and VR) were recorded. To compare latencies across low- and high-gambling baseline conditions, differences between drug and control (no injection) session median latencies were computed and subjected to three-way repeated-measures ANOVAs with “dose”, “series”, and “condition” (low- or high-gambling) as within-subject factors. In the cases of median FR and VR latencies, only data from forced-choice trials were analyzed because subjects sometimes exclusively chose only one alternative in all free-choice trials during a session.
Lastly, because D2/D3 agonists can increase perseverative responding (Boulougouris et al. 2009; Haluk and Floresco 2009), conditional probabilities of same-lever choice in transitions between the last forced- and the first free-choice trial were calculated and analyzed using a three-way ANOVA. This analysis was designed to evaluate the possibility that increased selections of a non-preferred lever (e.g., the VR lever in the low-gambling baseline) were due simply to increased perseveration on the lever that ended the final sequence of forced-choice trials.
With respect to all measures, on occasions where distributions violated assumptions of sphericity and were not amenable to transformations, F statistics were compared to critical values calculated using Greenhouse–Geisser adjusted degrees of freedom. To compute effect sizes, the formula for generalized eta squared, as suggested for use in repeated-measures ANOVA designs, was used (Bakeman 2005).
Results
Figure 1 (top graph) shows group mean percent VR choice (±SEM) as a function of PPX dose. In the low-gambling baseline condition, PPX significantly increased percent VR choice relative to saline levels [significant main effect of dose—F(4, 24) = 6.94, p = 0.001, \( \eta_G^2 = 0.{23} \)]. Post hoc Bonferroni-corrected comparisons showed percent VR choice was significantly elevated above VR choice in saline sessions across all PPX doses except 0.03 mg/kg (p = 0.16). Among PPX doses, however, there were no significant differences in percent VR choice (all p > 0.1). An effect of dosing series (i.e., first, second, or third exposure to saline and drug doses) was observed, suggesting sensitization to the effects of repeated PPX administration. Further analysis through Bonferroni-corrected pairwise comparisons revealed a significant increase in percent VR choice between the first and second exposure to the 0.3 mg/kg dose (p = 0.02). This same ANOVA suggested the possibility of a dose × series interaction; however, insufficient degrees of freedom precluded interpretation of this effect. In the high-gambling baseline condition, percent VR choice was unaffected by dose (p = 0.33) or series (p = 0.82) and there was no significant dose × series interaction (p = 0.75).
Percent choice for the VR alternative as a function of pramipexole dose in low- (bottom of graphs) and high-gambling (top of graphs) baseline conditions. The asterisk indicates doses which differed significantly from saline. In addition, maximum (Bl3), median (R3), and minimum (Bl4) low-gambling PPX responders (ranked by mean response across doses) are shown. C and V represent control (no injection) and saline vehicle, respectively. Error bars are SEM
Figure 1 also depicts representative individual-subject data. Specifically, subjects were rank ordered according to mean PPX response in the low-gambling baseline condition; those exhibiting maximum (Bl3), median (R3), and minimum (Bl4) drug responses are depicted. At this level of analysis, PPX produced a partial dose-effect in the low-gambling baseline condition for some individuals (e.g., Bl3), but less so for others (e.g., Bl4). For rat Bl4, the effect of PPX on choice was nonspecific in that choice in both the low- and high-gambling baseline conditions were shifted toward indifference. However, for the rats that selected the VR alternative more frequently following PPX injections, choice disruptions in the high-gambling baseline condition were rare.
Figure 2 (top panel) shows group mean differences between drug and control (no injection) session median latencies to press the center lever. The differences between these latencies increased as a function of dose [significant main effect of dose—F(4, 24) = 25.45, p < 0.001, \( \eta_G^2 = 0.{43} \); significant linear contrast—p = 0.02]. None of the interactions involving dose were significant (p > 0.09). Latency differences depended upon the dosing series and baseline condition [series × condition interaction—F(2, 12) = 6.99, p = 0.01, \( \eta_G^2 = 0.{12} \)]. The middle panel shows group mean differences between drug and control (no injection) median latencies to make the first response on the FR lever. Significant main effects of dose [F(4, 24) = 5.67, p = 0.002, \( \eta_G^2 = 0.{17} \)] and condition [F(1, 6) = 29.85, p = 0.002, \( \eta_G^2 = 0.{27} \)] were detected. A significant dose × condition interaction [F(4, 24) = 3.78, p = 0.02, \( \eta_G^2 = 0.{1} \)] revealed that PPX increased the already longer FR latencies in the high-gambling baseline condition more than it affected FR latencies in the low-gambling condition. Latency differences also increased as a function of dosing series [significant main effect of series—F(2, 12) = 4.05, p < 0.05, \( \eta_G^2 = 0.{05} \)], although none of the post hoc pairwise comparisons were significant. The bottom panel shows group mean differences between drug and control (no injection) median latencies to make the first response on the VR lever. A significant main effect of dose [F(1.44, 8.62) = 5.51, p = 0.04, \( \eta_G^2 = 0.{1} \)] was observed; however, neither a significant main effect of condition (p = 0.12) nor a significant dose × condition interaction (p = 0.07) was detected.
Group mean differences between median control (no injection) and drug or vehicle (V) latencies to press the center lever (top panel), the FR lever (middle panel), and the VR lever (bottom panel) (note differently scaled y-axes). The middle and bottom panels illustrate data collected on forced-choice trials only (see text for details). Latency differences from low- (left column) and high-gambling (right column) baseline conditions are shown as a function of pramipexole dose. Asterisks indicate significant effects of dose. Error bars are SEM
To investigate the perseverative effects of PPX, the conditional probability of making the first free choice on the lever that ended the series of forced-choice trials (i.e., “staying”) was calculated at each dose. Figure 3 shows group mean conditional probabilities of staying in the low- and high-gambling baseline conditions. Staying was unaffected by baseline condition (p > 0.8). Significant main effects of dose [F(4, 24) = 4.56, p < 0.01, \( \eta_G^2 = 0.{07} \)] and of series [F(2, 12) = 9.11, p < 0.01, \( \eta_G^2 = 0.{02} \)] were detected. Significant dose-related differences identified by post hoc Bonferroni-corrected pairwise comparisons were between saline and two PPX doses (0.1 mg/kg, p = 0.04; 0.18 mg/kg, p = 0.03), with the probability of staying higher following saline administration.
Group mean conditional probabilities of making the first free choice on the lever that ended the series of forced-choice trials (i.e., response perseveration) in low- (left graph) and high-gambling (right graph) baseline conditions. Conditional probabilities are plotted as a function of pramipexole dose with V representing saline vehicle. Error bars are SEM
Discussion
In the present study, a putative animal model of gambling was used to determine the effects of PPX, a D2/D3 dopamine agonist, on preference for a gambling-like (VR schedule) over a non-gambling (FR schedule) source of reinforcement when choosing to gamble resulted in reductions in food obtained. Acute administration of PPX increased choice of the gambling-like source of reinforcement (an average of 15% above saline). Furthermore, PPX’s effects increased with repeated testing, indicating a sensitization effect. In a control (high-gambling baseline) condition, the drug did not significantly affect choice behavior. Thus, the PPX-related increases in choice of the VR alternative are unlikely to be explained by nonspecific drug effects such as disrupted discrimination between response alternatives. This pattern of results appears consistent with the clinically documented coinciding events of emergent pathological gambling and initiation of PPX or other dopamine agonist regimens (Crockford et al. 2008), although caution is warranted when comparing these potentially convergent phenomena.
Consistent with previous findings that PPX (Lagos et al. 1998) and other D3-preferring agonists (Ouagazzal and Creese 2000) suppress locomotor activity in rats, PPX increased latencies to initiate new trials (center lever) and to begin responding on the FR and VR alternatives. Although generalizing across species is not straightforward, human reaction times also increase as a function of acute PPX dose (Hamidovic et al. 2008; Pizzagalli et al. 2008), suggesting a common effect of D3-preferring medications to decrease attention or motor activity in healthy subjects.
Given recent evidence that quinpirole, a D2/D3 dopamine agonist like PPX (but with lower relative preference for D3 compared to D2 receptors), inhibits reversal learning (i.e., promotes response perseveration) (Boulougouris et al. 2009; Haluk and Floresco 2009), we speculated that in drug sessions rats would be more likely to choose an alternative in a free-choice trial that had been the forced choice in the immediately preceding trial. This hypothesis was not supported. Indeed, the conditional probability of choosing the same lever across this forced-free transition was significantly lower at two doses of PPX (0.1 and 0.18 mg/kg) when compared to saline.
Together, these findings offer support for the hypothesis that PPX renders gambling-like schedules of reinforcement more attractive. That is, PPX increased rats’ preference for a less predictable source of food. This would be predicted if PPX increases sensitivity to reinforcer delay or the effort required to obtain the food reward. Previous studies (e.g., Field et al. 1996) have found that preference for VR schedules are strongly affected by the occasional reinforcer obtained after a single response (i.e., immediately and after the expenditure of minimal effort). If PPX increases the reinforcing efficacy of immediate or minimal-effort reinforcers, then in the present procedure this would be reflected in increased preference for the gambling-like alternative. Enhanced sensitivity to reinforcer delay is consistent with one prior study of the effects of PPX (Experiment 1 of Madden et al. 2010) but only trend-level findings in other experiments (Hamidovic et al. 2008; Experiment 2 of Madden et al.). No studies have to our knowledge examined the effects of PPX on sensitivity to effort manipulations.
A complimentary hypothesis comes from studies suggesting that dopamine agonist medications like PPX, while enhancing dopaminergic tone in general, may disrupt processes essential to reward-related learning and reward prediction (e.g., Pizzagalli et al. 2008). Learning from negative outcomes such as unexpected punishment (e.g., Cools et al. 2006) or reward omission (e.g., Frank et al. 2007) is also impaired by PPX in patients with PD. Along these lines, the results of the present study suggest that, through these disruptive effects, PPX may also desensitize an organism to the negative effects of large ratio requirements. When PPX was administered in the low-gambling baseline condition, rats were more likely to choose the gambling alternative, which occasionally required a greater number of responses (i.e., 33, 67, or 99) than did the non-gambling alternative (≤5). Perhaps PPX rendered these larger response requirements less aversive than during non-PPX sessions.
A few limitations of this research deserve comment. First, the function of the programmed response budget is unclear. For example, whether behavior was sensitive to the molar consequences of reductions in obtained food is unknown. Although budgetary manipulations of this kind are rich in face validity (i.e., topographically similar to budgets encountered in human choice contexts), their influence upon preference for gambling, especially when coupled with D2/D3 agonist medications, remains a topic for further investigation. Second, although reports of emergent ICDs have been most often reported in individuals with PD, we did not use Parkinsonian rats (i.e., intranigral injections of 6-OHDA). Future investigations of the effects of D2/D3 agonists among Parkinsonian animal models may benefit by conducting assessments of delay discounting before acute and daily dosing begins. Lastly, a graded dose–response function was obtained for latency measures but not for VR choice. While both D2 and D3 receptor stimulation may underlie inhibition of locomotor activity (Millan et al. 2004), sites of action modulating complex behavioral phenomena such as gambling preference are less certain (Fineberg et al. 2010). Co-administration of D2- or D3-preferring antagonists along with PPX may help to further elucidate the neurobiological basis for this clinically relevant finding.
In sum, the present study detected measurable increases in gambling-like behavior when PPX was administered acutely. Whether the effects are explicable in terms of deficient reward processing resulting in either marked insensitivity to larger ratio requirements or increased preference for unpredictable reward scheduling (or both) is unknown. The present findings raise the possibility that nonhuman animal laboratory models of gambling may have translational utility for investigating the effects of environmental and pharmacological interventions affecting human gambling.
References
Bakeman R (2005) Recommended effect size statistics for repeated measures designs. Behav Res Meth 37:379–384
Boulougouris V, Castañé A, Robbins TW (2009) Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 202:611–620
Breuer ME, Groenink L, Oosting RS, Buerger E, Korte M, Ferger B et al (2009) Antidepressant effects of pramipexole, a dopamine D3/D2 receptor agonist, and 7-OH-DPAT, a dopamine D3 receptor agonist, in olfactory bulbectomized rats. Eur J Pharmacol 616:134–140
Brown PL, Jenkins HM (1968) Autoshaping of the pigeon’s key-peck. J Exp Anal Behav 11:1–8
Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C et al (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 193:159–170
Collins GT, Tuccone A, Haji-Abdi F, Newman AH, Grundt P, Rice KC et al (2009) Proerectile effects of dopamine D2-like agonists are mediated by the D3 receptor in rats and mice. J Pharmacol Exp Ther 329:210–217
Cools R, Altamirano L, D’Esposito M (2006) Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia 44:1663–1673
Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH (2010) Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case–control study. Sleep 33:81–87
Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000) Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety 11:58–65
Crockford D, Quickfall J, Currie S, Furtado S, Suchowersky O, el-Guebaly N (2008) Prevalence of problem and pathological gambling in Parkinson’s disease. J Gambl Stud 24:411–422
Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE (2005) Pathological gambling caused by drugs used to treat Parkinson’s disease. Arch Neurol 62:1377–1381
Driver-Dunckley ED, Samanta J, Stacy M (2003) Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology 61:422–423
Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J et al (2007) Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol 30:249–255
Field DP, Tonneau F, Ahearn W, Hineline PN (1996) Preference between variable-ratio and fixed-ratio schedules: local and extended relations. J Exp Anal Behav 66:283–295
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A et al (2010) Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35:591–604
Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science 318:1309–1312
Giladi N, Weitzman N, Schreiber S, Shabtai H, Peretz C (2007) New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson’s disease: the role of dopamine agonist treatment and age at motor symptoms onset. J Psychopharmacol 21:501–506
Giovannoni G, O’Sullivan JD, Turner K, Manson AJ, Lees AJ (2000) Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 68:423–428
Grant JE, Schreiber L, Odlaug BL, Kim SW (2010) Pathologic gambling and bankruptcy. Compr Psychiatry 51:115–120
Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J et al (2006) Problematic gambling on dopamine agonists: not such a rarity. Mov Disord 21:2206–2208
Haluk DM, Floresco SB (2009) Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology 34:2041–2052
Hamidovic A, Kang UJ, de Wit H (2008) Effects of low to moderate doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol 28:45–51
Holman AJ (2009) Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. J Gambl Stud 25:425–431
Hursh SR (1984) Behavioral economics. J Exp Anal Behav 42:435–452
Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE (2005) Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord 11:381–386
Kvernmo T, Härtter S, Bürger E (2006) A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther 28:1065–1078
Lader M (2008) Antiparkinsonian medication and pathological gambling. CNS Drugs 22:407–416
Lagos P, Scorza C, Monti JM, Jantos H, Reyes-Parada M, Silveira R et al (1998) Effects of the D3 preferring agonist pramipexole on sleep and waking, locomotor activity and striatal dopamine release in rats. Eur Neuropsychopharmacol 8:113–120
Madden GJ, Johnson PS, Brewer AT, Pinkston JW, Fowler SC (2010) Effects of pramipexole on impulsive choice in male Wistar rats. Exp Clin Psychopharmacol 18:267–276
McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Bostwick MJ et al (2007) Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord 13:516–519
Millan MJ, Seguin L, Gubert A, Cussac D, Brocco M (2004) The role of dopamine D3 compared to D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology (Berl) 174:341–357
Molina JA, Saínz-Artiga MJ, Fraile A, Jiménez-Jiménez FJ, Villanueva C, Ortí-Pareja M et al (2000) Pathologic gambling in Parkinson’s disease: a behavioral manifestation of pharmacologic treatment? Mov Disord 15:869–872
Munhoz RP, Fabiani G, Becker N, Teive HA (2009) Increased frequency and range of sexual behavior in a patient with Parkinson’s disease after use of pramipexole: a case report. J Sex Med 6:1177–1180
Nirenberg MJ, Waters C (2006) Compulsive eating and weight gain related to dopamine agonist use. Mov Disord 21:524–529
Ouagazzal AM, Creese I (2000) Intra-accumbens infusion of D3 agonists reduces spontaneous and dopamine-induced locomotion. Pharmacol Biochem Behav 67:637–645
Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL et al (2008) Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 196:221–232
Riba J, Krämer UM, Heldmann M, Richter S, Münte TF (2008) Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS ONE 3:e2479
Sokoloff P, Giros B, Martres M, Bouthenet M, Schwartz J (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151
Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V et al (2010) Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 207:645–659
Zarate CA Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD et al (2004) Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 56:54–60
Acknowledgments
The research was supported by a grant from the National Institutes on Health: DA023564, and a report on the work was submitted by the first author in partial fulfillment of a Master of Arts degree from the Department of Applied Behavioral Science at the University of Kansas.
Portions of this research were presented at the 2008 meeting of the Mid-American Association for Behavior Analysis and the 2008 and 2009 meetings of the Association for Behavior Analysis International.
Pramipexole was provided under NIH grant DA 020669 (P.I., James H. Woods, University of Michigan, Ann Arbor). Jonathan Pinkston is now at the University of Texas Health Science Center at San Antonio.
Author information
Authors and Affiliations
Corresponding author
Additional information
Preparation of this manuscript was supported by a grant from the National Institutes on Health: DA023564. Reprints may be obtained from the first author.
Rights and permissions
About this article
Cite this article
Johnson, P.S., Madden, G.J., Brewer, A.T. et al. Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology 213, 11–18 (2011). https://doi.org/10.1007/s00213-010-2006-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2006-5