Abstract
Rationale
In rodents, prolonged maternal separation has been used as a model of developmentally early environmental stress to influence adult drug intake.
Objectives
The aim of the present study was to evaluate the long-term effects of prolonged maternal separation on alcohol consumption using two different self-administration procedures in mice: operant alcohol self-administration vs. three-bottle choice.
Materials and methods
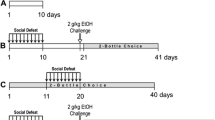
From postnatal day (PND) 1 to 14, pups were separated from the dam (maternal separation, MS) daily for 180 min or were left undisturbed, only handled during cage cleaning (animal facility rearing, AFR). On PND 60, they were assigned to one of two experimental manipulations: either a three-bottle choice or operant oral alcohol self-administration. In the three-bottle choice procedure, mice were given access to 6% or 10% alcohol or 0.05% saccharin solution for 2 h/day for 10 days. In the second experiment, mice were reinforced for nose poking by delivery of oral alcohol (6% or 10% in saccharin) or 0.05% saccharin solutions during daily 30-min sessions. Following the acquisition phase, “break points” were determined. Later, mice were allowed 1 h access to the reinforcing solution with no dosage limitation.
Results
In the three-bottle choice procedure, MS mice showed higher alcohol intake than AFR at the 10% alcohol concentration. In the operant alcohol self-administration, MS mice achieved higher alcohol intake than AFR at the concentrations 6% and 10% during the 1-h session.
Conclusions
The results demonstrate the long-term consequences of MS on alcohol intake in male mice, suggesting early life stress as a risk factor for alcohol consumption and abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to stressful events has been postulated to be intricately associated with increased vulnerability to the abuse of addictive substances (Koob and Kreek 2007; Sinha 2001). Several studies show a positive association between stress and increased alcohol-seeking and alcohol intake in alcoholics and social drinkers (Dawson et al. 2005; Kaufman et al. 2007; Linsky et al. 1985). However, attempts to model the effects of stress on alcohol intake in animals have yielded varying outcomes, which include both increased consumption and preference, as well as suppression of alcohol intake (Bowers et al. 1997; Chester et al. 2004; Lynch et al. 1999; Overstreet et al. 2007; Van Erp et al. 2001; Van Erp and Miczek 2001; Volpicelli et al. 1986). For example, restraint stress in rats (Bowers et al. 1997; Overstreet et al. 2007) and repeated social defeat stress in mice (Croft et al. 2005) were reported to produce increases in alcohol intake. On the other hand, rats exposed to social defeat stress presented a transient suppression of alcohol intake, as well as a reduced rate of alcohol reinforcements (Van Erp and Miczek 2001). In a recent study, repeated swim stress decreased alcohol intake in mice in a strain-dependent manner (Boyce-Rustay et al. 2008).
Some of the critical aspects to consider when analyzing the effects of stress on drug intake are the nature and characteristics of the stressor (Miczek et al. 2008; Pacak and Palkovits 2001). Each type of stressor (i.e., social defeat, foot shock, maternal separation, restraint, immobilization, sleep deprivation, etc.) presents its own behavioral and physiological profile and may produce different outcomes (Miczek et al. 2008; Meaney 2001; Miczek et al. 2004; Sinha 2001). Repeated maternal separation has been considered one of the most powerful stressors for an infant (Levine 1967; Levine 2001; Wigger and Neumann 1999), producing long-lasting neural and behavioral consequences, including an increased vulnerability to alcohol and drug abuse in adulthood (Gilmer and McKinney 2003; Moffett et al. 2007; Sanchez et al. 2001).
In rodents, maternal separation typically involves the daily removal of litters from the dams during the first 2 weeks of life, which corresponds to the “stress hyporesponsive period” of the pups (Levine 1957; Levine 1967; Plotsky and Meaney 1993). During this period, adequate maternal care is essential for the long-term regulation of the hypothalamic–pituitary–adrenal (HPA) axis and stress responses (see Levine 2005). A critical feature of repeated maternal separation pertains to the precise duration of the periods of separation (Levine 1957; Moffett et al. 2007; Plotsky and Meaney 1993). Short (i.e., 15 min/day) and prolonged (i.e., 180 or 360 min/day) separations may result in opposite outcomes in adulthood (Huot et al. 2001; Kawakami et al. 2007; Kikusui et al. 2005). In general, prolonged vs. short maternal separation are associated with hyperactivity vs. hypoactivity of the HPA axis and stress responses, respectively (Huot et al. 2001; Levine 1967; Meerlo et al. 1999; Plotsky and Meaney 1993).
In male rats, prolonged maternal separation can induce higher levels of alcohol consumption in adulthood (Huot et al. 2001; Ploj et al. 2003; Roman et al. 2005; Roman and Nylander 2005). Huot et al. (2001) reported that rats that were separated from their dams for 180 min/day (MS180) drank significantly more of an 8% alcohol/2.5% sucrose solution relative to those separated for 15 min/day (MS15) or the animal facility rearing (AFR) controls. MS180 rats consumed significant amounts of alcohol (4–5 g/kg/day, compared to less than 1 g/kg/day for AFR or MS15), and showed a reduced preference for the plain 2.5% sucrose solution in favor of the alcohol/sucrose solution (Huot et al. 2001). Additionally, Ploj et al. (2003) reported that MS rats (360 min separation from postnatal days 1–21) consumed more of a nonsweetened 8% ethanol solution when compared with MS15 or AFR rats. However, these effects may be gender-dependent (for example, female MS180 or MS360 rats do not show reliable increases in alcohol consumption), concentration-dependent (i.e., effect is usually more pronounced with solutions of increased alcohol concentrations); and time-dependent (e.g., Gustafsson et al. 2005; Gustafsson et al. 2007; Gustafsson and Nylander 2006; Marmendal et al. 2004; Roman et al. 2004; Roman et al. 2005).
The effects of maternal separation on alcohol intake have been mostly studied in rats, usually implementing 24-h access procedures to two-to-four bottles containing different concentrations of alcohol. The maternal separation procedure has been validated in mice (e.g., Parfitt et al. 2004; Romeo et al. 2003) and it was also shown to affect psychomotor responses to drugs, such as cocaine (Kikusui et al. 2005), or alcohol (Kawakami et al. 2007). In contrast to some evidence from rats, maternal separation did not significantly affect alcohol intake in C57BL/6J mice, using a two-bottle choice method (Advani et al. 2007). Further investigation of maternal separation stress in mice, as well as the implementation of different procedures for assessing motivational as well as consummatory aspects of alcohol intake would be instructive.
The aim of the present study was to evaluate the long-term effects of repeated prolonged maternal separation (MS180) on alcohol consumption using two different self-administration procedures in outbred mice: operant alcohol self-administration vs. three-bottle choice. In the present experiment, we used litters under standard animal facility conditions (AFR) to facilitate the comparison with other studies. Neither food nor fluid restrictions were implemented during the three-bottle choice or operant self-administration of alcohol in order to reduce the impact of additional sources of stress.
Materials and methods
Animals
Twelve breeding pairs of CFW mice were obtained from Charles River Breeding Labs (Wilmington, MA, USA). Breeding pairs were housed in clear polycarbonate cages (28 × 17 × 14 cm3) lined with pine shavings. Purina rodent chow and tap water were freely available through a stainless steel wire lid that covered the cage. The vivarium was maintained at 21 ± 1°C, humidity 30–40%. A 12-h light/dark photocycle was used with lights off at 0700 hours. The procedures followed the “Principles of Laboratory Animal Care” (NIH publication no. 86-23, 1996) and were approved by the Institutional Care and Use Committee (IACUC) of Tufts University.
Maternal separation
Pair-housed females were checked twice a day for newborn litters. The day of birth was considered to be postnatal day 0 (PND 0). On PND 1, litters were culled to ten pups to standardize litter size and to reduce some of the variability between litters. Half of the litters were removed from their nest for 3 h/day from PND 1 to PND 14 (between 0900 and 1400 hours; maternal separation, MS; n = 14 litters). During the maternal separation procedure, each litter was placed into a separate plastic container containing a layer of nest shavings. The containers were placed atop a heating pad set at nest temperature (32–34°C) to prevent any additional stress due to hypothermia. After 3 h, litters were placed back into the nest and reunited with their parents. AFR animals were used as controls (n = 14 litters). AFR pups were also culled to ten pups per litter and were only handled during cage cleaning once a week. Each original breeding pair of mice contributed with two or three litters to the study. If their first litter went through the MS procedure, the second litter would go through the AFR control procedure, and vice versa. On PND 21, litters were weaned. The females were removed and littermate males were housed in groups of five to six in large clear polycarbonate cages (46 × 24 × 15 cm3). On PND 55, mice were individually housed in clear polycarbonate cages (28 × 17 × 14 cm3). On PND 60, they were assigned either to the three-bottle choice experiment or to an operant alcohol self-administration procedure (see scheme in Fig. 1). For each experimental procedure, different animals were used. Each litter contributed with one to two individuals for each separate experiment or group.
Three-bottle choice procedure
On PND 60, MS (n = 10) and AFR (n = 10) mice were allowed 24 h access to three 50-mL acrylic bottles fitted with stainless steel sipper tubes with ball-valve nipples to minimize spillage. The three bottles contained 0.05% saccharin, which was used as vehicle for the ethanol solutions. The next 2 days (PND 61–62), the access to these bottles was restricted to 2 h/day, while the regular water bottle was available for the remaining 22 h. Starting on PND 63, mice had 2 h/day access to 6% and 10% ethanol solutions diluted in 0.05% saccharin and a third bottle with 0.05% saccharin solution. The amount of solution consumed by each animal was determined from PND 63 to PND 72 by weighing the bottles every day. All measurements were made during the dark phase between 1000 and 1200 hours. The fluid intake data were corrected for spillage using bottles that were identically handled and placed in an unoccupied home cage for the same period of time daily. Bottle positions were alternated daily. During all procedures, mice had free access to food and fluid was available at all times.
Operant ethanol self-administration procedure
Apparatus
An experimental panel was inserted into the middle of the home cage and affixed to the side walls with two thumb screws at the start of each session (Miczek and de Almeida 2001). On the left and right sides of the panel, nose poke sensors were mounted 3 cm above the floor with stimulus lights 5 cm above. In the center of the panel, a cup for fluid delivery was located in a recess and connected to a syringe pump. A house light provided illumination (all devices were from Med Associates, St. Albans, VT, USA). The devices in the panel as well as the pump were controlled by a PC interface and associated Windows Med-PC software.
Acquisition and maintenance of ethanol self-administration
Initially, each nose poke response in one assigned hole (alternated between left and right sides) was reinforced with the delivery of 0.05 mL saccharin (0.05%) (fixed ratio schedule of reinforcement; FR1). After 4–6 days on FR1, the requirement was increased gradually to FR3 in the course of daily 30-min sessions. All mice were kept on FR3 for four to six sessions before any alcohol was added to the solutions, as described below.
Three different sets of mice were used in this study; each set consisted of one MS and one AFR group (n = 10–12 per group). One set of mice had only access to 0.05% saccharin solution throughout the whole experiment. To the other two sets of animals, alcohol was gradually added to the saccharin solution in 2% steps, up to 6% (second set of mice) or 10% (third set of mice). The daily experimental sessions were reduced to a length that allowed the animals to consume 1.0 g/kg ethanol with a maximal limit of 30 min/session, and similar fluid volume for the animals with access to saccharin only. Mice had free access to food and water at all times, except for the duration of the self-administration sessions. Once mice reached their respective final alcohol solution (6% or 10%), they were maintained on that solution for 4–5 days before the next phase. In total, the acquisition/maintenance phase lasted 14–17 days for all groups. During maintenance, sessions were limited in dosage (1 g/kg alcohol), so that animals from both AFR and MS groups would have similar histories of alcohol exposure before the subsequent testing phases.
Progressive ratio schedule of reinforcement and unlimited intake
During exposure to a progressive ratio (PR) schedule of reinforcement, mice were allowed to self-administer the same solutions used for the maintenance phase: alcohol (6% or 10% in 0.05% saccharin) or saccharin (0.05%) for three sessions. The algorithm used for the progression of the ratio requirement was: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178… (Richardson and Roberts 1996). The last completed ratio, which resulted in the final reinforcement delivery, was defined as the break point (Hodos 1961). The PR session ended when no reinforcement was obtained for a 60-min period. PR sessions occurred every other day, alternating with maintenance sessions in which animals self-administered their respective solution on the FR3 schedule with a maximal limit of 1.0 g/kg of ethanol (or similar fluid volume for animals with access to saccharin) or 30 min/session, whichever happened first. Break points were determined in three PR sessions for each mouse. After the PR sessions, a final FR3 session was carried out in which animals had 60 min access to their respective solution with unlimited intake.
Blood alcohol determination
At the conclusion of the study, an additional session limited to 1.0 g/kg ethanol was conducted. Fifteen minutes after the end of the session, animals were briefly anesthetized with isoflurane and approximately 0.1 mL blood was collected from the retroorbital venous sinus capillary bed. Plasma alcohol concentrations were measured using ultraviolet spectroscopy using a nicotinamide adenine dinucleotide-alcohol dehydrogenase enzyme assay (Sigma Chemical, St. Louis, MO, USA), as previously described by Miczek and de Almeida (2001).
Statistical analysis
Alcohol intake (in grams per kilogram) from the three-bottle choice experiment was analyzed using three-way repeated-measures analyses of variance (ANOVA), considering the group (MS vs. AFR), solutions (6% vs. 10% alcohol), and days (grouped into two blocks of 5 days) as factors. Overall fluid intake (in milliliters per kilogram) was also analyzed with a similar three-way ANOVA with repeated measures with three levels for the fluid factor (saccharin, 6% and 10% alcohol). Results from the operant alcohol self-administration were analyzed using two-way ANOVA, considering solutions (saccharin, 6% or 10% ethanol) and group (MS vs. AFR) as independent factors. When significant main effects were observed in the global ANOVA (p < 0.05), one- or two-way ANOVAs and/or Tukey post hoc tests were carried out. With an a priori hypothesis that significant group effects might differentially affect the intake of the different solutions offered, post hoc tests were performed in order to detect these specific effects, even in the absence of significant interactions between the factors. Results are portrayed as the means±standard error of means (SEM).
Results
No significant difference in body weight was observed between MS and AFR mice in both experiments.
Three-bottle choice
In order to simplify the analysis, the results from the three-bottle choice experiment were pooled into two blocks of 5 days each (n = 10 mice per group). Alcohol intake was analyzed in grams per kilogram body weight for the two different alcohol concentrations (6% and 10%) in two 5-day blocks (Fig. 2). A three-way ANOVA with repeated measures detected significant group (F (1,18) = 4.61; p < 0.05) and day–block differences (F (1,18) = 6.13; p < 0.05) with no significant effects for concentration or interactions between the factors, despite a tendency for a group × alcohol concentration interaction (F (1,18) = 3.36, p = 0.08). Maternally separated mice showed overall higher alcohol intake than AFR mice (p < 0.05), and this effect was specific to the 10% alcohol solution (F (1,18) = 5.22; p < 0.05 for the group factor).
Alcohol intake (grams per kilogram body weight) by MS and AFR mice using a three-bottle choice procedure, averaged over two 5-day blocks. Alcohol solutions were diluted in 0.05% (w/v) saccharin. MS maternal separation (gray bars), AFR animal facility rearing (white bars). *p < 0.05 when compared to AFR group
Overall, there was no group or day–block differences in total fluid intake (AFR = 50.11 ± 8.7 and 58.58 ± 6.9 mL/kg; MS = 63.53 ± 7.11 and 71.64 ± 8.8 mL/kg, in the two 5-day blocks, respectively). As shown in Table 1, there were no group differences in terms of volume of fluid intake (in milliliters per kilogram body weight) of the three different solutions (0.05% saccharin, 6% or 10% alcohol in 0.05% saccharin). The three-way ANOVA with repeated measures detected significant effects for fluid (F (2,36) = 3.49, p < 0.05) and day–block (F (1,18) = 8.79, p < 0.01) with no interactions between the factors. Tukey post hoc tests failed to show significant differences for the intake of the fluids with only a trend for a lower intake of the 10% alcohol solution relative to the other two solutions (p = 0.056 relative to saccharin, p = 0.09 relative to the 6% alcohol solution). Overall, there was a higher fluid intake on the second day–block relative to the first one (p < 0.01).
When the percentage of volume consumed for each fluid over the total fluid intake was analyzed with a three-way ANOVA, there were no significant effects for group, fluid, day–blocks, or interactions between the factors, as shown in Table 1.
Operant alcohol self-administration
After approximately 13 self-administration sessions (range 12–14 days), mice were reliably performing nose poke responses on an FR3 schedule of reinforcement and self-administering the respective final testing solutions (6% or 10% alcohol in 0.05% saccharin or just 0.05% saccharin). During the last 3 days of maintenance, the sessions were terminated at 30 min or the equivalent to a 1.0 g/kg alcohol dose, whichever occurred first. During this phase, there were no differences in number of reinforcements or intake per session (milliliters per kilogram or grams per kilogram for alcohol solutions) between the groups. In the last 3 days of maintenance, saccharin-reinforced AFR mice obtained 10.7 (±1.07) while MS had 10.8 (±0.83) reinforcements. For the 6% and 10% alcohol groups, respectively, AFR obtained 9.8 (±0.96) and 8.7 (±1.39) reinforcements, while MS mice obtained an average of 12.5 (±0.41) and 10.5 (±1.07).
Progressive ratio schedule of reinforcement
Figure 3 shows the number of reinforcements obtained on a PR schedule of reinforcement in MS and AFR mice. The ANOVA did not detect significant effects for group, fluid, or interaction between factors. MS mice attained slightly higher, but statistically nonsignificant, break points at the 10% alcohol+saccharin solution relative to AFR mice.
Fluid intake on a 60-min unlimited dosage session
A two-way ANOVA detected an overall group effect on fluid intake [F (1,57) = 11.63, p < 0.01] during the 60-min session: in general, MS mice presented higher fluid intake (in milliliters per kilogram) than their AFR counterparts. No significant effects were detected for fluid concentration or interaction between group and fluid concentration on fluid intake. Separate ANOVAs for each fluid were then conducted and revealed no significant differences between MS and AFR mice in the intake of 0.05% saccharin solution (AFR = 38.8 ± 7.0 mL/kg vs. MS = 61.2 ± 12.2 mL/kg; F (1,20) = 2.54). However, MS groups consumed significantly more of the alcohol solutions when compared to AFR controls, 44.1 ± 7.5 vs. 28.1 ± 2.2 mL/kg of the 6% alcohol solution (F (1,18) = 5.56, p < 0.05) and 54.0 ± 14.3 vs. 20.4 ± 5.3 mL/kg of the 10% alcohol solution (F (1,19) = 5.78, p < 0.05), respectively. This increased alcohol intake by MS mice was reflected in higher doses (in grams per kilogram) of alcohol from both the 6% (F (1,18) = 5.56, p < 0.05) and the 10% concentrations (F (1,19) = 5.78, p < 0.05) relative to AFR animals, as portrayed in Fig. 4. Doses of alcohol achieved were based on an extrapolation from the number of reinforcements received by each animal, and no leftover fluid was observed in the delivery cup after termination of the session.
Alcohol intake by AFR (white bars) and MS (gray bars) mice in 60-min sessions with unlimited dosage, using operant self-administration panels inserted in the home cage. a and b Cumulative intake of the 6% alcohol/0.05% saccharin solution by individual AFR (a) and MS (b) mice. c Alcohol intake (in grams per kilogram) of 6% alcohol solution, presented as group averages (+SEM). d and e Cumulative intake of 10% alcohol/0.05% saccharin solution by individual AFR (d) and MS (e) mice. f Alcohol intake (in grams per kilogram) of the 10% alcohol solution, presented as group averages (+SEM). *p < 0.05
Blood alcohol determination
Blood alcohol concentrations were determined after a session with 1.0 g/kg alcohol intake for groups consuming 6% or 10% alcohol solutions. Since there were no significant differences detected by the ANOVA for group, fluid, or interaction between the factors, results were pooled together for both 6% and 10% solutions. AFR attained 55.7 ± 6.7 mg/dL and MS reached an average of 47.1 ± 7.5 mg/dL.
Discussion
Using a three-bottle choice procedure, we found that MS mice showed higher alcohol intake than AFR, especially at the higher alcohol concentration (10% alcohol solution). No differences between the groups were found for the other solutions (6% ethanol or 0.05% saccharin) or total fluid intake. The alcohol solutions were diluted in 0.05% saccharin, but there was no fading in or out of the alcohol/saccharin concentrations. In these conditions, mice promptly self-administered considerable amounts of alcohol (total of 2.0 g/kg/2-h session and above) within a short period of time, and differences between the AFR and MS were detectable within 10 days of exposure to the alcohol solutions.
Even though differences in methodology and animal species were used, our results are in agreement with previous studies in rats showing that prolonged periods (>1 h/day) of maternal separation during the first weeks of life result in higher alcohol intake and/or preference in male adult rats (Gustafsson and Nylander 2006; Huot et al. 2001; Ploj et al. 2003; Roman and Nylander 2005; but see Jaworski et al. 2005). For instance, Huot et al. (2001) showed that adult rats maternally separated for 180 min/day from postnatal days 2–14 displayed higher alcohol intake than AFR rats, when 8% ethanol was offered in a sucrose solution. However, MS female rats do not seem to show enhanced alcohol consumption, suggesting gender-dependent effects of maternal separation (Gustafsson et al. 2005; Marmendal et al. 2004; Roman et al. 2004; Roman et al. 2005). In mice, a recent study reported no major effects of maternal separation on alcohol intake in both male and female C57BL/6J mice, an alcohol-preferring strain (Advani et al. 2007). In that study, only when MS female mice were exposed to the additional stress of postweaning social isolation there was a significant increase in ethanol preference, but this was not observed in males (Advani et al. 2007).
Although most studies use two bottles for the free-choice procedure to assess alcohol intake in MS animals, we used three bottles instead (adapted from Gustafsson and Nylander 2006). Thus, it was possible to evaluate differences in intake and preference for different concentrations of alcohol between the MS180 and AFR mice. Although a clear alcohol preference was not detected in our study, differences between MS and AFR mice were particularly evident in intake of the solution with the highest concentration of alcohol (10%). In humans, consumption of high doses of alcohol usually precede the development of alcohol tolerance and dependence (Orr et al. 1997). Despite the fact that high alcohol doses may promote aversion due to intoxication (Hansen et al. 1994; Richter and Campbell 1940), the use of higher alcohol concentrations seemed to be more sensitive to detect enhanced vulnerability to alcohol intake in MS mice. The possibility exists that a decreased sensitivity to alcohol’s aversive effects may have contributed for the increased intake of 10% alcohol solution observed in maternally separated mice.
Another critical feature in the present study was the use of limited access procedures to assess alcohol intake (as in Martinetti et al. 2006; Thanos et al. 2004). In rats, higher alcohol intake was found under limited access conditions (4 h/day) when compared to a continuous access condition (23 h/day) (Murphy et al. 1986). In C57BL/6J mice, a daily 2-h access period with 15% alcohol resulted in substantial intake that correlated well with blood alcohol levels achieved immediately after the session. Longer access periods may result in larger cumulative intake, but the relationship to resultant blood alcohol levels—and possibly to behavioral and pharmacological effects of alcohol—is not as tight (Lopez and Becker 2005; Middaugh et al. 2003). In a preliminary study in our laboratory, the use of a continuous access (24 h/day) to three bottles yielded more variable and unreliable results regarding alcohol intake in outbred mice (data not shown). The use of limited (2 h) vs. 24-h access may be another critical difference between our study and that of Advani et al. (2007), which did not report significant main effects of rearing conditions on alcohol intake with male or female C57BL/6J mice.
In the present study, an alternative approach to measure alcohol intake was used in parallel to bottle-drinking, making use of operant self-administration procedures. Operant drug-reinforced behavior in primates, rodents, and other species is considered to be a useful procedure to characterize drugs of abuse because nearly all drugs abused by humans have been found to be reinforcing in animals of various species (Griffiths et al. 1980; Johanson and Schuster 1980). An important advantage to bottle-drinking, the current operant conditioning procedures allow for precise control of volume delivered and consumed with virtually no spillage. Furthermore, different schedules of reinforcement were used (i.e., fixed and progressive ratio) that permit assessment of motivational (as in the PR schedule of reinforcement) and consummatory patterns of alcohol self-administration (such as in the 1-h access condition with unlimited dosage).
During operant alcohol self-administration, MS mice presented higher alcohol intake than AFR at the concentrations 6% and 10% during the 1-h session with no differences between MS and AFR in their intake of 0.05% saccharin solution. Although there was an overall increase in fluid intake by MS mice (in volume per kilogram) during this session, this seems unlikely to be a nonselective effect. Only the alcohol, but not saccharin, intake was significantly elevated in maternally separated mice with over 150% increases in intake of the 10% solution relative to AFR animals. No differences in the PR break points were found between MS and AFR mice for any of these solutions. Although the MS experience did not clearly change “motivational” aspects of alcohol reinforcement as indexed by the break point results, it affected the consummatory aspects, leading to increased alcohol consumption when animals were allowed to consume unlimited dosage for 1 h. To our knowledge, this is the first study that demonstrated increased operant alcohol self-administration while assessing consequences of maternal separation in adult mice.
In our study, the lack of differences in blood alcohol levels between AFR and MS mice after the consumption of a fixed 1.0 g/kg dose of alcohol suggests that alterations in the metabolism of alcohol are unlikely to account for the differences in intake observed. In rat studies, increased alcohol intake has been associated with long-term neural and neuroendocrine adaptations promoted by maternal separation. For instance, MS180 rats show increased responsiveness to the effects of citalopram, a selective serotonin uptake inhibitor, in suppressing the firing rate of neurons in the dorsal raphe nucleus (Arborelius et al. 2004). Furthermore, treatment with paroxetine, another serotonin uptake inhibitor, reversed the increased anxiety-like behavior and increased alcohol consumption presented by MS rats (Huot et al. 2001).
A significant modulation of HPA axis activity is proposed to occur as a function of short vs. prolonged periods of maternal separation (Plotsky and Meaney 1993; Pryce and Feldon 2003). In general, prolonged MS procedures have been associated with increased corticotropin-releasing factor levels in hypothalamic and extrahypothalamic sites and exaggerated adrenocorticotropic hormone and corticosterone responses to a stressor (Lippmann et al. 2007; Plotsky et al. 2005; Plotsky and Meaney 1993; but see Roman et al. 2006). Thus, future research will aim to identify neuroendocrine mechanisms associated with the enhancement of alcohol intake in MS mice.
Thus, in the present study, both operant self-administration and home cage bottle-drinking procedures detected increased alcohol intake in adult male mice which had been submitted to repeated maternal separation procedure for 3 h/day in the first 2 weeks of life. The current results support the hypothesis that early life stress may be a risk factor for the development of increased alcohol consumption and abuse for males, in agreement with human and nonhuman primate studies (Higley et al. 1996; Kendler et al. 2000; Veijola et al. 2008). The current results further validate the use of mice in maternal separation studies, confirming this procedure as a model of early environmental influence on alcohol intake in adulthood (Huot et al. 2001; Matthews and Robbins 2003; Ploj et al. 2003; Roman and Nylander 2005).
References
Advani T, Hensler JG, Koek W (2007) Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol 10:595–607
Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB (2004) Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology 176:248–255
Bowers WJ, Sabongui AG, Amit Z (1997) The role of ethanol availability on stress-induced increases in ethanol consumption. Alcohol 14:551–556
Boyce-Rustay JM, Janos AL, Holmes A (2008) Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res 186:133–137
Chester JA, Blose AM, Zweifel M, Froehlich JC (2004) Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res 28:385–393
Croft AP, Brooks SP, Cole J, Little HJ (2005) Social defeat increases alcohol preference of C57BL/6J strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 183:163–170
Dawson DA, Grant BF, Stinson FS, Chou PS (2005) Psychopathology associated with drinking and alcohol use disorders in the college and general adult populations. Drug Alcohol Depend 77:139–150
Gilmer WS, McKinney WT (2003) Early experience and depressive disorders: human and non-human primate studies. J Affect Disord 75:97–113
Griffiths RR, Bigelow GE, Henningfield JE (1980) Similarities in animal and human drug-taking behavior. In: Mello NK (ed) Advances in substance abuse. JAI, Greenwich, pp 1–90
Gustafsson L, Nylander I (2006) Time-dependent alterations in ethanol intake in male wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol Clin Exp Res 30:2008–2016
Gustafsson L, Ploj K, Nylander I (2005) Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav 81:506–516
Gustafsson L, Zhou Q, Nylander I (2007) Ethanol-induced effects on opioid peptides in adult male Wistar rats are dependent on early environmental factors. Neuroscience 146:1137–1149
Hansen S, Fahlke C, Hard E, Engel JA (1994) Adrenal corticosteroids modulate the consumption of ethanol in the rat. In: Palomo T, Archer T (eds) Strategies for studying brain disorders: depressive, anxiety and drug abuse disorders. Farand, London, pp 465–479
Higley JD, Suomi SJ, Linnoila M (1996) A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res 20:643–650
Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:943–944
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 158:366–373
Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ (2005) Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 181:8–15
Johanson CE, Schuster CR (1980) Animal models of drug self-administration. In: Mello NK (ed) Advances in substance abuse. JAI, Greenwich, pp 219–297
Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J (2007) Genetic and environmental predictors of early alcohol use. Biol Psychiatry 61:1228–1234
Kawakami SE, Quadros IM, Takahashi S, Suchecki D (2007) Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice. Behav Brain Res 184:109–116
Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA (2000) Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 57:953–959
Kikusui T, Faccidomo S, Miczek KA (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology (Berl) 178:202–210
Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159
Levine S (1957) Infantile experience and resistance to physiological stress. Science 126:405
Levine S (1967) Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156:258–260
Levine S (2001) Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav 73:255–260
Levine S (2005) Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 30:939–946
Linsky AS, Straus MA, Colby JP Jr. (1985) Stressful events, stressful conditions and alcohol problems in the United States: a partial test of Bales’s theory. J Stud Alcohol 46:72–80
Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM (2007) Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25:3091–3098
Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696
Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME (1999) The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol 7:318–323
Marmendal M, Roman E, Eriksson CJ, Nylander I, Fahlke C (2004) Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol 45:140–152
Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG (2006) Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, nonpreferring, and Sprague-Dawley rats. Alcohol Clin Exp Res 30:836–843
Matthews K, Robbins TW (2003) Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev 27:45–55
Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–1192
Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM (1999) The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol 11:925–933
Miczek KA, de Almeida RMM (2001) Oral drug self-administration in the home cage of mice: alcohol- heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 157:421–429
Miczek KA, Covington HE, Nikulina EA, Hammer RP (2004) Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev 27:787–802
Miczek KA, Yap JJ, Convington HE (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. J Pharmacol Exp Ther (in press) doi:10.1016/j.pharmthera.2008.07.006
Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW (2003) Chronic ethanol consumption by C57BL/6J mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res 27:1892–1900
Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73:321–330
Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK (1986) Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol 3:331–336
Orr TE, Walters PA, Elkins RL (1997) Differences in free-choice ethanol acceptance between taste aversion-prone and taste aversion-resistant rats. Alcohol Clin Exp Res 21:1491–1496
Overstreet DH, Knapp DJ, Breese GR (2007) Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res 31:1473–1481
Pacak K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548
Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL (2004) Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6J mice. Brain Res 1016:111–118
Ploj K, Roman E, Nylander I (2003) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121:787–799
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200
Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ (2005) Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30:2192–2204
Pryce CR, Feldon J (2003) Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 27:57–71
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Richter CP, Campbell KH (1940) Alcohol taste thresholds and concentrations of solution preferred by rats. Science 91:507–508
Roman E, Nylander I (2005) The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress 8:157–174
Roman E, Ploj K, Nylander I (2004) Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol 33:31–39
Roman E, Gustafsson L, Hyytia P, Nylander I (2005) Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res 29:591–601
Roman E, Gustafsson L, Berg M, Nylander I (2006) Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav 50:736–747
Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG (2003) Anxiety and fear behaviors in adult male and female C57BL/6J mice are modulated by maternal separation. Horm Behav 43:561–567
Sanchez MM, Ladd CO, Plotsky PM (2001) Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 13:419–449
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359
Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND (2004) DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res 28:720–728
Van Erp AMM, Miczek KA (2001) Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav 73:301–311
Van Erp AMM, Tachi N, Miczek KA (2001) Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol 12:335–342
Veijola J, Laara E, Joukamaa M, Isohanni M, Hakko H, Haapea M, Pirkola S, Maki P (2008) Temporary parental separation at birth and substance use disorder in adulthood: a long-term follow-up of the Finnish Christmas Seal Home Children. Soc Psychiatry Psychiatr Epidemiol 43:11–17
Volpicelli JR, Davis MA, Olgin JE (1986) Naltrexone blocks the post-shock increase of ethanol consumption. Life Sci 38:841–847
Wigger A, Neumann ID (1999) Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 66:293–302
Acknowledgements
This research was supported by NIAAA research grant AA0013983 (KAM, PI). FCC was supported by a fellowship from CAPES (Brazil; process no. 2456/06-0). We thank Mr. J. Thomas Sopko for the outstanding technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruz, F.C., Quadros, I.M., da S. Planeta, C. et al. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology 201, 459–468 (2008). https://doi.org/10.1007/s00213-008-1307-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1307-4