Abstract
Background
Abuse of methylphenidate (Ritalin) is rising, particularly during adolescence and early adulthood, but the long-term effects of its abuse during adolescence are unclear.
Methods
In experiment 1, we examined the effect of adolescent methylphenidate self-administration (0.0625 mg/infusion), as compared with cocaine self-administration (0.125 mg/infusion), under a fixed ratio 1 schedule of reinforcement in male Sprague–Dawley rats during adolescence (postnatal day (PND) 32–47) on adult dopamine-mediated behaviors (PND >70). These included responding for a conditioned reward (CR), a measure of incentive motivation, and amphetamine-induced locomotor activity. In experiment 2, we aimed to replicate and enhance the effects observed in experiment 1, and we also examined the effects of methylphenidate self-administration during adolescence on adult amphetamine-induced zif268 messenger ribonucleic acid (mRNA) expression.
Results
Adolescent rats self-administered both cocaine and methylphenidate. There was no effect of adolescent drug self-administration on adult baseline or amphetamine-induced responding for a CR. However, both adolescent methylphenidate and cocaine self-administration increased amphetamine-induced locomotion. Adolescent methylphenidate self-administration also enhanced amphetamine-induced zif268 mRNA expression in the nucleus accumbens.
Conclusions
Our findings suggest that repeated, behaviorally contingent exposure to methylphenidate during adolescence enhances responsivity to the locomotor-stimulating and neuronal activating effects of amphetamine but not incentive motivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescence may be a period of vulnerability to the effects of drugs of abuse. In humans, exposure to drugs of abuse commonly first occurs during adolescence (Johanson and Fischman 1989), and adolescents progress from cocaine use to abuse more rapidly than adults (Estroff et al. 1989). Further, the age of first drug use inversely predicts the probability of subsequent drug dependence (Anthony and Petronis 1995; Kandel and Yamaguchi 1993). As summarized by Schramm-Sapyta et al. (2009), periadolescent rodents (approximate postnatal day (PND) 28–50) may be differentially sensitive to some but not all effects of drugs of abuse compared to adults (Badanich et al. 2006; Caster et al. 2007; Laviola and Adriani 1998; Laviola et al. 1995, 1999). Whether this differential sensitivity in adolescent rodents reflects a vulnerability to the effects of drugs of abuse is still unclear and requires further investigation. A factor that may contribute to a differential sensitivity to the effects of drugs of abuse in adolescence is the underdeveloped mesocorticolimbic dopamine (DA) system during this period (Andersen et al. 2000; Kalsbeek et al. 1988; Laviola et al. 1999; Teicher et al. 1995).
Methylphenidate, commonly prescribed for attention-deficit hyperactivity disorder (Scheffler et al. 2007), is used recreationally by adolescents. Like cocaine, methylphenidate increases mesocorticolimbic DA levels through blockade of the DA transporter (Volkow et al. 2001, 2002). Methylphenidate taken orally as prescribed has little abuse potential (Volkow et al. 1999, 2002), although the reinforcing effects of methylphenidate depend upon the route of administration. Intranasal methylphenidate produces a euphoric sensation or “high” in humans (Volkow and Swanson 2003; Volkow et al. 1998), and rats readily self-administer this drug intravenously (IV; Botly et al. 2008; Collins et al. 1984; Fletcher et al. 2006; Nielsen et al. 1984). The annual prevalence of illicit methylphenidate use, predominantly intranasal, in the USA is 4% and rising (Klein-Schwartz and McGrath 2003; McCabe et al. 2004). Despite this, the potential long-term effects of recreational methylphenidate use and abuse during adolescence are still unknown.
The ability for methylphenidate use to increase vulnerability to addiction may be dependent on the dose of methylphenidate. Studies using a high, recreationally relevant dose of methylphenidate (Morton and Stockton 2000) found that repeated noncontingent injections of methylphenidate (10 mg/kg) to male or female rats during early (PND 20–35) or late (PND 35–46) adolescence sensitized the locomotor activating effects of psychostimulants in adulthood (Achat-Mendes et al. 2003; Brandon et al. 2001; Valvassori et al. 2007; Wooters et al. 2006). In contrast, studies using repeated noncontingent administration of a low, clinically relevant dose of methylphenidate (2 mg/kg; Aoyama et al. 1990) found less consistent results (Achat-Mendes et al. 2003; Andersen et al. 2002; Bolanos et al. 2003; Brandon et al. 2001; Carlezon et al. 2003). Although these data suggest that recreational methylphenidate use may increase sensitivity to the effects of drugs of abuse in adulthood, the implications for methylphenidate abuse during adolescence are limited because all previous studies used noncontingent, intraperitoneally (IP) injected methylphenidate.
The present study examined whether adolescent rats would self-administer methylphenidate and whether this methylphenidate exposure affected DA-mediated behavior in adulthood and psychostimulant-induced gene expression in adulthood. Specifically, in experiment 1, we examined the effects of IV methylphenidate self-administration during adolescence (PND 32–47) on baseline and amphetamine-induced responding for a conditioned reward (CR) and locomotor activity. Responding for a CR is a measure of incentive motivation—the process whereby initially neutral environmental stimuli, such as lights and tones, acquire reinforcing or rewarding properties by association with primary rewards (Taylor and Robbins 1984). Dopamine plays a role in mediating incentive motivation (Beninger and Ranaldi 1992; Fletcher et al. 1998; Ranaldi and Beninger 1993; Taylor and Robbins 1984), and altered incentive motivational processes may be related to vulnerability to drug addiction (see Robinson and Berridge 2001 for review). The DA releaser amphetamine selectively enhances responding for a CR, and many of the behavioral effects of this drug, including locomotor activity, are sensitized in rats chronically pre-exposed to psychostimulants (Fletcher et al. 1998; Leith and Kuczenski 1981; Robinson and Becker 1986; Segal and Mandell 1974; Taylor and Robbins 1984). Therefore, both responding for a CR and locomotor activity were measured in the present experiments. A group of rats that self-administered cocaine was used as a control for psychostimulant self-administration because previous studies have shown that adolescents will self-administer cocaine (Frantz et al. 2006; Kantak et al. 2007; Li and Frantz 2009; Lynch 2008; Perry et al. 2007) and because of the neurochemical and pharmacological similarities between the two drugs. The main control group used in this experiment was rats that self-administered saccharin orally; in turn, this control was validated by comparing it to a group of naïve rats that had no surgery or self-administration experience. We found that methylphenidate self-administration in adolescence produced a sensitized locomotor response to amphetamine. Thus, in experiment 2, we aimed to replicate and possibly enhance this behavioral effect using a regimen of self-administration in which the dose of methylphenidate was continuously increased. Finally, we examined whether the sensitized response to amphetamine in rats with a history of methylphenidate self-administration was accompanied by changes in amphetamine-induced zif268 messenger ribonucleic acid (mRNA) expression in the mesocorticolimbic pathway. zif268 is an immediate early gene considered a marker of neuronal activation (Worley et al. 1991).
Materials and methods
Subjects
Pregnant Sprague–Dawley dams (gestational day 13) and male adult rats were obtained from Charles River Farms (St. Constant, QC, Canada). On PND 4, litters were culled to six male and four females. The colony was maintained on a 12-h reverse light–dark cycle (0700 to 1900 hours) and maintained at ∼22°C and humidity ∼50–60%. Procedures conformed to the guidelines of the Canadian Council on Animal Care and were approved by the Centre for Addiction and Mental Health Animal Care Committee.
Weaning and group assignment
On PND 21, each rat was pair-housed with a rat from a different litter; both were subsequently assigned to the same experimental group. A maximum of two rats per litter per group was used. In experiment 1, rats from 10 litters were assigned to the groups: methylphenidate-reinforced (n = 9), cocaine-reinforced (n = 10), or saccharin-reinforced (n = 10). In experiment 1, naïve control rats (n = 12) brought into the colony as adults, were pair-housed, and acclimatized for 1 week. This group did not undergo surgery or self-administration. In experiment 2, rats from seven litters were assigned to the groups: methylphenidate-reinforced (n = 11) or saccharin-reinforced (n = 6).
General experimental overview
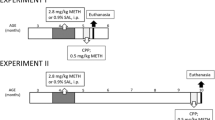
For an overview of both experiments, please see Fig. 1. Experiment 1 compared the long-term effects of self-administered methylphenidate, cocaine, and saccharin during adolescence on responding for a CR and amphetamine-induced locomotion in adulthood (>PND 70). The naïve group was used to validate the saccharin control group. Experiment 2 compared the effects of methylphenidate and saccharin self-administration on the same behaviors as experiment 1 and amphetamine-induced zif268 mRNA expression.
Experiment 1a and 2a: self-administration
Self-administration was conducted in 12 operant conditioning boxes (28 cm long × 21 cm wide × 21 cm high; Med. Associates, Inc., St. Albans, VT, USA). Two levers 4.5 cm wide and 7 cm above the floor were located 6.5 cm on either side of a central, recessed magazine. A liquid dispenser was used to deliver saccharin to the magazine. A counterbalanced arm held a fluid swivel above the ceiling of the chamber. The swivel was attached at one end by Tygon tubing to a syringe mounted on a motor-driven pump (Razel) located outside the chamber. At the other end of the swivel, a length of Tygon tubing encased by a stainless-steel tether allowed a catheter to be connected to the syringe via the swivel. Each chamber was illuminated by a houselight and housed in a sound-attenuating box equipped with a ventilating fan.
Lever training
Rats were food restricted from PND 21–26 (∼12 g per rat Purina rat chow). From PND 23–26, rats learned to lever press on a continuous reinforcement schedule for one 45-mg Noyes pellet per response. Each session terminated after 30 min or 50 pellets.
Surgery
On PND 27, rats weighing approximately 70 g were anesthetized with 60 mg/kg ketamine and 8 mg/kg xylazine. Silastic catheters with a 22-g guide cannulae on the terminal end were implanted into the right jugular vein and secured between the shoulder blades. After surgery, rats received penicillin (Derapen, 0.1 ml, IM; Ayerst Veterinary Laboratories, Guelph, ON, Canada) and buprenorphine (0.03 mg/kg, subcutaneously). Catheters were flushed daily with heparin (0.1 ml of 30 i.u./ml, IV; LEO Pharma, Thornhill, ON, Canada).
Self-administration
From PND 32–47, rats learned to lever press on a fixed ratio 1 schedule for one infusion per response in daily 3 h sessions. Sessions commenced with a priming infusion of 0.1 ml over 5 s for both drug self-administration groups or the delivery of 0.1 ml of saccharin for the saccharin-reinforced group. Initial doses in both experiments were 0.0625 mg/infusion of methylphenidate (Medisca, Montreal, QC, Canada), 0.125 mg/infusion of cocaine (Medisca, Montreal, QC, Canada), or 0.15% saccharin. No stimuli were paired with reinforcer delivery. Half the rats were trained to press the left lever, and the other half were trained to press the right lever for a reinforcer. After receiving the reinforcer, responses over the following 20 s were recorded but had no programmed consequence. The number of saccharin reinforcers was set to the mean number of drug infusions from the previous day to ensure that all groups received approximately the same number of reinforcers. In experiment 1a, after 12 sessions, the doses were increased to 0.125 mg/infusion for methylphenidate, 0.25 mg/infusion for cocaine, and 0.3% of saccharin for sessions 13–15. The original dose was reintroduced during the final session. Following the final session, all rats received a 0.1-ml IV infusion of methohexital (Brevital Sodium; 10 mg/ml; Monarch Pharmaceuticals, Bristol, TN, USA) to test catheter patency.
One aim of experiment 2 was to enhance the sensitized amphetamine response observed in experiment 1. Thus, in experiment 2a, the session length was increased to 4 h, and the doses were increased across self-administration. After 7 days at 0.0625 mg/infusion for methylphenidate and 0.15% for saccharin, doses were increased to 0.125 mg/infusion of methylphenidate and 0.3% saccharin for four sessions. The doses increased again to 0.25 mg/infusion of methylphenidate and 0.6% saccharin for the final four sessions.
Extinction
Responding was extinguished over 7 days in the self-administration boxes (PND 63–69). Each daily session consisted of three 1-h periods with the houselight illuminated; lever responses were not reinforced. Thirty-minute breaks separated these periods during which the houselight was turned off and responding had no programmed consequence.
Experiment 1b and 2b: responding for conditioned reward
Testing occurred in different boxes and at a different time of day from self-administration. Two levers were located 6.5 cm on either side of a central, recessed magazine positioned 3 cm from the floor of the chamber. A red stimulus light was located above each lever, and a sonalert (tone-generator) was located behind one stimulus light. Each chamber contained a solenoid operated water dispenser. Rats (PND 70) were restricted to 2 h access to water per day and on PND 72 underwent a single 40-min lever preference session where they were allowed to press either lever with no programmed consequence. The next day rats were placed in the chambers for one 15-min session with 2 ml of water in the magazine with response levers retracted.
Pavlovian conditioning
Conditioning occurred in 14 daily sessions (∼30 min) from PND 74–87. With the levers retracted, rats learned to associate the delivery of water (0.05 ml of tap water) with the conditioned stimulus (CS): a 5-s illumination of two red lights with the houselights turned off and during the last 0.5 s of the light presentation a tone sounded (2,900 Hz; 85 dB). At the end of the CS, 0.05 ml of water was delivered. The CS and reward were presented on a random time 60 s schedule (30 presentations/rewards per session). The main dependent measures were nose pokes in the magazine during the 5-s CS periods and nose pokes during the 5-s periods immediately prior to the onset of the conditioned stimulus (PCS).
Operant responding for conditioned reward
Both levers were extended into the chamber. For half the rats, a response on the left lever delivered the CS (now the CR) according to a random ratio 2 schedule (CR lever) and responses on the right lever had no programmed consequence (NCR lever) and vice versa for the other half. The CR lever assigned was the opposite of the lever associated with the reinforcer during self-administration. During this phase, rats were never reinforced with water. Rats (PND 88) were injected with 0.9% (1 ml/kg) saline in their home cage 15 min prior to the 40-min test session. The next day rats were injected with d-amphetamine sulfate (0.5 mg/kg, IP; US Pharmacopeia, Rockville, MD, USA) 15 min prior to testing. Dependent measures were responses on the CR and NCR levers.
Experiment 1c and 2c: amphetamine-induced locomotion
Locomotor activity was tested in a custom-built system consisting of 16 clear polycarbonate cages (25 cm wide × 20 cm high × 45 cm long) each with six infrared photocells spaced 7.5 cm apart and 2 cm above the floor positioned along the length of the cages. Rats (PND 90) were habituated to the cages over four 1-h consecutive daily sessions. The following day, rats were placed in the boxes for 30 min and then injected with 0.9% saline (1 ml/kg) prior to the 60-min test session. The next day, the same protocol was followed except rats received an injection of amphetamine (0.5 mg/kg, IP) immediately before testing. Locomotor activity was quantified as the number of beam breaks in 5-min intervals.
Experiment 2d: zif268 mRNA expression
Tissue preparation
At the end of locomotor activity testing, rats were decapitated 60 min after the injection of amphetamine. Brains were extracted and immediately frozen on dry ice and stored at −80°C until sectioning; 20-μm sections from 4.68 to 1.08 mm relative to bregma were taken at −20°C with a cryostat, thaw-mounted onto Superfrost/Plus slides (Fisher, Ottawa, ON, Canada), and stored at −80°C until processing.
In situ hybridization
35S-UTP-labeled riboprobes were prepared from primers complementary to GenBank # NM_012551 (bases 660–679), 5′- tcacctatactggccgcttc-3′ and (bases 1062–1043) 5′-aggtctccctgttgttgtgg-3′. Probes were diluted to a concentration of 200,000 cpm/μl in hybridization solution containing 50% formamide, 35% Denhardt’s solution, 10% dextran sulfate, 0.1× standard sodium citrate (SSC), salmon sperm DNA (300 μg/ml), yeast tRNA (100 μg/ml), and dithiothreitol (40 µM). Slides were incubated in plastic mailers overnight at 60°C. After hybridization, the sections were rinsed in 4× SSC at 60°C, treated in RNase A (20 µg/ml) solution at 45°C for 40 min, washed with agitation in decreasing concentrations of SSC containing 25 g/ml sodium thiosulfate, dipped in water, dehydrated in 70% ethanol, and air-dried. The slides were then exposed to Kodak Biomax film for 6 days at 4°C along with calibrated radioactivity standards.
Image analysis
Autoradiographs of brain sections were used to quantify mRNA levels with computer-assisted image analysis (MCID, InterFocus, Inc., Leiton, UK). Standard curves obtained from calibrated radioactive standards were used to convert film optical densities to micro-Curie per gram. The brain regions examined were the prefrontal cortex, the nucleus accumbens core and shell, and subdivisions of the caudate putamen: dorsolateral, dorsomedial, ventrolateral, and ventromedial. These regions were identified using the Paxinos and Watson atlas (1998). Each region was sampled by a blinded observer under uniform background illumination conditions in at least three sections. Densitometry data for each region were averaged across brain sections for a given subject, then for all subjects within a given treatment group.
Statistical analyses
All data were analyzed (using Statistica v7) by analysis of variance (ANOVA), followed where appropriate by post hoc tests using Fishers least significant difference test. For the drug self-administration experiments (1 and 2), the number of reinforcers, lever responses, and body weight were separately analyzed using the appropriate groups as a between-subjects factor and day as a within-subjects factor. In experiment 1 only, separate paired t tests compared the amount of drug (methylphenidate or cocaine) taken and changes in amount of drug taken after changes in infusion dose. For the Pavlovian phase of the conditioned reward experiments, the percentage of nose pokes into the magazine during the 5-s CS period (CS responses/total responses × 100) and the percentage of responding during the 5 s prior to the CS period (PCS responses/total responses × 100) were calculated. These data were analyzed using a three-way ANOVA with session and CS (CS/PCS) as within-subjects factors and group as a between-subjects factor. Responding for the CR were analyzed using a three-way ANOVA with drug (amphetamine or saline) and lever (CR, NCR) as within-subjects factors and group as the between-subject factor. Saline and amphetamine-induced locomotor activity was analyzed separately with two-way ANOVAs with group as the between-subject factor and time as the within-subjects factor. zif268 mRNA expression was analyzed with an ANOVA with group as the between-subject factor. Bonferroni corrections were applied in cases of multiple comparisons (e.g., self-administration and mRNA expression).
Results
Experiment 1: comparison of control groups
Saccharin-reinforced and naïve rats did not differ significantly from each other on any critical measures for responding for CR and locomotion (data not shown). These groups were combined into a control group resulting in group sizes of nine methylphenidate-reinforced, ten cocaine-reinforced, and 22 control rats.
Experiment 1a: self-administration and extinction
Body weight
Body weight did not vary by group. On PND 32, the average body weights (grams) were 99.2 ± 2.9, 106.7 ± 2.8, and 98.9 ± 2.8 for methylphenidate-, cocaine-, and saccharin-reinforced rats, respectively. By the end of self-administration (PND 47), the corresponding body weights (grams) were 144.9 ± 3.2, 157.3 ± 3.0, and 145.7 ± 3.0. Body weight increased over days (F (15, 390) = 139.03, p = 0.01). There was no main effect of group (F (30, 390) = 0.68, ns) and no group × day interaction (F (2, 26) = 2.59, ns).
Self-administration
Rats readily acquired methylphenidate and cocaine self-administration and responding for saccharin (Fig. 2a). For methylphenidate- and cocaine-reinforced rats, the number of reinforcers earned (F (5, 40) = 0.41, and F (5, 45) = 1.16, respectively; both ns) and the amount of drug taken (F (5, 40) = 0.51 and F (5, 45) = 2.16, respectively; both ns) were stable across sessions 7–12. In all sessions, rats in all groups responded significantly more on the lever delivering the drug rather than the lever with no programmed consequences (data not shown).
Adolescent methylphenidate, cocaine, and saccharin self-administration. a The number of reinforcers earned across the 16 self-administration sessions. The doses for the first 12 sessions were 0.0625 mg/infusion of methylphenidate (n = 9), 0.125 mg/infusion of cocaine (n = 10), and 0.15% saccharin (n = 10). For sessions 13–15, the doses were doubled to 0.125 mg/infusion of methylphenidate, 0.25 mg/infusion of cocaine, and 0.3% saccharin. The original doses were reinstated for the final session. b The corresponding amounts of drug earned in milligrams per kilogram for rats responding for methylphenidate and cocaine. Values are means ± SEM
Doubling the methylphenidate dose (0.0625 to 0.125 mg/infusion) initially decreased the number of earned reinforcers (t (8) = 3.42, p = 0.01; Fig. 2a) and the amount of drug infused (t (8) = 3.02, p = 0.016; Fig. 2b). By the third day at the higher dose (day 15), the number of earned infusions and amount of drug infused was similar to that seen at the original dose (t (8) = 0.31, ns and t (8) = 0.37, ns, respectively). When the original dose was reintroduced on day 16 (0.0625 mg/infusion), the number of earned infusions and the amount of drug infused were similar to that earned previously at the same dose (day 12; t (8) = 1.16, ns and t (8) = 0.38, ns, respectively). Doubling the cocaine dose (0.125 to 0.25 mg/infusion) initially decreased the number of earned infusions (t (9) = 4.63, p = 0.01; Fig. 2a) and the amount of drug infused (t (9) = 7.07, p = 0.01; Fig. 2b). By the third day at the higher dose (day 15), the number of earned infusions was similar to that seen at the original dose (t (9) = 0.22, ns and t (9) = 0.37, ns, respectively). When the dose of cocaine was decreased to the original dose on day 16 (0.125 mg/infusion), the number of infusions was significantly greater than on the last day at the same dose (day 12; t (9) = 2.39, p = 0.04), although this effect was no longer significant after the Bonferroni correction was applied (α = 0.016). Further, the amount of drug infused was similar to that earned on the last day at the same dose (t (9) = 1.69, ns). The total amount of drug consumed (milligrams per kilogram) across the 16 sessions of self-administration was 354.6 ± 41.4 for the methylphenidate-reinforced rats and 507.3 ± 15.2 for the cocaine-reinforced group.
Extinction
Following removal of the reinforcer responding decreased across days (F (6, 156) = 40.79, p < 0.01). There was no main effect of group and no significant day × group interaction (F (2, 26) = 0.26, ns and F (12, 156) = 0.61, ns, respectively). On day 1, the total numbers of responses across the three sessions were 57.3 ± 10.2, 53.8 ± 8.9, and 52.8 ± 5.7 for methylphenidate-, cocaine-, and saccharin-reinforced rats, respectively. By day 7, the total corresponding responses were 9.2 ± 2.2, 9.0 ± 2.2, and 12 ± 1.9 (data not shown).
Experiment 1b: responding for conditioned reward
Lever preference testing
Rats in all groups (F (2, 26) = 0.19, ns) did not show a lever preference (F (1, 26) = 2.14, ns; data not shown). There was no interaction between group and lever (F (2, 26) = 0.01, ns). Naïve rats also did not show a lever preference (t (11) = 2.14, p = ns).
Pavlovian conditioning
Rats responded more during CS periods than PCS periods (F (1, 23) = 89.53, p < 0.01), and this divergence increased across sessions (F (13, 299) = 10.08, p < 0.01). Rats responded more during PCS periods in session 1 (F (1, 38) = 9.92, p < 0.01), but from session two onward, responding was greater during CS periods than PCS periods (p < 0.01; sessions 2–14). This behavior did not differ across groups, and there were no interactions between group and session or CS (Fig. 3).
Approach behavior. Discriminated approach behavior during conditioning was not affected by previous adolescent drug experience. The proportion of nose poking during the conditioned stimulus periods (CS) as compared to 5-s periods immediately prior to the conditioned stimulus (PCS) was increased for all rats starting on session two (p < 0.05). Values are means ± SEM. Methylphenidate n = 9, cocaine n = 10, control n = 22
Operant responding for conditioned reward
Rats responded on the CR lever more than the NCR lever (F (1, 38) = 60.78, p < 0.01; Fig. 4) similarly across groups (F (2, 38) = 0.62, ns). Overall, amphetamine increased responding (F (1, 38) = 54.4, p < 0.01), and this effect was greater on the CR lever than the NCR lever (F (1, 38) = 39.91, p < 0.01). There was no three-way interaction between group, lever, and amphetamine (F (2, 38) = 0.08, ns); thus, the response-enhancing effect of amphetamine was similar in the three groups.
Responding for conditioned reward. Rats in all groups responded more on the CR than NCR lever (p < 0.05) and an amphetamine (AMPH) challenge (0.5 mg/kg) enhanced responding on the CR lever (**p < 0.01). Previous adolescent drug experience did not affect this behavior. a Control (n = 22), b methylphenidate (n = 9), c cocaine (n = 10). Values are means ± SEM. CR lever associated with the conditioned reward, NCR lever associated with no programmed consequences
Experiment 1c: amphetamine-induced locomotion
There were no group differences during the first exposure to the locomotor chambers, after saline injection (data not shown) and during the 30 min prior to amphetamine injection (Fig. 5). Figure 5 illustrates that amphetamine-induced locomotion was enhanced in methylphenidate- and cocaine-reinforced rats compared to control rats. Although the main effect of group was not significant (F (2, 38) = 1.28, ns), the interaction between group and time was significant (F (22, 418) = 2.48, p < 0.01) such that methylphenidate- and cocaine-reinforced rats had higher locomotor activity than control rats during the first 30 min after the amphetamine injection.
Amphetamine-induced locomotion. Compared to control rats (saccharin and naïve combined: n = 22) methylphenidate-reinforced (n = 9) and cocaine-reinforced (n = 10) rats exhibited an enhanced locomotor response for 30 min after an injection of amphetamine (0.5 mg/kg). Prior to injection, all groups had habituated and did not differ significantly in locomotor activity. Values are means ± SEM
Experiment 2a: self-administration and extinction
Body weight
On PND 32, the average body weights (grams) were 100.9 ± 4.4 and 116.6 ± 6.0 for methylphenidate- and saccharin-reinforced rats, respectively. By the end of self-administration (PND 47), the corresponding body weights (grams) were 142.5 ± 3.1 and 161.4 ± 4.2 Overall, the methylphenidate-reinforced rats weighed less than saccharin-reinforced rats (F (1, 15) = 14.76, p < 0.01).
Self-administration
Rats in all groups responded significantly more on the lever delivering the reinforcer rather than the lever with no programmed consequence (data not shown). Figure 6a illustrates that across all sessions, the number of earned methylphenidate infusions was similar across infusion doses. Figure 6b shows that the amount of methylphenidate taken increased with increasing doses. These observations are borne out by statistical analyses of the daily averages for number of infusions and the amount of drug earned. For methylphenidate-reinforced rats, the number of infusions was stable across dose (F (2, 20) = 0.26, ns; Fig. 6a), but the amount of drug taken increased with dose (F (2, 20) = 9.99, p < 0.01; Fig. 6b). The total amount of methylphenidate taken (milligrams per kilogram) across the 16 sessions of self-administration was 515 ± 60.1.
Adolescent methylphenidate and saccharin self-administration. a The number of reinforcers earned increased as a function of dose for methylphenidate (0.0625, 0.125, and 0.25 mg/infusion; n = 11) and saccharin (0.15, 0.3, 0.6%; n = 6) (p < 0.05). b The amount of drug as a function of body weight increased with dose (0.0625, 0.125, 0.25 mg/infusion) for methylphenidate. Values are means ± SEM. PND 36 data are excluded because of a computer malfunction during testing which led to no drug infused in some animals
Extinction
Following removal of the reinforcer, responding decreased across days (F (6, 96) = 30.74, p < 0.01) in both groups (F (1, 16) = 0.24, ns; data not shown). On day 1, the total numbers of responses across the three sessions were 54.8 ± 6.1 and 58.5 ± 8.7 for methylphenidate- and saccharin-reinforced rats, respectively. By day 7, the total corresponding numbers of responses were 2.4 ± 0.4 and 2.40 ± 1.0.
Experiment 2b: responding for conditioned reward
Lever preference testing
Rats did not exhibit a lever preference on first exposure to the test boxes (F (1, 15) = 1.31, ns) regardless of group (F (1, 15) = 0.22, ns; data not shown). There was no lever × group interaction (F (1, 15) = 0.1, ns).
Pavlovian conditioning
Rats in all groups responded more during CS periods than PCS periods (F (1, 15) = 113.36, p < 0.01), and this divergence increased across sessions (F (13, 195) = 16.94, p < 0.01). This behavior did not differ between groups (data not shown—similar to Fig. 3).
Operant responding for conditioned reward
Rats responded on the CR lever more than the NCR lever (F (1, 15) = 21.56, p < 0.01), and this effect was similar across groups (F (1, 15) = 1.70, ns). Overall, amphetamine increased responding (F (1, 15) = 25.48, p < 0.01), and this effect was greater on the CR lever than the NCR lever (F (1, 15) = 18.15, p < 0.01; data not shown—similar to Fig. 4). There was also no three-way interaction between group, lever, and amphetamine (F (1, 15) = 4.5, ns); thus, the response-enhancing effect of amphetamine was similar in the two groups.
Experiment 2c: amphetamine-induced locomotion
There were no group differences during the first exposure to the locomotor chambers, after the saline injection (data not shown) or during the 30 min prior to amphetamine (Fig. 7). Although the main effect of group was not significant (F (1, 14) = 3.4, ns), the interaction between group and time was significant (F (11, 154) = 1.94, p = 0.04) such that methylphenidate-reinforced rats had higher locomotor activity than saccharin-reinforced rats during the first 30 min after the amphetamine injection.
Amphetamine-induced locomotion. Compared to control rats (n = 6), methylphenidate-reinforced rats (n = 10) exhibited a sensitized locomotor response to an injection of amphetamine (0.5 mg/kg) during the first 30-min postinjection. Prior to injection, all groups had habituated and did not differ significantly in locomotor activity. Values are means ± SEM
Experiment 3: zif268 mRNA expression
Methylphenidate-reinforced rats tended to have higher zif268 mRNA expression than control rats in all brain regions analyzed (Fig. 8). This effect was only significant in the nucleus accumbens core and shell (t (13) = 2.1, p = 0.05 and t (13) = 2.4, p = 0.03, respectively). However, when the Bonferroni correction was applied (α = 0.004), these effects were no longer significant. Thus, methylphenidate self-administration during adolescence marginally enhanced amphetamine-induced neuronal activation in the nucleus accumbens.
Amphetamine-induced zif268 mRNA expression. Compared to control rats (n = 6), methylphenidate-reinforced rats (n = 10) had enhanced amphetamine-induced zif268 mRNA expression in the nucleus accumbens core and shell. Values are means ± SEM. PFC prefrontal cortex, NAS nucleus accumbens shell, NAC nucleus accumbens core, DM dorsomedial caudate, DL dorsomedial caudate, VM ventromedial caudate and VL ventrolateral caudate
Discussion
This study found that methylphenidate and cocaine self-administration during adolescence (PND 32–47) enhanced amphetamine-induced locomotion in adulthood. Methylphenidate also enhanced accumbal neuronal activation. Neither drug affected learning about a CS that predicted reinforcement or responding for the CS when it functioned as a CR in adulthood. The data suggest that some aspects of mesocorticolimbic DA function are altered by psychostimulant self-administration during adolescence.
Methylphenidate and cocaine self-administration
Adolescent rats self-administer drugs of abuse such as cocaine and amphetamine (Frantz et al. 2006; Kantak et al. 2007; Kerstetter and Kantak 2007; Lynch 2008; Perry et al. 2007; Shahbazi et al. 2008). Adult rats self-administer methylphenidate (Botly et al. 2008; Collins et al. 1984; Marusich and Bardo 2009; Nielsen et al. 1984), and the current study demonstrated that adolescent rats also self-administer this drug. This finding may be important since adolescence is when methylphenidate is most commonly abused (Babcock and Byrne 2000; Klein-Schwartz and McGrath 2003). Two aspects of the results indicate that methylphenidate and cocaine controlled behavior in the adolescent rats: Rats responded more on the active lever than on an inactive lever, and when drug was removed, responding decreased rapidly.
Manipulating methylphenidate or cocaine doses did not alter responding in a way consistent with the patterns observed in adult rats where generally clear dose–response relationships are found (e.g., Botly et al. 2008; Pickens and Thompson 1968; Yokel and Pickens 1973). This lack of dose dependence during adolescence is consistent with some, but not all, reports in the literature. For example, Shram et al. (2007) reported nicotine dose dependence in adults but not in adolescents. Similarly, Frantz et al. (2006) did not find a dose-dependent relationship in adolescent rats acquiring cocaine self-administration. Conversely, Kantak et al. (2007) reported a dose-dependent change in responding in adolescent rats self-administering cocaine using a within-session descending dose procedure. Differences in procedural factors, reinforcement schedule, drug, age, and strain could contribute to these variable findings. The issue of how, or whether, drug intake is regulated during adolescence remains unresolved. Although this issue was not the focus of the present experiments, the pattern of responding observed, coupled with previous reports in the literature, suggests that adolescent rats may regulate their drug intake differently than adults. Research directly comparing responses to self-administration drug dose manipulation in adolescent and adult rats is necessary to address the possibility that psychostimulant intake regulation differs between adolescent and adult rats.
Behavioral and neuronal sensitization to amphetamine
A neutral environmental stimulus can gain incentive salience when paired with a primary reinforcer such as water or food. This incentive salience can be demonstrated if the stimulus (CS) functions as a CR that elicits operant responding. Psychomotor stimulants enhance responding for a CR (Fletcher et al. 1998; Taylor and Robbins 1984). In the present study, we found that rats that self-administered methylphenidate and cocaine during adolescence did not differ from control animals on any aspect of performance of a test of responding for CR in adulthood. Thus, all groups of rats showed equivalent discriminated approach behavior to the food magazine during the Pavlovian phase of training and equivalent operant responding for the CR. Amphetamine enhanced CR responding but did so to the same extent in all groups. Overall, our results indicate that incentive motivational processes, at least those related to a nondrug primary reinforcer, were unaltered by self-administration of methylphenidate or cocaine.
The long-term effects of methylphenidate administration on adult reward and motivational processes are unclear. Repeated administration of methylphenidate (2 mg/kg; IP) from PND 20–35 reduced cocaine conditioned place preference (CPP; Andersen et al. 2002; Carlezon et al. 2003) and sucrose preference in adulthood. This implies that this apparent reduction in reward-related behavior applies to drug and nondrug rewards (Bolanos et al. 2003). Conversely, in other studies, noncontigent exposure of methylphenidate (2 mg/kg; IP) to adolescent male rats enhanced cocaine self-administration acquisition (Brandon et al. 2001) and enhanced reinstatement of an extinguished CPP to cocaine when treated between PND 26–32 (Achat-Mendes et al. 2003). Thus, across various studies, the effects of adolescent exposure to methylphenidate are variable, and no clear picture has emerged. Differences in the methylphenidate dose, the timing of methylphenidate exposure during adolescence, and the route and contingency of injection (IP versus IV) may contribute to these mixed findings.
In contrast to the results of the experiments involving responding for CR, cocaine and methylphenidate self-administration during adolescence induced a modest sensitized locomotor response to an amphetamine challenge in adulthood. These results are consistent with those of previous studies in which repeated administration of a high dose of methylphenidate (10 mg/kg) to male and female rats during both early (PND 20–35) and late (PND 35–46) adolescence sensitized the locomotor-stimulating effects of cocaine and amphetamine (Achat-Mendes et al. 2003; Brandon et al. 2001; Valvassori et al. 2007; Wooters et al. 2006). Thus, several studies have now shown that response-contingent or noncontingent exposure to methylphenidate during adolescence produces at least some behavioral evidence of sensitization to an amphetamine challenge. This implies that methylphenidate or cocaine self-administration induces long-term functional alterations in the activity of mesocorticolimbic DA systems.
It should also be noted that the locomotor effect reported in this study was observed in two separate experiments with small procedural variations related to dose and session length. These modifications to the second experiment were intended to enhance the magnitude of the sensitized locomotor response. An overall increase in the amount of methylphenidate self-administered did not translate into a statistically enhanced sensitized locomotor response; although as seen in Figs. 5 and 7, a trend toward increased amphetamine-induced locomotor activity in the second experiment is evident.
Procedural factors may explain the apparent lack of a sensitized response for a CR with the presence of a sensitized locomotor response in the same rats in the present study. Amphetamine (0.5 mg/kg) was administered 15 min prior to CR testing to ensure testing commenced during the peak of amphetamine effects. This interval was chosen based on previous behavioral data from our lab (Tenn et al. 2003, 2005) and previous neurochemical findings (Di Chiara and Imperato 1988; McKittrick and Abercrombie 2007; Pum et al. 2007). Psychostimulant self-administration during adolescence may shift the peak of amphetamine effects; thus, injecting amphetamine immediately prior to the session may have revealed group differences. A shift in the peak of amphetamine effects may also have contributed to the increased amphetamine-induced locomotion 10 min postinjection in the rats that previously self-administered psychostimulants. Further, greater differences in zif268 mRNA expression may have been observed if measured at another time point. Context also may have played a role in the lack of expression of a sensitized responding for CR, particularly time of day (Arvanitogiannis et al. 2000). Amphetamine-induced a modest sensitized locomotor response when the drug challenge was at the same time of day as self-administration in a very different context.
The enhanced amphetamine-induced locomotor response observed in rats that self-administered methylphenidate was accompanied by enhanced neuronal activity in the nucleus accumbens. Amphetamine-induced zif268 mRNA expression in the nucleus accumbens was enhanced in methylphenidate-exposed rats compared to saccharin-reinforced rats (experiment 2d). zif268 is an established marker of neuronal activation because the zif268 protein is rapidly responsive to changes in membrane activity and levels of zif268 mRNA and protein are correlated to synaptic activity (Worley et al. 1991). Therefore, increased zif268 mRNA expression indicates a functional change in this region of the mesocorticolimbic pathway. Repeated methylphenidate exposure during adolescence blunts immediate early gene expression (e.g., c-fos and zif268) in the striatum and nucleus accumbens when the challenge is on the day following the termination of the sensitization regimen (Brandon and Steiner 2003). In contrast, our results showed that when challenged with amphetamine, weeks after methylphenidate self-administration during adolescence zif268 expression was increased in the nucleus accumbens. These opposite findings are likely related to the timing of the drug challenge in relation to the sensitizing drug regimen. Regardless, the modest sensitized locomotion and zif268 mRNA expression effects in the present findings likely reflect some underlying change in mesocorticolimbic DA pathway function.
Possible mechanisms
Long-term psychostimulant-induced behavioral changes likely result from, in part, altered mesocorticolimbic DA function. Relatively less research in this area has focused on methylphenidate. Neuroplastic alterations induced by cocaine and amphetamine include alterations to cell morphology (Robinson et al. 2001; Robinson and Kolb 1999), neurochemistry (Chefer et al. 2000; Kalivas and Duffy 1990; Kalivas and Stewart 1991; Robinson and Becker 1982; Sorg et al. 1997), and receptor sensitivity (Henry and White 1991). Sensitization alters the expression of genes other than zif268, including genes related to DA and its receptors (Izawa et al. 2006; Mead et al. 2002; Xu et al. 1994; Zhang et al. 2005), and other transcription factors indirectly related to dopaminergic function (Adriani et al. 2006; Brenhouse and Stellar 2006; Filip et al. 2006; Izawa et al. 2006; Mattson et al. 2005). These psychostimulant-induced alterations in transcription factor expression are likely mediated via the dopamine D1 and D2 receptor (Bhat and Baraban 1993; Graybiel et al. 1990; Yano et al. 2006; Young et al. 1991). Therefore, the amphetamine-induced sensitized locomotor and neuronal activating responses after adolescent psychostimulant exposure in the present studies were likely mediated by such long-term functional alterations to the mesocorticolimbic DA pathway.
Conclusions and implications
Psychostimulant abuse during adolescence may increase the likelihood of addiction in adulthood by altering mesocorticolimbic DA system function. Increased vulnerability to addiction may result in part from sensitization of this system (Robinson and Berridge 2000). We report modest sensitization to the psychomotor and neuronal activating effects of amphetamine but no alterations in responding for CR after methylphenidate self-administration during adolescence. These data raise some concern about the potential long-term effects of recreational methylphenidate use, although more research is needed to determine if recreational methylphenidate use may result in increased vulnerability to addiction. This question is particularly important given the rising number of adolescents recreationally using this drug (Klein-Schwartz and McGrath 2003; McCabe et al. 2004). However, these findings may not have any direct implications for the long-term effects of clinical methylphenidate use because our methodology involved IV self-administered methylphenidate at doses that are likely outside the clinical range (Aoyama et al. 1990).
References
Achat-Mendes C, Anderson KL, Itzhak Y (2003) Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology 45:106–115
Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C (2006) Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology 31:1946–1956
Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37:167–169
Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA Jr (2002) Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci 5:13–14
Anthony JC, Petronis KR (1995) Early-onset drug use and risk of later drug problems. Drug Alcohol Depend 40:9–15
Aoyama T, Kotaki H, Iga T (1990) Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. J Pharmacobiodyn 13:647–652
Arvanitogiannis A, Sullivan J, Amir S (2000) Time acts as a conditioned stimulus to control behavioral sensitization to amphetamine in rats. Neuroscience 101:1–3
Babcock Q, Byrne T (2000) Student perceptions of methylphenidate abuse at a public liberal arts college. J Am Coll Health 49:143–145
Badanich KA, Adler KJ, Kirstein CL (2006) Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol 550:95–106
Beninger RJ, Ranaldi R (1992) The effects of amphetamine, apomorphine, SKF 38393, quinpirole and bromocriptine on responding for conditioned reward in rats. Behav Pharmacol 3:155–163
Bhat RV, Baraban JM (1993) Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther 267:496–505
Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ (2003) Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry 54:1317–1329
Botly LC, Burton CL, Rizos Z, Fletcher PJ (2008) Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology (Berl) 199:55–66
Brandon CL, Steiner H (2003) Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J NeuroSci 18:1584–1592
Brandon CL, Marinelli M, Baker LK, White FJ (2001) Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology 25:651–661
Brenhouse HC, Stellar JR (2006) c-Fos and deltaFosB expression are differentially altered in distinct subregions of the nucleus accumbens shell in cocaine-sensitized rats. Neuroscience 137:773–780
Carlezon WA Jr, Mague SD, Andersen SL (2003) Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry 54:1330–1337
Caster JM, Walker QD, Kuhn CM (2007) A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 193:247–260
Chefer VI, Moron JA, Hope B, Rea W, Shippenberg TS (2000) Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience 101:619–627
Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR (1984) Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 82:6–13
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278
Estroff TW, Schwartz RH, Hoffmann NG (1989) Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 28:550–555
Filip M, Faron-Gorecka A, Kusmider M, Golda A, Frankowska M, Dziedzicka-Wasylewska M (2006) Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res 1071:218–225
Fletcher PJ, Korth KM, Sabijan MS, DeSousa NJ (1998) Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Res 805:29–40
Fletcher PJ, Botly LC, Rizos Z (2006) Characterization of methylphenidate self-administration and reinstatement in the rat. Society for Neuroscience, Atlanta
Frantz KJ, O’Dell LE, Parsons LH (2006) Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 32:625–637
Graybiel AM, Moratalla R, Robertson HA (1990) Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87:6912–6916
Henry DJ, White FJ (1991) Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther 258:882–890
Izawa R, Jaber M, Deroche-Gamonet V, Sillaber I, Kellendonk C, Le Moal M, Tronche F, Piazza PV (2006) Gene expression regulation following behavioral sensitization to cocaine in transgenic mice lacking the glucocorticoid receptor in the brain. Neuroscience 137:915–924
Johanson CE, Fischman MW (1989) The pharmacology of cocaine related to its abuse. Pharmacol Rev 41:3–52
Kalivas PW, Duffy P (1990) Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 5:48–58
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB (1988) Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol 269:58–72
Kandel D, Yamaguchi K (1993) From beer to crack: developmental patterns of drug involvement. Am J Public Health 83:851–855
Kantak KM, Goodrich CM, Uribe V (2007) Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus). Exp Clin Psychopharmacol 15:37–47
Kerstetter KA, Kantak KM (2007) Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 194:403–411
Klein-Schwartz W, McGrath J (2003) Poison centers’ experience with methylphenidate abuse in pre-teens and adolescents. J Am Acad Child Adolesc Psych 42:288–294
Laviola G, Adriani W (1998) Evaluation of unconditioned novelty-seeking and d-amphetamine-conditioned motivation in mice. Pharmacol Biochem Behav 59:1011–1020
Laviola G, Wood RD, Kuhn C, Francis R, Spear LP (1995) Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther 275:345–357
Laviola G, Adriani W, Terranova ML, Gerra G (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010
Leith NJ, Kuczenski R (1981) Chronic amphetamine: tolerance and reverse tolerance reflect different behavioral actions of the drug. Pharmacol Biochem Behav 15:399–404
Li C, Frantz KJ (2009) Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 204:725–733
Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197:237–246
Marusich JA, Bardo MT (2009) Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol 20:447–454
Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT (2005) Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem 95:1481–1494
McCabe SE, Teter CJ, Boyd CJ, Guthrie SK (2004) Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health 35:501–504
McKittrick CR, Abercrombie ED (2007) Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem 100:1247–1256
Mead AN, Rocha BA, Donovan DM, Katz JL (2002) Intravenous cocaine induced-activity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur J NeuroSci 16:514–520
Morton WA, Stockton GG (2000) Methylphenidate abuse and psychiatric side effects. Prim Care Companion J Clin Psychiat 2:159–164
Nielsen JA, Duda NJ, Mokler DJ, Moore KE (1984) Self-administration of central stimulants by rats: a comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav 20:227–232
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Perry JL, Anderson MM, Nelson SE, Carroll ME (2007) Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav 91:126–133
Pickens R, Thompson T (1968) Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther 161:122–129
Pum M, Carey RJ, Huston JP, Muller CP (2007) Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 193:375–390
Ranaldi R, Beninger RJ (1993) Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology (Berl) 113:110–118
Robinson TE, Becker JB (1982) Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol 85:253–254
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95(Suppl 2):S91–S117
Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J NeuroSci 11:1598–1604
Robinson TE, Gorny G, Mitton E, Kolb B (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266
Scheffler RM, Hinshaw SP, Modrek S, Levine P (2007) The global market for ADHD medications. Health Aff (Millwood) 26:450–457
Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM (2009) Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 206:1–21
Segal DS, Mandell AJ (1974) Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2:249–255
Shahbazi M, Moffett AM, Williams BF, Frantz KJ (2008) Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 196:71–81
Shram MJ, Funk D, Li Z, Le AD (2007) Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology 33:739–748
Sorg BA, Davidson DL, Kalivas PW, Prasad BM (1997) Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther 281:54–61
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 84:405–412
Teicher MH, Andersen SL, Hostetter JC Jr (1995) Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89:167–172
Tenn CC, Fletcher PJ, Kapur S (2003) Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res 64:103–114
Tenn CC, Fletcher PJ, Kapur S (2005) A putative animal model of the “prodromal” state of schizophrenia. Biol Psychiatry 57:586–593
Valvassori SS, Frey BN, Martins MR, Reus GZ, Schimidtz F, Inacio CG, Kapczinski F, Quevedo J (2007) Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol 18:205–212
Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N (1998) Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 155:1325–1331
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Dewey SL, Hitzemann R, Gifford AN, Pappas NR (1999) Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther 288:14–20
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:RC121
Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ (2002) Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol 12:557–566
Wooters TE, Dwoskin LP, Bardo MT (2006) Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 188:18–27
Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM (1991) Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci USA 88:5106–5110
Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S (1994) Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell 79:945–955
Yano M, Beverley JA, Steiner H (2006) Inhibition of methylphenidate-induced gene expression in the striatum by local blockade of D1 dopamine receptors: interhemispheric effects. Neuroscience 140:699–709
Yokel RA, Pickens R (1973) Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther 187:27–33
Young ST, Porrino LJ, Iadarola MJ (1991) Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA 88:1291–1295
Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M (2005) Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology 30:1443–1454
Acknowledgments
The authors thank Drs. Suzanne Erb and Megan Shram for their advice on procedural aspects of this work and Judy Sinyard, Roger Raymond, Mustansir Diwan, and Silvia Isabella for technical assistance. This work was supported by a Masters Award from the Canadian Institutes of Health Research Masters Award to CLB and a Discovery Grant from Natural Sciences and Engeneering Research Council of Canada to PJF.
Ethical standards
Experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Procedures were approved by the CAMH Animal Care Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burton, C.L., Nobrega, J.N. & Fletcher, P.J. The effects of adolescent methylphenidate self-administration on responding for a conditioned reward, amphetamine-induced locomotor activity, and neuronal activation. Psychopharmacology 208, 455–468 (2010). https://doi.org/10.1007/s00213-009-1745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1745-7