Abstract
Rationale and objectives
Psychological dependence is one of the worst side effects of morphine. It limits the clinical availability of morphine and non-patient morphine users suffer from addiction. An analgesic, which is more potent than morphine but without the liability of psychological dependence, has long been sought in the clinic. We have recently developed a new μ-opioid receptor agonist, Nα-amidino-Tyr-D-Arg-Phe-β-Ala (amidino-TAPA), as a potent analgesic with an antinociceptive profile that is distinct from morphine, including the release of endogenous κ-opioid peptides. The activation of κ-opioid receptors has been suggested to suppress the development of psychological dependence by μ-opioid receptor agonists. In the present study, the psychological dependence liability and the related locomotor-enhancing effect of amidino-TAPA were evaluated.
Results
Amidino-TAPA injected subcutaneously produced an extremely potent and longer lasting antinociception than morphine in ddY mice, prodynorphin-knockout mice, and wild-type C57BL/6J mice. Unlike subcutaneously injected morphine, which had potent locomotor-enhancing and rewarding effects at antinociceptive doses in ddY mice, amidino-TAPA injected subcutaneously did not induce significant locomotor-enhancing and rewarding effects at antinociceptive or even higher doses in ddY mice. In wild-type C57BL/6J mice, amidino-TAPA showed the same pharmacological profile (potent antinociception, lack of locomotor-enhancing and rewarding effects) as in ddY mice. However, amidino-TAPA produced potent locomotor-enhancing and rewarding effects at antinociceptive doses in prodynorphin-knockout mice.
Conclusions
The present results suggest that amidino-TAPA is a potent analgesic without the liability of psychological dependence because it releases endogenous κ-opioid peptides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
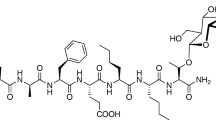
Nα-amidino-Tyr-D-Arg-Phe-β-Ala (amidino-TAPA) is an N-terminal tetrapeptide analog of dermorphin (Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2). Because of its high affinity and selectivity for μ-opioid receptors, amidino-TAPA produces an extreme potent and long-lasting antinociception in mice (Ogawa et al. 2002a, b; Mizoguchi et al. 2007). Its potency for antinociception in mice is approximately 10 times and 400 times higher than that of morphine by the subcutaneous (s.c.) and intrathecal injection routes, respectively (Mizoguchi et al. 2007; Ogawa et al. 2002a, b; Sato et al. 1999). Unlike most peptidic analgesics, which are generally ineffective by oral administration, amidino-TAPA is effective for antinociception by oral administration with a slightly higher potency than morphine (Ogawa et al. 2002a, b). Interestingly, amidino-TAPA has an antinociceptive mechanism in the spinal cord that is distinct from the traditional μ-opioid receptor agonist [D-Ala2-N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO) (Mizoguchi et al. 2007). Unlike DAMGO, the antinociception induced by amidino-TAPA is mediated through the release in the spinal cord of the endogenous κ-opioid peptides dynorphin A, dynorphin B, and α-neo-endorphin and the endogenous δ-opioid peptide [Leu5]enkephalin, which are released by the activation of μ-opioid receptors.
Psychological dependence is one of the major side effects of narcotic analgesics, such as morphine, and it limits distribution and clinical applications of narcotic analgesics. The development of psychological dependence on narcotic analgesics is generally induced by the repeated reinforcement of their rewarding effect via μ-opioid receptors (Herz and Spanagel 1995). It is well known that the rewarding effect of μ-opioid receptor agonists is mediated by the disinhibition of mesolimbic dopaminergic neurons, which projects from the ventral tegmental area to the nucleus accumbens (Spanagel et al. 1992). The activation of μ-opioid receptors located on the γ-aminobutyric acid (GABA)ergic neurons in the ventral tegmental area by μ-opioid receptor agonists causes the reduction of GABA release in this area, which subsequently induces the disinhibition of mesolimbic dopaminergic neurons and the enhancement of dopamine release from these neurons in the nucleus accumbens (Johnson and North 1992a). On the terminal of these mesolimbic dopaminergic neurons in the nucleus accumbens, dynorphinergic nerve fibers that inhibit the release of dopamine are localized (Spanagel et al. 1990, 1992). Therefore, the activation of κ-opioid receptors suppresses the development of psychological dependence to μ-opioid receptor agonists (Funada et al. 1993). Interestingly, the same type of neuronal network regulates the nigrostriatal dopaminergic neurons, which project from the substantia nigra to the striatum (Enrico et al. 1998). By the same mechanism as the mesolimbic dopaminergic neurons, the activation of μ-opioid receptors by μ-opioid receptor agonists in the substantia nigra promotes disinhibition of nigrostriatal dopaminergic neuron and increases the release of dopamine in the striatum, which results in enhancement of locomotor activity (Johnson and North 1992b). Like the rewarding effect in the nucleus accumbens, the locomotor-enhancing effect of μ-opioid receptor agonists is also regulated by the inhibitory neurons of the κ-opioid system in the striatum (Narita et al. 1993).
We have recently developed amidino-TAPA as a new analgesic that has a pharmacological profile that is distinct from the traditional μ-opioid receptor agonists, including the release of endogenous κ-opioid peptides. The evidence strongly suggests that amidino-TAPA has less psychological dependence liability. In the present study, the rewarding and locomotor-enhancing effects of amidino-TAPA injected s.c. were evaluated in mice.
Materials and methods
All experiments were performed following the approval of the Ethics Committee for Animal Experiments at Tohoku Pharmaceutical University and according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number and any suffering of the animals used in the following experiments.
Animals
Male ddY mice (Japan SLC, Hamamatsu, Japan), C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA), and prodynorphin-knockout mice (B6.129S4-Pdyntm1Ute; The Jackson Laboratory) weighing 22–25 g were used. The prodynorphin-knockout mice, which originated on a B6/129 (C57BL/6J × 129S6/SvEvTac) background, have been backcrossed for eight generation onto the C57BL/6J background by the supplier. Therefore, C57BL/6J mice were used as the wild-type mice for the prodynorphin-knockout mice. The deletion of the prodynorphin gene in the prodynorphin-knockout mice was confirmed with the polymerase chain reaction before they were used. The animals were housed in a room maintained at 22–23°C and 50–60% relative humidity with an alternating 12-h light/dark cycle. Food and water were available ad libitum. Mice were used only once.
Antinociception
Antinociception was determined with the thermal tail-flick test (D’Amour and Smith 1941). For measurement of the latency of the tail-flick response, mice were gently held by hand with their tail positioned in an apparatus (Ugo Basile, Italy) for radiant heat stimulation on the ventral surface of the tail. The intensity of the heat stimulus was adjusted so that the animal withdrew its tail after 2.5–3.5 s. The inhibition of the tail-flick response was expressed as the percent maximal possible effect, %MPE, which was calculated as follows: [(T 1 − T 0) / (T 2 − T 0)] × 100, where T 0 and T 1 are the tail-flick latencies before and after the treatments, respectively, and T 2 is the cutoff time, set at 10 s to avoid injury to the tail. The antinociceptive effect was represented as the mean ± SEM for at least 10 mice. The dose–response curves and ED50 values with their 95% confidence intervals were calculated with a computer-associated curve-fitting program (GraphPad Prism; GraphPad Software, Inc., San Diego, CA, USA).
Conditioned place preference
The conditioned place preference (CPP) test was conducted by the method of Bardo et al. (1984) with a minor modification (Suzuki et al. 1999). The apparatus consisted of a shuttle box (15 cm wide × 30 cm long × 15 cm high), which was made of acrylic resin board and divided into two equal-sized compartments. One compartment was white with a textured floor, and the other was black with a smooth floor to create equally preferred compartments. The pre-conditioning test and the post-conditioning test were performed as follows: the partition separating the two compartments was raised to 7 cm above the floor, a neutral platform was inserted along the seam separating the compartments, and mice that had not been treated with either drugs or saline were then placed on the platform. The time spent in each compartment during a 900-s session was recorded automatically using an infrared sensor beam (KN-80, Natsume Seisakusyo Co., Tokyo, Japan). Twelve mice in each group were used for the pre-conditioning test, and eight mice in each group, which had no strong preference (less than 100 s more time spent in one compartment than the other) for either the white or black compartment, were selected for the conditioning session. The mice used in the conditioning session, spent 446.4 ± 15.41 s in the white compartment and 453.6 ± 15.47 s in the black compartment in the pre-conditioning test. Groups of mice were allocated to their respective conditioning compartments based on their pre-conditioning test data, and conditioning sessions (three for drug and three for saline) were conducted once per day for 6 days. Immediately after the s.c. treatment with morphine or saline, or 60 min after the s.c. treatment with amidino-TAPA or saline, these animals were placed for 1 h in the compartment opposite to that in which they had spent more time during the pre-conditioning test. On alternate days, these animals received saline or drugs and then were placed in the other compartment for 1 h. On day 7, the post-conditioning test was performed. The CPP score represents the time spent in the drug-conditioned compartment minus that spent in the saline-conditioned compartment. All sessions were conducted under conditions of dim illumination (200 lx lamp) and white masking noise.
Locomotor activity
The locomotor activity of mice was measured with an activity monitoring system (NS-AS01; Neuroscience Inc., Tokyo, Japan). Briefly, the activity monitor was composed of an infrared ray sensor place over an open-top box (34.5 cm wide × 40.3 cm long × 17.7 cm high), a single amplification circuit, and a control circuit. The sensor can detect the movement of animals on the basis of released infrared rays associated with their body temperature. Counts of locomotor activity were collected in 10-min intervals for 3 h before treatment for habituation and for 3 or 6 h after the treatment with morphine or amidino-TAPA, respectively. The data were analyzed with a computer-associated analyzing system (Multidigital 32-port Counter System; Neuroscience Inc.)
Drugs
The drugs used were amidino-TAPA (Peptide Institute, Osaka, Japan), morphine hydrochloride (Sankyo, Tokyo, Japan), and naloxone hydrochloride (Sigma-Aldrich, St. Louis, MO, USA).
Statistical analyses
The statistical significance of differences between the groups was assessed with a one-way analysis of variance (ANOVA) followed by Dunnett’s test or with a two-way ANOVA followed by Bonferroni’s test.
Results
The antinociceptions induced by morphine and amidino-TAPA
Groups of ddY mice were injected s.c. with saline, morphine (2.5–5.0 mg/kg), or amidino-TAPA (0.17–0.52 mg/kg), and the inhibition of the tail-flick response induced by morphine or amidino-TAPA was measured. The s.c. administration of morphine or amidino-TAPA resulted in a dose-dependent antinociception (Fig. 1a, b). The antinociceptive effect of amidino-TAPA started 30 min after the treatment, reached its maximal effect at 90 min, and then disappeared slowly by 360 min. The antinociceptive effect of amidino-TAPA was more prolonged than that of morphine, which disappeared by 120 min after the treatment. The ED50 values of morphine and amidino-TAPA for antinociception at the peak-effect time were 3.028 and 0.2336 mg/kg, respectively (Fig. 1c). The antinociceptive effect of amidino-TAPA was approximately 13 times more potent than that of morphine in ddY mice.
Antinociceptive effects of morphine and amidino-TAPA in ddY mice. Groups of mice were injected s.c. with saline, morphine (a 2.5–5.0 mg/kg), or amidino-TAPA (b 0.17–0.52 mg/kg), and inhibition of the tail-flick response induced by morphine or amidino-TAPA was measured for 120 or 360 min. The antinociceptive effect was represented as the mean ± SEM for at least 10 mice. The statistical significance of the differences between the groups was assessed with a two-way ANOVA followed by Bonferroni’s test. The dose–response curves and ED50 values with their 95% confidence intervals (c) for morphine and amidino-TAPA at their peak-effect time were calculated with GraphPad Prism, a computer-associated curve-fitting program. a The F value of the two-way ANOVA for morphine was F[3, 252] = 42.37 (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001 vs. the saline-treated group. b The F value of the two-way ANOVA for amidino-TAPA was F[3, 324] = 46.66 (p < 0.001). **p < 0.01, ***p < 0.001 vs. the saline-treated group. c The ED50 values for morphine and amidino-TAPA with their 95% confidence intervals were 3.028 (2.692–3.406) and 0.2336 (0.1692–0.3226) mg/kg, respectively
We previously reported that intrathecally administered amidino-TAPA causes the release of the endogenous κ-opioid peptides dynorphin A, dynorphin B, or α-neo-endorphin in the spinal cord to produce the antinociception (Mizoguchi et al. 2007). To elucidate the involvement of endogenous κ-opioid peptides in the antinociception induced by s.c.-administered amidino-TAPA, prodynorphin-knockout mice, which lack the precursor for the endogenous κ-opioid peptides, and wild-type C57BL/6J mice were used. Groups of C57BL/6J mice and prodynorphin-knockout mice were injected s.c. with saline, morphine (1.3–3.5 mg/kg), or amidino-TAPA (0.14–0.40 mg/kg), and the inhibition of the tail-flick response induced by morphine or amidino-TAPA was measured. In both C57BL/6J mice and prodynorphin-knockout mice, the s.c.-administered morphine or amidino-TAPA resulted in a dose-dependent antinociception with the same antinociceptive profiles observed in ddY mice (Fig. 2a–d). The ED50 value of morphine in C57BL/6J mice and prodynorphin-knockout mice for antinociception at the peak-effect time was 1.992 and 2.116 mg/kg, respectively, whereas the ED50 value of amidino-TAPA in C57BL/6J mice and prodynorphin-knockout mice for antinociception at the peak-effect time was 0.2374 and 0.2727 mg/kg, respectively (Fig. 2e). The antinociceptive effects of both morphine and amidino-TAPA were equipotent in C57BL/6J mice and prodynorphin-knockout mice.
Antinociceptive effects of morphine and amidino-TAPA in C57BL/6J mice and prodynorphin-knockout mice. Groups of C57BL/6J mice (a and c) and prodynorphin-knockout mice (b and d) were injected s.c. with saline, morphine (a and b 1.3–3.5 mg/kg), or amidino-TAPA (c and d 0.14–0.40 mg/kg), and inhibition of the tail-flick response induced by morphine or amidino-TAPA was measured for 180 and 360 min. The antinociceptive effect was represented as the mean ± SEM for at least 10 mice. The statistical significance of the differences between the groups was assessed with a two-way ANOVA followed by Bonferroni’s test. The dose–response curves and ED50 values with their 95% confidence intervals (e) for morphine and amidino-TAPA at their peak-effect time were calculated with GraphPad Prism, a computer-associated curve-fitting program. a The F value of the two-way ANOVA for morphine in C57BL/6J mice was F[4, 360] = 65.54 (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001 vs. the saline-treated group. b The F value of the two-way ANOVA for morphine in prodynorphin-knockout mice was F[4, 360] = 61.43 (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001 vs. the saline-treated group. c The F value of the two-way ANOVA for amidino-TAPA in C57BL/6J mice was F[4, 405] = 96.35 (p < 0.001). **p < 0.01, ***p < 0.001 vs. the saline-treated group. d The F value of the two-way ANOVA for amidino-TAPA in prodynorphin-knockout mice was F[3, 324] = 145.5 (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001 vs. the saline-treated group. e The ED50 values for morphine with its 95% confidence interval in C57BL/6J mice and prodynorphin-knockout mice were 1.992 (1.655–2.399) and 2.116 (1.756–2.548) mg/kg, respectively. The ED50 values for amidino-TAPA with its 95% confidence intervals in C57BL/6J mice and prodynorphin-knockout mice were 0.2374 (0.1926–0.2926) and 0.2727 (0.1112–0.6688) mg/kg, respectively
Rewarding effect of morphine and amidino-TAPA
The rewarding effects of morphine and amidino-TAPA were evaluated with the CPP test in ddY mice. Groups of ddY mice were conditioned for 6 days to the black and white compartments with saline, morphine (1.3–5.0 mg/kg, s.c.), or amidino-TAPA (0.30–0.90 mg/kg, s.c.), and on day 7, the greater (or less) amount of time spent in drug-conditioned compartment than saline-conditioned compartment during a 900-s test session without any injection was recorded as the CPP score. Morphine resulted in a dose-dependent increase in the CPP score and showed a significant CPP score compared to saline at an 80–90% analgesic dose (5 mg/kg), which is indicated by an arrow (Fig. 3a). On the contrary, amidino-TAPA did not show a significant CPP score at an 80–90% analgesic dose (0.52 mg/kg) or even at higher doses (Fig. 3b).
Rewarding effects of morphine and amidino-TAPA on conditioned place preference (CPP) in ddY mice. In conditioning sessions (three for drug and three for saline), groups of mice were conditioned to the black and white compartments for 1 h with saline and various s.c. doses of morphine (a 1.3–5.0 mg/kg) or amidino-TAPA (b 0.30–0.90 mg/kg). During the test session, the time spent in each compartment during a 900-s session was recorded without any injection. The rewarding effect of morphine or amidino-TAPA is represented as the CPP score, which is the time spent in the drug-conditioned compartment minus the time spent in the saline-conditioned compartment. The arrows indicate the doses that show an 80–90% antinociception. Each column represents the mean ± SEM for 7–16 mice. The statistical significance of the differences between the groups was assessed with a one-way ANOVA followed by the Dunnett’s test. a The F value of the one-way ANOVA for morphine was F[3, 35] = 5.807 (p < 0.01). *p < 0.05, **p < 0.01 vs. the saline-conditioned group. b The F value of the one-way ANOVA for amidino-TAPA was F[3, 36] = 0.1839 (p = 0.9066)
To elucidate the involvement of endogenous κ-opioid peptides in the lack of a rewarding effect of s.c.-administered amidino-TAPA, prodynorphin-knockout mice and C57BL/6J mice were used for the CPP test. In both C57BL/6J mice and prodynorphin-knockout mice, morphine resulted in a dose-dependent increase in the CPP score and showed a significant CPP score at an 80–90% analgesic dose (3.5 mg/kg), as observed in ddY mice (Fig. 4a, b). In prodynorphin-knockout mice, the morphine (3.5 mg/kg)-induced significant CPP score was completely eliminated by the concomitant administration of the μ-opioid receptor antagonist naloxone (0.5 mg/kg), which did not show any effect on the CPP score by itself (Fig. 4b). In C57BL/6J mice, amidino-TAPA did not show a significant CPP score at an 80–90% analgesic dose (0.4 mg/kg) or even at higher doses, as observed in ddY mice (Fig. 4c). However, amidino-TAPA caused a dose-dependent increase in the CPP score in prodynorphin-knockout mice and showed a significant CPP score at an 80–90% analgesic dose (0.4 mg/kg) and at higher doses (Fig. 4d). The amidino-TAPA (0.8 mg/kg)-induced significant CPP score in prodynorphin-knockout mice was completely eliminated by the concomitant administration of naloxone (0.5 mg/kg).
Rewarding effects of morphine and amidino-TAPA on conditioned place preference (CPP) in C57BL/6J mice and prodynorphin-knockout mice. During conditioning sessions (three for drug and three for saline), groups of C57BL/6J mice (a and c) and prodynorphin-knockout mice (b and d) were conditioned to the black and white compartments for 1 h with saline and various s.c. doses of morphine (a and b 1.8–3.5 mg/kg) or amidino-TAPA (c and d 0.2–0.8 mg/kg). Naloxone (0.5 mg/kg) was concomitantly administered s.c. with saline, morphine, or amidino-TAPA. On the test session, the time spent in each compartment during a 900-s session was recorded without any injection. The rewarding effect of morphine or amidino-TAPA is represented as the CPP score, which is the time spent in the drug-conditioned compartment minus the time spent in the saline-conditioned compartment. The arrows indicate the doses that show an 80–90% antinociception. Each column represents the mean ± SEM for 7–16 mice. The statistical significance of the differences between the groups was assessed with a one-way ANOVA followed by the Dunnett’s test. a The F value of the one-way ANOVA for morphine in C57BL/6J mice was F[3, 36] = 3.817 (p < 0.05). **p < 0.01 vs. the saline-conditioned group. b The F value of the one-way ANOVA for morphine in prodynorphin-knockout mice was F[5, 63] = 4.702 (p < 0.01). **p < 0.01 vs. the saline-conditioned group. c The F value of the one-way ANOVA for amidino-TAPA in C57BL/6J mice was F[3, 26] = 0.5052 (p = 0.6821). d The F value of the one-way ANOVA for amidino-TAPA in prodynorphin-knockout mice was F[5, 62] = 3.905 (p < 0.01). *p < 0.05 vs. the saline-conditioned group
Locomotor-enhancing effect of morphine and amidino-TAPA
Groups of ddY mice were injected s.c. with saline, morphine (2.5–5.0 mg/kg), or amidino-TAPA (0.30–0.90 mg/kg), and the locomotor activity was measured for 180 or 360 min. Morphine increased the locomotor activity in a dose-dependent manner and showed a significant locomotor enhancement at an 80–90% analgesic dose (5 mg/kg), which is indicated by an arrow (Fig. 5a). On the contrary, amidino-TAPA did not increase locomotor activity at an 80–90% analgesic dose (0.52 mg/kg) or even at higher doses (Fig. 5b).
Locomotor-enhancing effects of morphine and amidino-TAPA in ddY mice. Groups of mice were injected s.c. with saline, morphine (a 2.5–5.0 mg/kg), or amidino-TAPA (b 0.30–0.90 mg/kg), and the locomotor activity was measured for 180 or 360 min. The arrows indicate the doses that show an 80–90% antinociception. Each column represents the mean ± SEM for at least 10 mice. The statistical significance of the differences between the groups was assessed with a one-way ANOVA followed by Dunnett’s test. a The F value of the one-way ANOVA for morphine was F[3, 46] = 6.383 (p < 0.01). **p < 0.01 vs. the saline-treated group. b The F value of the one-way ANOVA for amidino-TAPA was F[3, 35] = 3.597 (p < 0.05)
To elucidate the involvement of endogenous κ-opioid peptides in the lack of a locomotor-enhancing effect of s.c.-administered amidino-TAPA, prodynorphin-knockout mice and C57BL/6J mice were used. In both C57BL/6J mice and prodynorphin-knockout mice, morphine increased the locomotor activity in a dose-dependent manner and showed a significant locomotor enhancement at 5.0 mg/kg, which is a 1.4-fold higher dose than an 80–90% analgesic dose (Fig. 6a, b). In prodynorphin-knockout mice, morphine (5.0 mg/kg)-induced locomotor enhancement was completely eliminated by concomitant administration of naloxone (0.5 mg/kg), which did not show any effect on locomotor activity by itself (Fig. 6b). In C57BL/6J mice, amidino-TAPA did not increase locomotor activity at doses up to 0.80 mg/kg, which is a 2-fold higher dose than an 80–90% analgesic dose (Fig. 6c). However, amidino-TAPA increased locomotor activity in a dose-dependent manner in prodynorphin-knockout mice and showed a significant locomotor enhancement at 0.80 mg/kg (Fig. 6d). Amidino-TAPA (0.80 mg/kg)-induced locomotor enhancement in prodynorphin-knockout mice was completely eliminated by concomitant administration of naloxone (0.5 mg/kg).
Locomotor-enhancing effects of morphine and amidino-TAPA in C57BL/6J mice and prodynorphin-knockout mice. Groups of C57BL/6J mice (a and c) and prodynorphin-knockout mice (b and d) were injected s.c. with saline, morphine (a and b 3.5–5.0 mg/kg), or amidino-TAPA (c and d 0.40–0.80 mg/kg), and the locomotor activity was measured for 180 or 360 min. Naloxone (0.5 mg/kg) was concomitantly administered s.c. with saline, morphine, or amidino-TAPA. The arrows indicate the doses that show an 80–90% antinociception. Each column represents the mean ± SEM for at least 10 mice. The statistical significance of the differences between the groups was assessed with a one-way ANOVA followed by Dunnett’s test. a The F value of the one-way ANOVA for morphine in C57BL/6J mice was F[3, 36] = 9.558 (p < 0.001). **p < 0.01 vs. the saline-treated group. b The F value of the one-way ANOVA for morphine in prodynorphin-knockout mice was F[5, 54] = 28.54 (p < 0.001). **p < 0.01 vs. the saline-treated group. c The F value of the one-way ANOVA for amidino-TAPA in C57BL/6J mice was F[3, 36] = 0.5544 (p = 0.6485). d The F value of the one-way ANOVA for amidino-TAPA in prodynorphin-knockout mice was F[5, 54] = 11.68 (p < 0.001). **p < 0.01 vs. the saline-treated group
Discussion
The μ-opioid receptor agonists that are commonly used in the clinic as narcotic analgesics have both potent analgesic and rewarding effects. Since the rewarding effect as well as the analgesic effect of narcotic analgesics is mediated through μ-opioid receptors (Herz and Spanagel 1995), there are currently no opioid analgesics available in the clinic that have high potency and efficacy for antinociception without a rewarding effect. Amidino-TAPA is a selective μ-opioid receptor agonist that we have recently developed as a new peptidic analgesic (Ogawa et al. 2002a, b; Mizoguchi et al. 2007). In the present study, amidino-TAPA injected s.c. was approximately 13 times more potent than morphine in a behavioral assay for antinociception. Generally, peptidic analgesics are ineffective after peripheral injection because they are rapidly degraded by enzymes and penetrate poorly into the central nervous system through the blood–brain barrier. However, unlike other peptidic analgesics, amidino-TAPA is very resistant to enzymatic degradation and is transported into the central nervous system through the blood–brain barrier via the adsorption-mediated endocytosis system (Deguchi et al. 2004). The distinct pharmacokinetic profile of amidino-TAPA could lead to extremely potent antinociception after peripheral injection. It should be noted from the present study that unlike morphine, which showed remarkable rewarding and locomotor-enhancing effects at analgesic doses, amidino-TAPA injected s.c. did not show any rewarding effect or locomotor-enhancing effect at analgesic or even higher doses. We have previously reported that the endogenous μ-opioid peptide endomorphin-2 injected intracerebroventricularly showed a potent antinociceptive effect without a rewarding effect (Tseng et al. 2000; Narita et al. 2001). However, endomorphin-2 is ineffective via peripheral injection routes. Therefore, the present study suggests that amidino-TAPA is the first μ-opioid receptor agonist, which lacks the rewarding effect, but is effective after peripheral injection.
As we reported previously, amidino-TAPA has a pharmacological profile that is distinct from the traditional μ-opioid receptor agonists, including the release of endogenous κ-opioid peptides via activation of μ-opioid receptors (Mizoguchi et al. 2007). The activation of κ-opioid receptors is well known to suppress the development of psychological dependence on and the locomotor-enhancing effect of μ-opioid receptor agonists (Funada et al. 1993; Narita et al. 1993). In fact, endomorphin-2 given intracerebroventricularly, which causes the release of dynorphin A via activation of μ-opioid receptors at a supraspinal site (Tseng et al. 2000; Sakurada et al. 2008), does not show a remarkable rewarding effect, but produces a prominent aversive effect rather than a rewarding effect (Narita et al. 2001; Wu et al. 2004). The activation of κ-opioid receptors by dynorphins or other κ-opioid receptor agonists in the nucleus accumbens leads to the decreased release of dopamine from the mesolimbic dopaminergic neuron and shows an aversive effect (Spanagel et al. 1992). Indeed, the endomorphin-2-induced aversive effect was completely suppressed by a κ-opioid receptor antagonist or an antiserum against dynorphin A (Narita et al. 2001; Wu et al. 2004). Moreover, the aversive effect of endomorphin-2 microinjected into the nucleus accumbens shell was completely suppressed by co-microinjection of the μ-opioid receptor antagonist CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) or antiserum against dynorphin A (Terashvili et al. 2004). The evidence clearly suggests that increased release of dynorphin A via activation of μ-opioid receptors in the nucleus accumbens is involved in the aversive effect (or lack of the remarkable rewarding effect) of endomorphin-2. The lack of the rewarding effect of amidino-TAPA observed in the present study may be mediated by the same mechanism as for endomorphin-2. Therefore, in the present study, to confirm the involvement of endogenous κ-opioid peptides in the lack of a rewarding effect and a locomotor-enhancing effect of amidino-TAPA, prodynorphin-knockout mice, which lack the prodynorphin gene (Sharifi et al. 2001), were used. In the prodynorphin-knockout mice, amidino-TAPA produced remarkable rewarding and locomotor-enhancing effects at doses of amidino-TAPA that did not show any rewarding effect or locomotor-enhancing effect in wild-type C57BL/6J mice. The rewarding and locomotor-enhancing effects of amidino-TAPA observed in prodynorphin-knockout mice were completely abolished by the μ-opioid receptor antagonist naloxone. The evidence clearly suggests that although the selective μ-opioid receptor agonist amidino-TAPA has rewarding and locomotor-enhancing effects by stimulating μ-opioid receptors, the distinct pharmacological effect of amidino-TAPA, i.e., the release of endogenous κ-opioid peptides, may block its rewarding effect and locomotor-enhancing effect by stimulating κ-opioid receptors. Endomorphin-2 causes the release of only dynorphin A as an endogenous κ-opioid peptide (Tseng et al. 2000; Sakurada et al. 2001, 2008), whereas amidino-TAPA can cause the release of not only dynorphin A but also dynorphin B and α-neo-endorphin (Mizoguchi et al. 2007). At present, it is not clear which endogenous κ-opioid peptides are involved in the lack of rewarding and locomotor-enhancing effects of amidino-TAPA. Extensive research will be required to make clear the exact mechanisms for the lack of rewarding and locomotor-enhancing effects by amidino-TAPA.
Our previous finding that the antinociceptive effect of intrathecally administered amidino-TAPA was significantly suppressed in prodynorphin-knockout mice compared to wild-type mice suggests that the spinal antinociceptive effect of amidino-TAPA is mediated partially through the spinal release of dynorphins (Mizoguchi et al. 2007). However, in the present study, the antinociceptive effect of amidino-TAPA injected s.c. was unexpectedly not reduced in prodynorphin-knockout mice. The present evidence suggests that the release of dynorphins is not the main component for the production of antinociception for s.c.-administered amidino-TAPA. At present, the reason for this apparent discrepancy is not clear. Unlike the spinal cord, the regions involved in the antinociception induced by s.c.-administered amidino-TAPA may not contain the special μ-opioid receptors involved in the release of dynorphins.
In contrast to endomorphin-2, which produces a prominent aversive effect rather than a rewarding effect (Narita et al. 2001; Wu et al. 2004), amidino-TAPA did not show either a rewarding effect or an aversive effect. Amidino-TAPA causes the release of dynorphin A, dynorphin B, α-neo-endorphin, and [Leu5]enkephalin via activation of μ-opioid receptors (Mizoguchi et al. 2007), whereas endomorphin-2 causes the release of dynorphin A and [Met5]enkephalin but not dynorphin B, α-neo-endorphin, or [Leu5]enkephalin (Tseng et al. 2000; Sakurada et al. 2001). The evidence clearly suggests that amidino-TAPA stimulates more or different types of μ-opioid receptors (μ-opioid receptor subclasses) than endomorphin-2. In fact, amidino-TAPA-induced antinociception is suppressed by the selective μ2-opioid receptor antagonist D-Pro2-Tyr-W-MIF-1, which is ineffective against endomorphin-2-induced antinociception (Watanabe et al. 2005; unpublished observation). By activating more or different types of μ-opioid receptors, amidino-TAPA may show a neutral effect on motivation, i.e., may not show either a rewarding effect or an aversive effect. The aversive effect of a medicine seriously affects a patient’s compliance to a regular schedule of medication. From this point of view, amidino-TAPA may be better than endomorphin-2 as a potent analgesic that lacks a rewarding effect.
In conclusion, unlike the traditional narcotic analgesic morphine, the selective μ-opioid receptor agonist amidino-TAPA did not show a rewarding effect or a locomotor-enhancing effect after peripheral injection. The release of endogenous κ-opioid peptides via activation of μ-opioid receptors, identified as a pharmacological profile of amidino-TAPA distinct from morphine, may be responsible for the lack of rewarding and locomotor-enhancing effects of amidino-TAPA.
References
Bardo MT, Miller JS, Neisewander JL (1984) Conditioned place preference with morphine: the effect of extinction training on the reinforcing CR. Pharmacol Biochem Behav 21:545–549
D’Amour FE, Smith DL (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
Deguchi Y, Naito Y, Ohtsuki S, Miyakawa Y, Morimoto K, Hosoya K, Sakurada S, Terasaki T (2004) Blood–brain barrier permeability of novel [D-Arg2]dermorphin (1-4) analogs: transport property is related to the slow onset of antinociceptive activity in the central nervous system. J Pharmacol Exp Ther 310:177–184
Enrico P, Mura MA, Esposito G, Serra P, Migheli R, De Natale G, Desole MS, Miele M, Miele E (1998) Effect of naloxone on morphine-induced changes in striatal dopamine metabolism and glutamate, ascorbic acid and uric acid release in freely moving rats. Brain Res 797:94–102
Funada M, Suzuki T, Narita M, Misawa M, Nagase H (1993) Blockade of morphine reward through the activation of κ-opioid receptors in mice. Neuropharmacology 32:1315–1323
Herz A, Spanagel R (1995) Endogenous opioids and addiction. In: Tseng LF (ed) The pharmacology of opioid peptides. Harwood Academic, Singapore, pp 445–462
Johnson SW, North RA (1992a) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Johnson SW, North RA (1992b) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol (London) 450:455–468
Mizoguchi H, Watanabe C, Watanabe H, Moriyama K, Sato B, Ohwada K, Yonezawa A, Sakurada T, Sakurada S (2007) Involvement of endogenous opioid peptides in the antinociception induced by the novel dermorphin tetrapeptide analog amidino-TAPA. Eur J Pharmacol 560:150–159
Narita M, Takahashi Y, Takamori K, Funada M, Suzuki T, Misawa M, Nagase H (1993) Effects of κ-agonist on the antinociception and locomotor enhancing action induced by morphine in mice. Jpn J Pharmacol 62:15–24
Narita M, Ozaki S, Ioka M, Mizoguchi H, Nagase H, Tseng LF, Suzuki T (2001) Different motivational effects induced by the endogenous μ-opioid receptor ligands endomorphin-1 and -2 in the mouse. Neuroscience 105:213–218
Ogawa T, Miyamae T, Murayama K, Okuyama K, Okayama T, Hagiwara M, Sakurada S, Morikawa T (2002a) Synthesis and structure–activity relationships of an orally available and long-acting analgesic peptide, Nα-amidino-Tyr-D-Arg-Phe-MeβAla-OH (ADAMB). J Med Chem 45:5081–5089
Ogawa T, Miyamae T, Okayama T, Hagiwara M, Sakurada S, Morikawa T (2002b) Structure–activity relationships (SAR) of [D-Arg2]dermorphin(1-4) analogues, Nα-amidino-Tyr-D-Arg-Phe-X. Chem Pharm Bull 50:771–780
Sakurada S, Hayashi T, Yuhki M, Orito T, Zadina JE, Kastin AJ, Fujimura T, Murayama K, Sakurada C, Sakurada T, Narita M, Suzuki T, Tan-no K, Tseng LF (2001) Differential antinociceptive effects induced by intrathecally administered endomorphin-1 and endomorphin-2 in the mouse. Eur J Pharmacol 427:203–210
Sakurada S, Sawai T, Mizoguchi H, Watanabe H, Watanabe C, Yonezawa A, Morimoto M, Sato T, Komatsu T, Sakurada T (2008) Possible involvement of dynorphin A release via μ1-opioid receptor on supraspinal antinociception of endomorphin-2. Peptides 29:1554–1560
Sato T, Sakurada S, Takahashi N, Sakurada T, Tan-No K, Wako K, Kisara K (1999) Contribution of spinal μ1-opioid receptors to morphine-induced antinociception. Eur J Pharmacol 369:183–187
Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U (2001) Generation of dynorphin knockout mice. Mol Brain Res 86:70–75
Spanagel R, Herz A, Shippenberg TS (1990) The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 55:1734–1740
Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050
Suzuki T, Kishimoto Y, Misawa M, Nagase H, Takeda F (1999) Role of the κ-opioid system in the attenuation of the morphine-induced place preference under chronic pain. Life Sci 64:PL1-7
Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF (2004) Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther 309:816–824
Tseng LF, Narita M, Suganuma C, Mizoguchi H, Ohsawa M, Nagase H, Kampine JP (2000) Differential antinociceptive effects of endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther 292:576–583
Watanabe H, Nakayama D, Ito K, Watanabe C, Mizoguchi H, Fujimura T, Murayama K, Kawamura S, Sato T, Sakurada C, Sakurada T, Sakurada S (2005) A Tyr-W-MIF-1 analog containing D-Pro2 acts as a selective μ2-opioid receptor antagonist in the mouse. J Pharmacol Exp Ther 312:1075–1081
Wu HE, MacDougall RS, Clithero AD, Leitermann RJ, Terashvili M, Tseng LF (2004) Opposite conditioned place preference responses to endomorphin-1 and endomorphin-2 in the mouse. Neurosci Lett 365:157–161
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) [KAKENHI 21600013 and 22600009] from the Japan Society for the Promotion of Science and a Matching Fund Subsidy for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizoguchi, H., Watanabe, C., Osada, S. et al. Lack of a rewarding effect and a locomotor-enhancing effect of the selective μ-opioid receptor agonist amidino-TAPA. Psychopharmacology 212, 215–225 (2010). https://doi.org/10.1007/s00213-010-1946-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1946-0