Abstract
Rationale
Negative mood states are characterized by both stress hormone dysregulation and serotonergic dysfunction, reflected by altered thalamic serotonin transporter (5-HTT) levels. However, so far, no study examined the individual association between cortisol response and cerebral in vivo 5-HTT levels in patients suffering from negative mood states.
Objective
The objective of this cross-sectional study was to assess the interrelation of cortisol response, thalamic 5-HTT levels, and anxiety in healthy subjects and two previously published samples of patients with unipolar major depression (UMD) and obsessive–compulsive disorder (OCD), controlling for age, gender, 5-HTT genotype, smoking, and seasonality.
Methods
Regional 5-HTT levels and cortisol response to dexamethasone-corticotropin (Dex-CRH) challenge were assessed in consecutive samples of medication-free patients suffering from UMD (N = 10) and OCD (N = 10), and 20 healthy volunteers. The intervention used was combined Dex-CRH test and [11C]DASB positron emission tomography. The main outcome measures were: 5-HTT binding potential (BPND) in a predefined thalamic ROI, cortisol response defined as the maximum cortisol increase in the combined Dex-CRH-test, and state of anxiety from the state-trait-anxiety inventory.
Results
Reduced thalamic 5-HTT BPND was associated with increased cortisol response (r = −0.35, p < 0.05; in patients: r = −0.53, p < 0.01) and with increased state anxiety (r = −0.46, p < 0.01), surviving correction for age, gender, 5-HTT genotype, smoking, and seasonality (p < 0.05). The 5-HTT genotype, on the contrary, was not significantly associated with cortisol response (p = 0.19) or negative mood (p = 0.23).
Conclusion
The association between stress hormone response, thalamic 5-HTT levels, and anxiety in patients suffering from negative mood states suggests an interaction between two major mechanisms implicated in negative mood states in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic life stress has been associated with altered cortisol response (Carpenter et al. 2007; Heim et al. 2000) and an increased risk to develop depression (Risch et al. 2009). Human and animal studies of chronic stress suggest that dysfunction of the hypothalmic-pituitary-adrenal (HPA) axis is a hallmark of negative mood states such as depression (Delgado et al. 1994; Luby et al. 2003; Posener et al. 2000; Ströhle and Holsboer 2003; Vreeburg et al. 2009) and obsessive–compulsive disorder (OCD)(Altemus et al. 1992; Kluge et al. 2007). At the same time, studies using positron emission tomography (PET) and single photon emission computed tomography have shown altered serotonin transporter (5-HTT) levels in various subcortical and cortical brain regions in patients with negative mood states. In patients with depression, particularly with comorbid anxiety (Malison et al. 1998; et al. 2000; Parsey et al. 2006; Oquendo et al. 2007; Reimold et al. 2008), and in OCD (Hesse et al. 2005; Reimold et al. 2007; Zitterl et al. 2007; but see Simpson et al. 2003; Pogarell et al. 2003; Matsumoto et al. 2010), decreased 5-HTT levels were observed in 5-HTT rich subcortical brain regions, which in turn correlated with behavioral measures of anxiety (Reimold et al. 2008) and OCD severity, respectively (Hesse et al. 2005; Reimold et al. 2007; Zitterl et al. 2007).
While animal studies suggest that serotonergic neurotransmission may be affected by the stress hormone axis (Lanfumey et al. 2008), so far, only a very limited number of studies directly looked at the interaction between 5-HTT and cortisol responses. One animal experiment showed a decrease in 5-HTT levels following cortisol application (Slotkin et al. 1997) and one human study was restricted to healthy control subjects and alcohol-dependent patients (Heinz et al. 2002), and assessed baseline cortisol levels rather than cortisol response as measured with the dexamethasone-corticotropin-releasing hormone (Dex-CRH) test, which has been suggested as a more precise assessment of HPA axis function (Heim et al. 2000; Ströhle and Holsboer 2003).
Recent evidence indicates that 5-HTT genotype (Barr et al. 2004) as well as other candidate genes of monoaminergic systems (Jabbi et al. 2007) may affect HPA axis function. In addition, there have been reports of interactions between 5-HTT levels and genotype, HPA axis dysregulation, and negative mood states (Caspi et al. 2003; Gotlib et al. 2008; Lanfumey et al. 2008). However, an interaction effect of stressful life events and 5-HTT genotype on the risk to develop major depression could not be replicated in a recent meta-analysis (Risch et al. 2009). Therefore, in this study, we explored whether individual differences in stress hormone response interact with 5-HTT availability and anxiety independent of 5-HTT genotype and other confounding variables such as age, gender (Staley et al. 2006), smoking status (Staley et al. 2001), and seasonal effects (Kalbitzer et al. 2009; Praschak-Rieder et al. 2008). Specifically, we tested whether cortisol response as measured with the Dex-CRH test is correlated with 5-HTT availability, as measured with [¹¹C]N,N-Dimethyl-2(-2-amino-4-cyanophenylthio)-benzylamine ([11C]DASB) PET in our sample of 20 unmedicated patients suffering from negative mood states (unipolar major depression (UMD), Reimold et al. 2008; OCD, Reimold et al. 2007) and 20 controls. Furthermore, we tested the effect of 5-HTT availability and cortisol response on anxiety, controlling for age, gender, smoking status, seasonality, and 5-HTT genotype.
Materials and methods
Patients and control subjects
Twenty patients with negative mood states (mean age 46.0 years, SD = 9.1 years; ten female, ten male; ten suffering from UMD and ten suffering from OCD) were included in this study. UMD or OCD was diagnosed according to International Classification of Disease-10 and Diagnostic and Statistical Manual for Mental Disorder (DSM)-IV criteria. Patients had no other psychiatric axis I disorder, no past history of drug dependence or current drug abuse (random urine drug testing and Structured Clinical Interview for DSM-IV (SCID)-interview; First et al. 1998; First et al. 2001) except for smoking. All patients were recruited before starting antidepressant medication (N = 11) or during a washout period (N = 8) before switching antidepressants; participation in the study was not the reason for withdrawal from antidepressant treatment. Patients had received either monotherapies or combinations of mirtazapine (N = 2), selective serotonin reuptake inhibitors (citalopram N = 5, sertraline N = 1, fluvoxamine N = 1), tricyclic antidepressants (trimipramine N = 1, doxepine N = 1) or reboxetine (N = 1). Withdrawal from antidepressants started at least five plasma half-lives (range 5–17 days, mean 9 days, SD = 4 days) before PET scans. Two patients were on neuroleptics (olanzapine, sulpiride, or promethazine), but had stopped intake more than 8 days before PET scanning. The severity of state anxiety in patients with negative mood states was measured with Spielberger's state-trait-anxiety inventory (STAI; Laux et al. 2001). 5-HTT levels in the thalamus were previously found to be reduced in both patient groups (UMD) (Reimold et al. 2007, 2008).
Twenty age-matched healthy subjects (mean age 44.2 years, SD = 9.8 years; ten female, ten male) served as control subjects. They had no psychiatric axis I or II disorder (SCID I & II-interview; First et al. 1998, 2001). The study was approved by the Ethics Committee of the Universities of Heidelberg and Tübingen and was in accordance with the Helsinki Declaration. After a complete description of the study to the subjects, written informed consent was obtained.
Stress hormone response
Dex-CRH tests were performed within 3–5 days after PET scanning as previously described (Ströhle et al. 1998). Patients were pretreated with 1.5 mg of oral dexamethasone at 2300 hours; the following day, 100 μg of human CRH was administered intravenously at 1500 hours. Blood was drawn directly before CRH administration and after 30, 45, 60, and 75 min through intravenous catheters. Plasma cortisol concentration was assessed with a commercially available radioimmunoassay (RIA) (MP Biomedicals LLC, Solon, Ohio, USA). For each patient, baseline cortisol levels were subtracted from maximum levels after CRH application.
PET methods
[¹¹C]N,N-Dimethyl-2(-2-amino-4-cyanophenylthio)-benzylamine ([11C]DASB) (Houle et al. 2000) was synthesized as previously described (Solbach et al. 2004). After intravenous bolus injection of 20 mCi [11C]DASB, the distribution of cerebral radioactivity was measured with a GE Advance PET scanner (GE Medical Systems, Milwaukee, Wisconsin, axial field of view 15 cm) in two-dimension acquisition mode. For attenuation correction, a transmission scan with 500,000 kilo counts was used. Three external markers were attached to the skull to support realignment. We used filtered back projection (128 × 128 pixel = 30 cm) with a Hanning filter (cut-off 4.6 mm) to reconstruct our images. Realignment, stereotactic normalization, and Gaussian smoothing (10-mm full width half maximum) were done with MATLAB and the software SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). For stereotactic normalization, we compared early summation images (0–5 min p.i.) with the standard PET perfusion template provided with statistical parametrical mapping (SPM2, Wellcome Department, London, UK). After spatial normalization and Gaussian smoothing (10 mm FWHM), DASB-binding potential BPND, a measure proportional to the density of 5-HTT (Ichise et al. 2003; Innis et al. 2007; Laruelle et al. 2002), was calculated for each voxel and for three-dimensional ROI using the “multilinear reference tissue model 2” (MRTM2) with the cerebellum (excluding the vermis) as a reference region. The washout from the cerebellum, which is required for this analysis, was estimated as previously described (Reimold et al. 2007). For ROI analysis, we used the same thalamus ROI (2 × 0.98 ml) as in our previous publications (Reimold et al. 2007, 2008). For voxelwise analysis, we used SPM2 including the publicly available toolbox MASCOI (Reimold et al. 2006).
Genotyping
We assessed the genotype of the 5-HTT regulatory region (5-HTTLPR) (Lesch et al. 1996), and an A < G substitution in the 5-HTT gene (Hu et al. 2006) with polymerase chain reaction (PCR) using oligonucleotide primers (stpr5, 5′-GGCGTTGCCGCTCTGAATGC; int1, 5′-CAGGGGAGATCCTGGGAGGA), as described previously (Heinz et al. 2000; Heinz et al. 2005). PCR amplification was carried out in a final volume of 30 µl consisting of 50 ng of genomic DNA, 2.5 mM of deoxyribonucleotides (dGTP/7-deaza-2′-dGTP = 1/1), 0.1 µg of sense and antisense primers, 10 mM of tris-HCl (pH 8.3), 50 mM of KCl, 1.5 mM of MgCl2, and 1 U of Taq DNA polymerase. Annealing was carried out at 61°C for 30 s, extension at 72°C for 1 min, and denaturation at 95°C for 30 s for 35 cycles. For 5′ nuclease genotyping of SCL6A4 A and G alleles, dye-labeled probes and oligonucleotide primers (F: GCAACCTCCCAGCAACTCCC TGTA; R: GAGGTGCAGGGGGATGCTGGAA) were designed to optimize allele discrimination using Primer Express Software (ABI, Foster City, CA 94404, USA). The fluorogenic probes were labeled at the 5′ end with either FAM or VIC (A: 6FAM-CCCCCCTGCACCCCCAGCATCCC MGB; G: VIC-CCCCTGCACCCCCGGCATCCCC MGB).
Statistical analysis
Group differences in 5-HTT BPND (healthy controls versus patients with negative mood states (UMD and OCD)) were assessed with a general linear model (multiple regression) controlling for age, gender, smoking status, seasonality, and 5-HTT genotype. To correct for seasonality in a linear model, we calculated a daylight index from the date of the PET scan by means of a sinusoidal function with its maximum in June and July, and a minimum in January and December (Kalbitzer et al. 2009; Praschak-Rieder et al. 2008). To correct for genotype, we used the number of LA alleles (zero, one, or two) without implicit ordering (i.e., modeling type nominal).
Correlations between behavioral measures of anxiety, cortisol response, 5-HTT BPND, and 5-HTT genotype were calculated with a simple regression analysis, as well as with a general linear model controlling for the above named factors and diagnosis (control versus UMD versus OCD), testing the hypothesis that decreased 5-HTT BPND are associated with increased anxiety and increased cortisol response. In one patient (OCD; 41 years, female) and one control subject (45 years, male), anxiety scores and 5-HTTLPR genotype were not available; in these two subjects and in two other control subjects (44 years, female; 40 years, male), the Dex-CRH test could not be performed. Therefore, these four subjects were excluded from the respective correlation analyses.
For statistical hypothesis testing, we chose an ROI-based approach (thalamus ROI as described above) for two reasons: first, 5-HTT BPND is known to be correlated across various brain regions, a condition that is difficult to account for with respect to multiple comparisons, particularly in the framework of voxelwise statistics. Second, aim of this paper was to further elucidate previously published results from an (a priori) thalamus ROI by investigating the impact of HPA reactivity on the observed correlations. For explorative ROI analysis, we included an ROI of the midbrain/raphe region, the amygdala (Reimold et al. 2008), and the putamen.
To exploratively look for further brain regions involved and to locate more precisely the significant effects, we calculated a voxelwise statistical analysis with SPM2 and the publicly available toolbox MASCOI (Reimold et al. 2006). SPM's general linear model was set up so that it corresponded to that in the ROI analyses described above. For all SPM analyses, we used a voxel-level threshold of p = 0.01 (search volume: mean BPND > 0.1) and used SPM's built-in correction for multiple testing (family-wise error, FWE, p < 0.05). After completing the SPM analysis, we calculated “masked contrast images” as previously described (Reimold et al. 2006), using the SPM toolbox MASCOI. This had the effect of improving spatial precision and of allowing a quantitative color scale rather than a statistical one. We also explored interaction effects of 5-HTT genotype and cortisol response on 5-HTT BPND. All abovementioned analyses were carried out using JMP 7.0.2, SAS Institute, Inc.
Results
Group differences in clinical variables and 5-HTT BPND
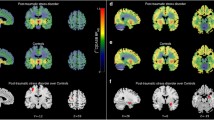
Table 1 gives an overview of the demographic and clinical variables of the study participants. There were no statistically significant differences in age, gender, and smoking status between patients and controls (all p > 0.19). Severity of state anxiety (STAI) was significantly increased in patients suffering from UMD or OCD compared with healthy control subjects (two sample t test: t = 5.31, df = 36, p < 0.0001). In patients (UMD or OCD), thalamic 5-HTT BPND (1.10 ± 0.23) was reduced by about 20% (controls: 1.36 ± 0.18; two sample t test: t = 4.05, df = 38, p < 0.0001; controlling for age, gender, smoking status, seasonality, and genotype: t = 3.45, df = 30, p < 0.001; Fig. 1). Thalamic 5-HTT BPND was not associated with time since last selective serotonin uptake inhibitor intake measured in plasma half-lives (p = 0.47). In the voxelwise analysis, using a voxel-level threshold of p = 0.01, we found a single cluster (8.1 ml) that was predominantly located in the bilateral thalamus (where we found the maximum t value Tmax = 4.0 at MNI 6/−22/6), and included adjacent parts of the midbrain (where we found the maximum contrast at MNI −2/−24/−2). This cluster survived SPM's correction for multiple comparisons (p < 0.05 FWE corrected). No voxels outside this cluster met the threshold of p < 0.01.
Group differences in 5-HTT levels. Top: ROI analysis shows reduced serotonin transporter levels binding potential (BPND) in the thalamus of 20 subjects with negative mood states versus 20 matched healthy controls, measured with PET and the radioligand [11C]DASB. Bottom: Voxelbased analysis (SPM2&MASCOI) shows a large cluster extending from the midbrain to the bilateral thalamus, surviving correction for multiple testing (p < 0.05 FWE corrected). UMD indicates patients suffering from unipolar major depression, OCD indicates obsessive–compulsive disorder, and control indicates healthy controls
5-HTT BPND and cortisol response
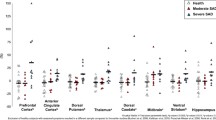
Statistical analysis of the whole study population (Table 2, Fig. 2) showed that reduced 5-HTT BPND in the thalamus was associated with an increased cortisol response (simple regression: r = −0.35, t = 2.18, p = 0.018, df = 34), surviving correction for diagnosis, age, gender, smoking status, seasonality, and genotype (partial correlation r = −0.38, t = 2.11, p = 0.022, df = 26, together explaining 67% of BPND variance). Of the covariates, only diagnosis (Least-squares means: control, 1.328; UMD, 1.093; OCD, 1.145; p = 0.005), age (p < 0.001) and gender (female < male; p = 0.030) reached statistical significance.
Association between 5-HTT levels and cortisol response. In patients with UMD and OCD as well as in healthy controls, lower 5-HTT levels (BPND) in the thalamus (a priori ROI analysis) were associated with a higher cortisol response (Dex-CRH test). The slope of the depicted regression line (p = 0.002) originates from a linear regression analysis of all three groups, including an additional diagnosis effect. The association between 5-HTT BPND and cortisol response survived correction for age, gender, smoking status, seasonality, and 5-HTT genotype in a multiple regression analysis (p = 0.02)
Analyzing patients and controls separately, this association was specifically present in patients (simple regression: r = −0.54, t = 2.62, p = 0.009, df = 17; correcting for all abovementioned covariates: partial correlation r = −0.57, t = 2.19, p = 0.026, df = 10, together explaining 62% of BPND variance) as compared to controls (simple regression: r = −.33, t = 1.36, p = 0.10, df = 15; correcting for the abovementioned covariates: partial correlation r = 0.04, t = 0.13, p = 0.451, df = 9, together explaining 68% of BPND variance). Interaction analysis, however, revealed no significant differences between patients and controls (comparison of slopes, correcting for all abovementioned covariates: t = 1.17, p = 0.25).
No significant 5-HTT genotype effect was observed in any of the abovementioned multiple regression analyses (p > 0.58).
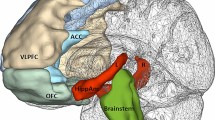
Voxelwise explorative analysis of the correlation between 5-HTT BPND and cortisol response (controlling for diagnosis, age, gender, and smoking status, df = 29) confirmed ROI-based findings and revealed a large cluster ranging over thalamus, parahippocampal gyrus and amygdala, striatum, anterior cingulate, and parts of the frontal cortex (Fig. 3). Applying a subcortical a priori mask, the abovementioned cluster survived correction for multiple testing (p < 0.05 FWE corrected). The highest t value (Tmax = 4.03) was observed in the right striatum, the highest slope of the regression line (CONmax = 1.55 ml/µg) in the midbrain at MNI 4/−28/−6.
Association between 5-HTT levels and cortisol response (voxelwise analysis). Voxelwise multiple regression analysis (SPM2&MASCOI) revealed a large contiguous cluster with a negative correlation between 5-HTT BPND and cortisol response (corrected for diagnosis, age, gender, and smoking status). Applying a subcortical a priori mask (all cortical findings are considered explorative), this cluster survived correction for multiple testing (p < 0.05; FWE corrected)
5-HTT BPND and anxiety
Among all subjects, increased state anxiety was associated with decreased 5-HTT BPND in the thalamus (simple regression: r = −0.46, t = 3.08, p = 0.002, df = 36; controlling for diagnosis, age, gender, smoking status, seasonality, and genotype: partial correlation: r = −0.45, t = 2.67, p = 0.006, df = 26, together explaining 70% of BPND variance). After excluding the control subjects from the analysis, this correlation remained significant in the multiple regression analysis (partial correlation: r = −0.56, t = 2.15, p = 0.03; whole model R 2 = 0.61, df = 10).
Again, 5-HTT genotype showed no significant effect in the abovementioned multiple regression analysis, neither in the whole study population (p = 0.43) nor when patients (p = 0.48) or controls (p = 0.66) were assessed separately.
5-HTT BPND, cortisol response, and anxiety
Expressing thalamic 5-HTT BPND as a function of both cortisol response and anxiety multiple regression analysis revealed independent effects of cortisol response (partial correlation r = −0.43, t = 2.70, p < 0.01) and anxiety, respectively (partial correlation r = −0.53, t = 3.62, p < 0.001), together explaining 37% of thalamic 5-HTT BPND variance (df = 33). Both effects remained independently significant after correcting for diagnosis, age, gender, smoking, seasonality, and genotype (cortisol response: r = −0.35, t = 1.75, p = 0.046; anxiety r = −0.57, t = 4.46, p < 0.0001; whole model R 2 = 0.71, df = 25). Interestingly, the correlation between cortisol response and anxiety was much weaker and not significant, neither in the whole study population (simple regression, r = −0.05; partial correlation, correcting for 5-HTT BPND effects, r = −0.263), nor in patients (simple regression, r = 0.00; partial correlation, r = −0.25) or controls (simple regression, r = 0.01;.partial correlation, r = 0.23), separately.
Genotype effects
SS, SL, and LALA subjects did not differ significantly with respect to 5-HTT BPND in the thalamus (ANOVA: F = 0.063, p = 0.94, mean ± standard error: SS, 1.25 ± 0.10; SL, 1.22 ± 0.05; LL, 1.21 ± 0.09). Also, there was no significant effect of the number of LA alleles (0, 1, or two; modeling type, nominal) on thalamic 5-HTT BP ND in a multiple regression analysis controlling for diagnosis, age, gender, smoking status, and seasonality (p = 0.70; least-squares means: 1.25, SS; 1.18, SL; 1.19, LL).
Cortisol response was also not affected by 5-HTT genotype (ANOVA, p = 0.50; multiple regression, p = 0.44), neither was anxiety (ANOVA, p = 0.71; multiple regression p = 0.72).
However, subsequent interaction analysis revealed a significant genotype-by-cortisol response interaction (t = 2.49, p < 0.05): while LALA homozygotes and SLA/LGLA heterozygotes displayed a negative correlation between 5-HTT BPND and cortisol response, no such correlation was found in subjects carrying two reduction-of-function alleles (SS or SLG), owing to the fact that all patients with this genotype displayed a low cortisol response and low thalamic 5-HTT BPND. However, this interaction effect accounted for less than 3% of the variance in 5-HTT BPND in the whole study population.
Discussion
It has long been suggested that serotonin dysfunction, potentially arising from 5-HTT-mediated stress sensitivity, contributes to major depression (Caspi et al. 2003); however, a recent meta-analysis did not confirm the presence of 5-HTT genotype effects on the risk to develop depression, but did confirm an effect of cumulative life stress on subsequent depression (Risch et al. 2009). Our finding is in accordance with the important role ascribed to stress hormone dysregulation in affective disorders: reduced 5-HTT in the brainstem or thalamus have repeatedly been associated with affective disorders such as UMD, anxiety, and OCD (Malison et al. 1998; Hesse et al. 2005; Parsey et al. 2006; Zitterl et al. 2007; Oquendo et al. 2007; Reimold et al. 2007, 2008; Willeit et al. 2000). Also, stress hormone dysregulation is a mainstay of neurobiological research in major depression (Carpenter et al. 2007; Heim et al. 2000; Ising et al. 2005; Ströhle and Holsboer 2003). Our data suggest that (1) there is indeed an association between 5-HTT levels (but not 5-HTT genotype per se) and negative mood states, even if covariates such as age, gender, seasonality, and smoking are taken into account, and (2) there is an association between stress hormone axis reactivity and 5-HTT levels, suggesting that HPA axis dysregulation accounts for a sizeable proportion of the effects of altered serotonergic neurotransmission on anxiety in patients suffering from negative mood states. Furthermore, the effect of cortisol response on 5-HTT levels interacted with 5-HTT genotype, but this interaction explained only a very small amount of the variance in the whole study population. Specifically, HPA axis dysregulation, as measured with the Dex-CRH test, accounted for about 15% of the variance in central 5-HTT BPND, indicating a large effect according to Cohen (1992), while the genotype effect explained less than 3% of the variance, indicating a small effect. This latter finding underlines that straightforward associations between 5-HTT genotype and stress exposure may fail to reveal significant correlations with negative mood states, because individual differences in HPA axis reactivity may significantly affect the serotonergic neurotransmission and anxiety. Animal experiments with corticoid application and one imaging study measuring baseline cortisol levels in alcoholics and controls (Heinz et al. 2002; Lanfumey et al. 2008; Slotkin et al. 1997) both suggest that high cortisol levels exert negative effects on serotonergic neurotransmission. Therefore, our findings suggest that elevated stress hormone responses in affective disorders interfere with serotonergic neurotransmission and this causes negative mood states, but longitudinal studies are needed to confirm this hypothesis. To the best of our knowledge, this is the first study which shows that cortisol response following Dex-CRH challenge correlates negatively with 5-HTT levels in the human thalamus, and in light of the association between 5-HTT levels and state anxiety, this finding suggests a mechanism how HPA dysfunction may affect mood states via effects on serotonergic neurotransmission.
Post-mortem studies on 5-HTT density have yielded inconsistent results. While there is some evidence from post-mortem studies that 5-HTT density is decreased in subjects with a history of depression (Cannon et al. 2007) other studies did not find increased 5-HTT density in major depression, but only in depressed suicide victims (for review, see Stockmeier 2003), and it has been suggested that an increase in 5-HTT density may go along with a decrease in serotonergic raphe neurons (Arango et al. 2001), suggesting that additional factors may explain some of the variance of alterations in 5-HTT binding. In addition, neuroimaging studies so far yielded seemingly contradictory results in depression and OCD. In depressed patients, some studies reported unaltered even with increased 5-HTT levels (Meyer et al. 2004; Cannon et al. 2006, 2007). Still, the findings of Meyer and coworkers do not contradict an association between 5-HTT reductions and negative mood states, particularly anxiety, insofar as patients with comorbid anxiety had been excluded from this study; in the patient sample of Cannon and coworkers, anxiety ratings were also rather low. Furthermore, Cannon et al. do report a negative correlation between 5-HTT levels in the thalamus and depression severity, that is, patients with higher depression ratings showed relatively lower 5-HTT levels in the thalamus. In OCD, some studies reported unaltered or even increased 5-HTT levels in 5-HTT-rich subcortical regions (Simpson et al. 2003; Matsumoto et al. 2010), a finding that remains to be further elucidated and may be associated with age of OCD onset. Interestingly, all studies that reported decreased 5-HTT levels also observed a dependence on disease severity in that patients with higher OCD scores showed more pronounced 5-HTT reductions. In the light of the correlation between HPA reactivity and 5-HTT levels observed in the present study, it seems conceivable that discrepancies in the literature are, at least partly, mediated by HPA reactivity.
Several limitations of our study need to be addressed. All patients suffering from negative mood states included in the study were medication-free for five plasma half-lives. Still, it has to be mentioned that plasma levels might not necessarily reflect brain kinetics (cf. Tauscher et al. 2002) so that a small amount of residual 5-HTT blockade cannot fully be ruled out in some of our patients. Furthermore, animal studies have suggested an association between chronic antidepressant treatment and decreased 5-HTT gene expression (Lesch et al. 1993) and decreased 5-HTT density (Benmansour et al. 1999); however, Parsey et al. (2006) did not observe 5-HTT reductions in 13 patients with recent antidepressant use as compared to 12 antidepressant naive patients and we observed no significant association between previous antidepressant treatment or duration of drug-free period and 5-HTT alterations.
Overall, sample size was limited. Due to the small sample size, we were unable to specifically test for gender effects, which may well play into the associations presented here (Staley et al. 2001, 2006). However, gender was used as a covariate in all analyses presented, and the pattern of results remained the same when controlling for other potential confounds, including, age, seasonality, and smoking. Data from our cross-sectional study cannot be taken to infer causality, and both longitudinal and intervention studies are needed to further elucidate the interplay between serotonergic neurotransmission, HPA axis dysregulation, and anxiety.
Altogether, our findings link two mainstays of neurobiological research in negative mood states: it suggests that disinhibition of HPA axis activity, which is frequently found in UMD and OCD (Gillespie and Nemeroff 2005; Ising et al. 2005; Kluge et al. 2007) correlates with low thalamic 5-HTT levels and that the reduction in 5-HTT levels (and not genotype per se) contributes to increased anxiety (Reimold et al. 2008). This link between 5-HTT levels and stress hormone response suggests that individual differences in HPA axis regulation exert an effect on serotonin dysfunction, which may interact with the effects of life stress exposure on negative mood states.
References
Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A (1999) Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci 19:10494–10501
Altemus M, Pigott T, Kalogeras KT et al (1992) Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive–compulsive disorder. Arch Gen Psychiatry 49:9–20
Arango V, Underwood MD, Boldrini M et al (2001) Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 25:892–903
Barr CS, Newman TK, Shannon C et al (2004) Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biol Psychiatry 55:733–738
Cannon DM, Ichise M, Fromm SJ et al (2006) Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry 60:207–217
Cannon DM, Ichise M, Rollis D et al (2007) Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry 62:870–877
Carpenter LL, Carvalho JP, Tyrka AR et al (2007) Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry 62:1080–1087
Caspi A, Sugden K, Moffitt TE et al (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Delgado PL, Price LH, Miller HL et al (1994) Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry 51:865–874
First MB, Spitzer RL, Gibbon M, Williams J (1998) Structured clinical interview for DSM-IV personality disorders (SCID-II). American Psychiatric Press, Washington
First MB, Spitzer RL, Gibbon M, Williams J (2001) Structured clinical interview for DSM-IV-TR axis I disorders. research version, patient edition with psychotic screen. Biometric Research, New York State Psychiatric Institute, New York
Gillespie CF, Nemeroff CB (2005) Hypercortisolemia and depression. Psychosom Med 67(Suppl 1):S26–S28
Gotlib IA, Joormann J, Minor KL, Hallmayer J (2008) HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry 63:847–851
Heim C, Newport DJ, Heit S et al (2000) Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284:592–597
Heinz A, Jones DW, Mazzanti C et al (2000) A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 47:643–649
Heinz A, Jones DW, Bissette G et al (2002) Relationship between cortisol and serotonin metabolites and transporters in alcoholism [correction of alcolholism]. Pharmacopsychiatry 35:127–134
Heinz A, Braus DF, Smolka MN et al (2005) Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8:20–21
Hesse S, Müller U, Lincke T et al (2005) Serotonin and dopamine transporter imaging in patients with obsessive–compulsive disorder. Psychiatry Res Neuroimaging 140:63–72
Houle S, Ginovart N, Hussey D, Meyer J, Wilson A (2000) Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med 27:1719–1722
Hu XZ, Lipsky RH, Zhu G et al (2006) Serotonin transporter promoter gain-of-function genotypes are linked to obsessive–compulsive disorder. Am J Hum Genet 78:815–826
Ichise M, Liow JS, Lu JQ et al (2003) Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112
Innis RB, Cunningham VJ, Delforge J et al (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Ising M, Künzel HE, Binder EB, Nickel T, Modell S, Holsboer F (2005) The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1085–1093
Jabbi M, Korf J, Kema IP et al (2007) Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry 12:483–490
Kalbitzer J, Erritzoe D, Holst KK et al (2009) Seasonal changes in brain serotonin transporter binding in short 5-HTTLPR-allele carriers but not in long-allele homozygotes. http://hdl.handle.net/10101/npre.2008.2259.1. Accessed 10 Oct 2009
Kluge M, Schüssler P, Künzel HE, Dresler M, Yassouridis A, Steiger A (2007) Increased nocturnal secretion of ACTH and cortisol in obsessive–compulsive disorder. J Psychiatr Res 41:928–933
Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M (2008) Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev 32:1174–1184
Laruelle M, Slifstein M, Huang Y (2002) Positron emission tomography: imaging and quantification of neurotransporter availability. Methods 27:287–299
Laux LT, Glanzmann LT, Schaffner P, Spielberger CD (2001) Das State-Trait-Angstinventar [The state trait anxiety inventory] (STAI). Beltz, Weinheim
Lesch KP, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL (1993) Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Brain Res Mol Brain Res 17:31–35
Lesch KP, Bengel D, Heils A et al (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E (2003) Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry 60:1248–1255
Malison RT, Price LH, Berman R et al (1998) Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3-beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 44:1090–1098
Matsumoto R, Ichise M, Ito H et al (2010) Reduced serotonin transporter binding in the insular cortex in patients with obsessive–compulsive disorder: A [11C]DASB PET study. NeuroImage 49(2010):121–126
Meyer JH, Houle S, Sagrati S et al (2004) Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry 61:1271–1279
Oquendo MA, Hastings RS, Huang YY et al (2007) Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry 64:201–208
Parsey RV, Hastings RS, Oquendo MA et al (2006) Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry 163(1):52–58
Pogarell O, Hamann C, Pöpperl G et al (2003) Elevated brain serotonin transporter availability in patients with obsessive–compulsive disorder. Biol Psychiatry 54:1406–1413
Posener JA, DeBattista C, Williams GH et al (2000) 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry 57:755–760
Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH (2008) Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry 65:1072–1078
Reimold M, Slifstein M, Heinz A, Mueller-Schauenburg W, Bares R (2006) Effect of spatial smoothing on t-maps: arguments for going back from t-maps to masked contrast images. J Cereb Blood Flow Metab 26:751–759
Reimold M, Smolka MN, Zimmer A et al (2007) Reduced availability of serotonin transporters in obsessive–compulsive disorder correlates with symptom severity - a [(11)C]DASB PET study. J Neural Transm 114:1603–1609
Reimold M, Batra A, Knobel A et al (2008) Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression - a [11C]DASB PET study. Mol Psychiatry 13:606–613
Risch N, Herrell R, Lehner T et al (2009) Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 301:2462–2471
Simpson HB, Lombardo I, Slifstein M et al (2003) Serotonin transporters in obsessive–compulsive disorder: a positron emission tomography study with [11C]McN 5652. Biol Psychiatry 54:1414–1421
Slotkin TA, McCook EC, Ritchie JC, Caroll BJ, Seidler FJ (1997) Serotonin transporter expression in rat brain regions and blood platelets: aging and glucorticoid effects. Biol Psychiatry 41:172–183
Solbach C, Reischl G, Machulla HJ (2004) Determination of reaction parameters for the synthesis of the serotonin transporter ligand [11C]DASB: application to a remotely controlled high yield synthesis. Radiochim Acta 92:341–344
Staley JK, Krishnan-Sarin S, Zoghbi S et al (2001) Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 41:275–284
Staley JK, Sanacora G, Tamagnan G et al (2006) Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 59:40–47
Stockmeier CA (2003) Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res 37(5):357–373
Ströhle A, Holsboer F (2003) Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry 36:S207–S214
Ströhle A, Kellner M, Holsboer F, Wiedemann K (1998) Atrial natriuretic hormone decreases endocrine response to a combined dexamethasone-corticotropin-releasing hormone test. Biol Psychiatry 43:371–375
Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S (2002) Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 7:317–321
Vreeburg SA, Hoogendijk WJ, van Pelt J et al (2009) Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626
Willeit M, Praschak-Rieder N, Neumeister A et al (2000) [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry 47:482–489
Zitterl W, Aigner M, Stompe T et al (2007) [123I]-beta-CIT SPECT imaging shows reduced thalamus–hypothalamus serotonin transporter availability in 24 drug-free obsessive–compulsive checkers. Neuropsychopharmacology 32:1661–1668
Acknowledgments
The study was supported by grants from the Deutsche Forschungsgemeinschaft (He 2597/7-3, Sm 80/2-2, and Re 1472/6-2) and supported in part by the German Ministry for Education and Research (01GS08159), and the National Genome Research Network (NGFN 01GS08148). We would like to thank Professors Florian Holsboer and Jeff Meyer for their helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reimold, Knobel, and Rapp contributed equally to this work.
Rights and permissions
About this article
Cite this article
Reimold, M., Knobel, A., Rapp, M.A. et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology 213, 563–572 (2011). https://doi.org/10.1007/s00213-010-1903-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1903-y