Abstract
Rationale
To date, there is no medication specifically approved for cocaine addiction. Agonist medications are used clinically in the treatment of other addictions, which suggests that this method of drug therapy could potentially be successful in treating cocaine addiction as well.
Objective
The objective of this study was to determine the effect of extended d-amphetamine treatment on responding on a progressive ratio (PR) schedule reinforced by cocaine.
Materials and methods
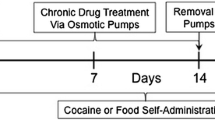
Rats were trained to self-administer cocaine (0.19, 0.38, 0.75, or 1.5 mg/kg/injection) or food on a PR schedule. After stable baseline breakpoints (the number of reinforcers earned in one session) were established over 3 days, animals were implanted with osmotic mini-pumps that continuously delivered d-amphetamine (5 mg/kg/day) for a duration of either 7 or 14 days. Breakpoints were then determined during and/or after this treatment period.
Results
Rats demonstrated dose-dependent decreases in cocaine-reinforced responding over the d-amphetamine treatment period. Breakpoints for doses of 0.75 mg/kg/injection and below decreased significantly when compared to baseline and remained decreased for up to 14 days after mini-pump removal, whereas those for the highest dose of cocaine remained unchanged. Additionally, d-amphetamine treatment during a 14-day abstinence period from cocaine self-administration had no effect on breakpoints when tested the day after mini-pump removal.
Conclusions
These data suggest that the reduction in cocaine-reinforced responding after continuous d-amphetamine treatment cannot be accounted for by tolerance alone. Instead, the roles of learning and the interaction between cocaine and d-amphetamine must be considered and examined in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, there is no medication approved by the FDA for the treatment of cocaine addiction (Preti 2007; Vocci et al. 2005); however, considerable effort continues to be directed toward the development of a pharmacological intervention to aid addicts in abstaining from cocaine use. A number of drugs and novel immunotherapies have shown promising results in clinical studies (O’Brien 2005; Vocci and Elkashef 2005), and further confirmatory trials are underway (Karila et al. 2008; Preti 2007).
One strategy that has been proposed is an agonist-like, replacement therapy for psychostimulant dependence (Grabowski et al. 2004b; Shearer 2008). This approach involves the use of a long-acting agonist with similar actions to cocaine in order to decrease cocaine craving and reduce cocaine use. This concept draws on studies showing beneficial effects of methadone or levo-alpha-acetyl-methadol treatment in the control of opiate dependence (Kreek and Vocci 2002) and the use of the nicotine patch to help with tobacco smoking cessation (Fiore 2000). To date, three double-blind randomized trials have provided promising clinical evidence that d-amphetamine may reduce cocaine intake and/or craving in cocaine-dependent individuals (Grabowski et al. 2001; Grabowski et al. 2004a; Shearer et al. 2003).
Preclinical studies have helped to clarify that the route and duration of d-amphetamine administration determines whether increases or decreases in cocaine-reinforced responding are observed. Repeated intermittent exposure to psychostimulants such as d-amphetamine has been shown to lead to increased locomotor activity, termed behavioral sensitization (Kalivas and Stewart 1991). This activity has been thought of as a manifestation of increased sensitivity to the drug and may underlie certain aspects of psychostimulant addiction (Robinson and Berridge 2001; Wise and Bozarth 1987). Treatment regimens that produce a sensitized locomotor response are also associated with increases in cocaine self-administration reinforced on a progressive ratio (PR) schedule (i.e., higher breakpoints), suggesting that d-amphetamine treatment actually increases the reinforcing strength of cocaine (Lorrain et al. 2000; Vezina et al. 2002). Furthermore, acute d-amphetamine treatment produces a leftward shift in the cocaine dose–response curve in discrimination (Li et al. 2006) and fixed ratio (Barrett et al. 2004) self-administration studies and acts as a ‘priming’ stimulus to reinstate cocaine-reinforced responding (Lynch et al. 1998; Schenk and Partridge 1999). While rodent studies using acute IP treatments show an augmentation of the reinforcing effects of cocaine, other studies using different routes of administration or more prolonged treatments have found that d-amphetamine leads to a decrease in cocaine intake. For example, twice daily SC injections for 7 days decreased responding for cocaine in rats (Peltier et al. 1996). In monkeys, oral d-amphetamine pretreatment decreased responding for a sweetened cocaine fluid (Foltin and Evans 1999). Similarly, both IM (Glowa et al. 1995) and IV (Mansbach and Balster 1993) d-amphetamine pretreatment decreased responding for IV cocaine. Thus, it appears that route and duration of d-amphetamine treatment have substantial influences on cocaine-reinforced responding and should be considered when studying the therapeutic potential of d-amphetamine.
Negus and Mello have examined the effect of slow IV infusions of d-amphetamine on cocaine self-administration in rhesus monkeys in order to test the hypothesis that constant blood levels of d-amphetamine over a prolonged period of time might have a therapeutic effect. They showed that d-amphetamine treatment decreased not only cocaine preference in a food-drug choice procedure (Negus 2003) but also cocaine self-administration reinforced under PR or second-order schedules (Negus and Mello 2003a, 2003b). Food intake was only transiently decreased by this treatment method (Negus and Mello 2003b), suggesting the possibility that the effect on cocaine might be relatively specific.
The present experiments were designed to adapt the primate model used by Negus and Mello (2003a) to rodents, and we confirm that slow delivery of d-amphetamine via osmotic mini-pump for 7 or 14 days decreases cocaine-reinforced responding under a PR schedule. To further characterize this treatment effect, we also assessed cocaine-reinforced responding in animals that had gone through a 14-day d-amphetamine treatment period in the absence of cocaine self-administration.
Materials and methods
Animals
Male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing approximately 350 g at the start of the experiments were used as subjects. Throughout the experiments, rats were maintained on a reverse 12-h light/dark cycle (lights on at 3 p.m.) with food and water available ad libitum. All rats were habituated to this schedule for a minimum of 3 days before entering the experiment. Animals in the cocaine self-administration experiments were housed individually in stainless steel custom-made experimental chambers (30 × 30 × 30 cm), whereas animals in the experiment using food as a reinforcer were pair-housed in polycarbonate cages.
Surgery
Prior to the beginning of the study, rats in the cocaine self-administration experiment were anesthetized via an IP injection of ketamine (100 mg/kg) and xylazine (8 mg/kg) and implanted with a chronically indwelling Silastic® jugular catheter (CamCaths, Cambridgeshire, UK). The catheter was connected to a plastic anchor that exited through the skin on the dorsal surface in the region of the scapulae. Tygon® tubing (enclosed by a stainless steel protective tether) was used to connect the catheter with a counterbalanced fluid swivel (Instech Laboratories, Plymouth Meeting, PA, USA) mounted above the experimental chamber. Tygon tubing also connected the swivel to an infusion pump (Razel Scientific Instruments, Stamford, Conn., USA). Animals were allowed 3–5 days to recover from surgery before beginning self-administration. Catheters were flushed daily throughout the experiments with heparinized saline in order to maintain patency.
After stable self-administration behavior was established (as described in the experiments below), all animals were implanted SC with an osmotic mini-pump (Alzet Model 2001, Durect, Cupertino, CA, USA; see Theeuwes and Yum 1976) filled with d-amphetamine or saline. Briefly, animals were anesthetized with a mixture of oxygen, nitrogen, and halothane (4%) and ventilated during surgery using halothane (1.5%). An incision in the skin was made between the scapulae (rostral to the plastic catheter anchor), and the mini-pump was inserted with the flow moderator pointing rostrally. The wound was closed using nylon sutures. The pump was removed 7 days later using the same procedure. Animals receiving 14 days of treatment were implanted with a second mini-pump, which was removed after 7 additional days.
Behavioral training
Cocaine self-administration
Experiments were conducted 7 days per week, and each daily session started at 10 a.m. The start of a session was indicated by extension of a single active lever into the experimental chamber. During training, cocaine (1.5 mg/kg/injection) was available on a fixed ratio 1 (FR1) schedule of reinforcement and was injected over approximately 4–5 s (depending on body weight) upon depression of the lever. Following each response, the lever was retracted and a light was illuminated for a 20-s post-response time-out period. Each training session lasted until 40 injections had been self-administered within 6 h, at which time the lever was retracted until the start of the next daily session. Self-administration training was termed complete following five consecutive daily sessions, during which the animal self-administered all 40 injections while maintaining consistent post-infusion pauses in responding between each of the injections.
Food-reinforced responding
Rats were removed from their home cages to be tested individually in experimental chambers (see above) 7 days per week. The extension of a single lever signaled the beginning of each session, and upon depression of this lever, one 45 mg sucrose pellet (Noyes) was delivered into a food hopper. The lever was then retracted, and a light was illuminated for a 20-s time-out period. Rats were trained to respond for sucrose pellets on an FR1 schedule, and training was considered to be complete following five consecutive daily sessions, during which the animal obtained 100 sucrose pellets within a 2-h testing period.
PR schedule of reinforcement
The PR schedule has proved useful in the study of treatments which might affect the reinforcing strength of cocaine. Responding on a PR schedule is sensitive to dose (0.19–1.5 mg/kg/inj), producing an ascending dose–response curve (Ward et al. 2005), which can be shifted by pharmacological, neurotoxic, and hormonal manipulations (Negus 2003; Roberts et al. 1989a; Roberts et al. 1989b). Following training, rats responded for cocaine or food (sucrose pellets) under a PR schedule. Under these conditions, delivery of the reinforcer was contingent upon an increasing number of responses incremented through the following ratio progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc. (Richardson and Roberts 1996). Breakpoints were defined in the 6-h cocaine experiments as the number of ratios completed (i.e., the number of reinforcers delivered) before a 1-h period in which no reinforcement was delivered. While animals typically reach breakpoints for the highest dose of cocaine (1.5 mg/kg/inj) within 3 h, pilot studies showed that animals normally reached breakpoints for sucrose pellets (45 mg) within 30 to 60 min regardless of session length. For this reason, each food-reinforcement session lasted only 2 h, and breakpoints were defined as the number of ratios completed (i.e., the number of sucrose pellets obtained) before a 15-min period in which no reinforcement was delivered. In all cases, animals reached their breakpoints before the end of the session.
Experiment 1: Effect of 7 days of d-amphetamine treatment on the dose/response curve for self-administered cocaine
The effect of 5 mg/kg/day SC infusion of d-amphetamine via osmotic mini-pump on cocaine-reinforced responding was investigated. After the training criterion was reached, animals were assigned to one of four groups (N = 8), each having access to a different unit dose of cocaine (0.19, 0.38, 0.75, and 1.5 mg/kg/inj) for the remainder of the experiment. Immediately following completion of a 3-day baseline period on the PR schedule, during which self-administration behavior did not vary by more than three breakpoints, animals were implanted with an osmotic mini-pump as described above (see “Surgery”). The mini-pumps were filled with d-amphetamine at a concentration which would result in the delivery of 5 mg/kg over 24 h, as determined by each subject’s body weight at the time of implantation. Following a 7-day treatment period (during which cocaine self-administration took place), the mini-pump was removed. Animals then completed daily PR testing for an additional 7 days to monitor the effect of discontinuation of d-amphetamine treatment on cocaine intake.
Experiment 2: Effect of 14 days of d-amphetamine treatment on cocaine self-administration
Experiment 2a: Cocaine self-administration before, during, and after an extended d-amphetamine treatment period (14 days)
This experiment extended experiment 1 by lengthening both the treatment period and the post-treatment period from 7 to 14 days and by incorporating saline mini-pump control groups. After the completion of training, baseline breakpoints on the PR schedule for either 0.75 or 1.5 mg/kg/inj cocaine were established in four groups of rats as in “Experiment 1”. All rats were then implanted with two consecutive 7-day mini-pumps containing either d-amphetamine (0.75 group: N = 9; 1.5 group: N = 8) or saline (0.75 group: N = 10; 1.5 group: N = 10). Breakpoints were evaluated during this 14-day treatment period. During the post-treatment period, rats self-administering 1.5 mg/kg/inj cocaine were evaluated for 7 days (as in “Experiment 1”), and rats self-administering 0.75 mg/kg/inj cocaine were evaluated for 14 days.
Experiment 2b: Cocaine self-administration before and after, but not during, a 14-day d-amphetamine treatment period
Similar to “Experiment 2a”, two groups of rats (N = 8) were trained, and stable baseline breakpoints for 0.75 mg/kg/inj cocaine were determined prior to the implantation of two consecutive 7-day mini-pumps containing either 5 mg/kg/day d-amphetamine or saline. During the 14-day treatment period, rats remained in their home experimental chambers where their catheters were flushed daily with heparinized saline (to maintain patency), but they did not have access to cocaine at this time. Cocaine self-administration resumed the morning after the second mini-pump was removed, and breakpoints for 0.75 mg/kg/inj cocaine were then evaluated throughout a 14-day post-treatment period.
Experiment 3: Effect of continuous d-amphetamine treatment on food-reinforced responding
The effect of 5 mg/kg/day SC infusion of d-amphetamine on food-reinforced responding on a PR schedule was investigated. Following training, baseline breakpoints reinforced by one 45 mg sucrose pellet on a PR schedule were determined in two groups of rats (N = 8). One group was then implanted with d-amphetamine (5 mg/kg/day) mini-pumps, and one group was implanted with saline mini-pumps. All mini-pumps were replaced after 7 days, and the subsequent mini-pumps were removed after an additional 7 days (see above). Breakpoints were assessed for 14 days during the treatment period and 14 days after removal of the second mini-pump.
Testing was conducted under conditions of 20-h food deprivation. Following each daily food-reinforcement session, rats were allowed unlimited access to rat chow for 2 h before being returned to the home cage. This procedure results in modest weight gain and a consistent deprivation state during each daily test (Roberts et al. 1996). The amount of food consumed as well as daily body weights were recorded for each animal.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse, Rockville, MD, USA. Cocaine was dissolved in sterile 0.9% saline (containing heparin, 10 USP units/ml) in concentrations of 0.625, 1.25, 2.5, and 5 mg/ml (expressed as the salt) and passed through a microfilter. d-Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline.
Data analysis
Conceptually, the final ratio (i.e., the response requirement for the last obtained reinforcer) is the measure of interest; however, final ratios taken from an exponential series usually violate the assumption of homogeneity of variance required of an analysis of variance (ANOVA; see Richardson and Roberts 1996). A log transformation can be performed on the final ratio values in order to conform to the assumptions of parametric statistics. In essence, this transformation simply yields values equivalent to the ordinal values of the final ratios (i.e., the number of reinforcers delivered). For this reason, the number of injections or sucrose pellets (breakpoint) was used as the dependent measure. All PR data were analyzed using a two-way ANOVA with repeated measures (SYSTAT Software). Paired t tests were used to analyze weight changes in each group of animals. Values of p < 0.05 were considered statistically significant, and Bonferroni tests were used in post hoc analyses.
Results
Animals acquired cocaine self-administration behavior after an average of 6.8 (±0.6) days on an FR1 schedule (data not shown).
Experiment 1
Figure 1a illustrates the effect of a constant infusion of d-amphetamine (5 mg/kg/day) via an osmotic mini-pump for 7 days on the cocaine dose–response curve. The mean breakpoints during the 3-day baseline testing period were compared to the mean breakpoints of the final 3 days of d-amphetamine treatment in this analysis. Repeated measures ANOVA revealed a significant effect of cocaine DOSE [F(3, 28) = 6.42, p < 0.01] and d-amphetamine TREATMENT [F(1, 28) = 24.7, p < 0.001] as well as a DOSE × TREATMENT interaction [F(3, 28) = 4.40, p < 0.05] thus indicating that the effect of d-amphetamine depended on the dose of cocaine self-administered. Inspection of Fig. 1a reveals that self-administration was greatly reduced at the lowest dose tested and was unaffected at the highest dose. Post hoc Bonferroni analysis confirmed a significant difference at the 0.19 mg/kg/inj dose (p < 0.001).
Effect of a continuous 7-day infusion of d-amphetamine (5 mg/kg/day) on the dose–response curve for cocaine self-administration reinforced on a PR schedule. a Points represent the mean (±SEM) breakpoints for cocaine averaged from a 3-day baseline period (open circles) or the last 3 days of d-amphetamine treatment (closed circles). Asterisk (*) indicates a significant difference from baseline. b Points represent the mean (±SEM) breakpoints measured in groups of animals (N = 8) self-administering various doses of cocaine. Days 1–3 represent a baseline period. Shaded portion (days 4–10) indicates the period during which the animals received a constant infusion of d-amphetamine. Days 11–17 represent the post-treatment period. The final ratio values corresponding to breakpoints are represented on the right y axis
Figure 1b illustrates the effect of d-amphetamine mini-pumps on cocaine self-administration over time. Repeated measures ANOVA, including the last day of baseline and the 7-day treatment period, revealed a significant effect of cocaine DOSE [F(3, 28) = 5.86, p < 0.01]. The main effect of DAY was statistically significant [F(7, 196) = 7.33, p < 0.001] as well as the DOSE × DAY interaction [F(21, 196) = 2.12, p < 0.01], indicating that the magnitude of change produced by 7 days of d-amphetamine depended on the unit injection dose of cocaine. In agreement with Fig. 1a, Fig. 1b suggests that the d-amphetamine mini-pump produced a gradual decrease in breakpoints at the lower cocaine doses, whereas responding reinforced by the highest dose of cocaine (1.5 mg/kg/inj) was not significantly affected. In the post-treatment recovery analysis, there was a significant effect of DOSE [F(3, 28) = 8.30, p < 0.001] and DAY [F(7, 196) = 9.02, p < 0.001], but no interaction between the two factors.
Experiment 2
Figure 2a depicts the effect of extended treatment (14 days) with continuous d-amphetamine or saline on responding for 1.5 mg/kg/injection cocaine. Repeated measures ANOVA, including the last day of baseline and the 14-day treatment period, failed to find significant main effects of either TREATMENT or DAY, but revealed a significant TREATMENT × DAY interaction [F(14, 224) = 2.58, p < 0.01] for groups self-administering 1.5 mg/kg/inj cocaine. This is accounted for by the small transient increase in breakpoints for the d-amphetamine group on days 2–4 of the treatment period. There were no significant findings in the post-treatment recovery analysis for groups self-administering 1.5 mg/kg/inj cocaine.
Effect of a continuous 14-day infusion of d-amphetamine (5 mg/kg/day) on cocaine self-administration reinforced on a PR schedule. Points represent the mean (±SEM) breakpoints measured in groups of animals self-administering either a 1.5 mg/kg/inj cocaine or b 0.75 mg/kg/inj cocaine. Days 1–3 represent a baseline period. Shaded portion (days 4–17) indicates the period during which the animals received either a constant infusion of d-amphetamine (closed symbols) or saline (open symbols). Days 18–31 represent the post-treatment period. Circles and squares represent animals that self-administered cocaine throughout the experiment, and triangles represent animals that did not have access to cocaine during the 14-day treatment period. The final ratio values corresponding to breakpoints are represented on the right y axis
Figure 2b shows the effect of a 14-day treatment period with continuous d-amphetamine or saline on responding for 0.75 mg/kg/inj cocaine that occurred during and/or after the treatment period. As expected, all four groups had statistically similar baseline breakpoints. Repeated measures ANOVA, including the last baseline day and the 14-day treatment period for groups self-administering cocaine during the mini-pump treatment period, revealed a significant effect of mini-pump TREATMENT [F(1, 17) = 28.72, p < 0.001]. The main effect of DAY was statistically significant [F(14, 238) = 8.68, p < 0.001] as well as the TREATMENT × DAY interaction [F(14, 238) = 7.58, p< 0.001], indicating that breakpoints decreased more over time in animals treated with d-amphetamine. Repeated measures ANOVA during the post-treatment period for all four groups of animals revealed a significant effect of GROUP [F(3, 28) = 9.33, p < 0.001] and a GROUP × DAY interaction [F(39, 364) = 2.17, p < 0.001]. Inspection of Fig. 2b shows that animals that did not self-administer cocaine while being treated with d-amphetamine were unaffected by the d-amphetamine alone as their breakpoints were similar to those reached by animals that received saline mini-pumps. Post hoc analysis revealed that post-treatment breakpoints were significantly lower in animals that self-administered cocaine while being treated with d-amphetamine.
Experiment 3
Figure 3 illustrates the effect of 14 days of continuous d-amphetamine treatment on food-reinforced responding on a PR schedule over time, as compared to saline treatment. Repeated measures ANOVA, including the final baseline day and the 14-day treatment period, found significant main effects of both d-amphetamine TREATMENT [F(1, 14) = 7.98, p < 0.05] and DAY [F(14, 196) = 2.16, p < 0.05], but no significant TREATMENT × DAY interaction [F(14, 196) = 1.57, p < 0.10], which indicates that the d-amphetamine-treated animals consistently reached higher breakpoints than the saline-treated animals throughout the treatment period. Similarly, post-treatment recovery analysis revealed main effects for TREATMENT [F(1, 14) = 5.37, p < 0.05] and DAY [F(14, 196) = 2.37, p < 0.01], but no significant TREATMENT × DAY interaction [F(14, 196) = 1.07, ns], indicating that breakpoints for d-amphetamine-treated rats remained elevated above those for saline-treated rats for 14 days after mini-pump removal.
Effect of a continuous 14-day infusion of d-amphetamine (5 mg/kg/day) or saline on food intake reinforced on a PR schedule. Points represent the mean (±SEM) breakpoints measured in two groups of animals (N = 8) responding for 45 mg sucrose pellets on a PR schedule. Days 1–3 represent a baseline period. Shaded portion (days 4–17) indicates the period during which the animals received either a constant infusion of d-amphetamine (closed circles) or saline (open circles). Days 18–31 represent the post-treatment period. The final ratio values corresponding to breakpoints are represented on the right y axis
Body weights before implantation and after removal of the mini-pumps were compared. Paired t tests did not reveal any change in weight as a result of 7 days of d-amphetamine treatment for animals self-administering any dose of cocaine in experiment 1. As shown in Table 1, the baseline weights of d-amphetamine-treated animals self-administering 0.75 mg/kg/inj cocaine or food for 14 days did not significantly differ from their corresponding saline control groups before mini-pump implantation. Paired t tests revealed a significant increase in weight for animals self-administering 0.75 mg/kg/inj cocaine regardless of mini-pump treatment [d-amphetamine: t = −2.67, df = 8, p < 0.05; saline: t = −5.34, df = 9, p < 0.001]. For animals responding for food, there was a significant decrease in weight only in animals treated with d-amphetamine [t = 4.27, df = 7, p < 0.01].
Discussion
The present experiments explored the effects of a slow subcutaneous infusion of d-amphetamine, via osmotic mini-pump, on cocaine self-administration reinforced under a PR schedule. Breakpoints for only the lowest dose of cocaine (0.19 mg/kg/inj) were significantly decreased by 7 days of d-amphetamine treatment. A trend was observed with moderate doses, which failed to reach significance, and the highest dose (1.5 mg/kg/inj) remained unaffected. Extending the treatment period to 14 days significantly decreased breakpoints for a moderately high dose of cocaine (0.75 mg/kg/inj), but again, breakpoints for the highest dose of cocaine remained unchanged. Moreover, 14 days of d-amphetamine treatment when animals did not have access to cocaine had no effect on subsequent cocaine self-administration.
The present experiments replicate, in rats, a phenomenon reported by Negus and Mello (2003a) in rhesus monkeys given extended treatment with d-amphetamine. In that study, d-amphetamine (0.01–0.1 mg/kg/h) was delivered every 20 min over 10 days using a double lumen IV catheter, while subjects self-administered a single dose of cocaine (0.032 mg/kg/inj) under a PR schedule. d-Amphetamine treatment was shown to produce a dose-dependent decrease in cocaine-reinforced responding. Negus and Mello (2003b) also showed a greater reduction in responding for cocaine doses on the low end of the dose–response curve during d-amphetamine treatment using a second-order schedule, a procedure that the authors consider to reveal similar information about potential pharmacotherapies as PR (Negus and Mello 2003a). Cocaine-maintained responding in both of these studies appeared to progressively decrease over the d-amphetamine treatment period, similar to findings in the present study.
The results from the present study, as well as those from the studies mentioned above (Negus and Mello 2003a, 2003b), raise a question as to how d-amphetamine treatment leads to decreased cocaine self-administration in animals. One possibility, suggested by Peltier et al. (1996), is that extended exposure to d-amphetamine causes cross-tolerance to the reinforcing effects of cocaine. In their study, d-amphetamine was administered by a subcutaneous injection (3.2 mg/kg) every 12 h for 7 days, and post-treatment breakpoints for cocaine were reduced when compared to baseline. These results are similar to those from the present study; both sets of data showed that d-amphetamine treatment produced the greatest effect at the lowest unit injection dose of cocaine.
Strictly speaking, cross-tolerance implies that tolerance to a specific effect of one drug has an impact on the same effect of another drug. Neither of the studies mentioned above (Negus and Mello 2003a; Peltier et al. 1996) nor the present study specifically evaluated the reinforcing effects of continuously delivered d-amphetamine, so any reference to cross-tolerance is merely a speculation. Regardless, the general idea of tolerance would not appear to completely account for the data from the present study. If exposure to d-amphetamine created tolerance to the reinforcing effects of cocaine, one would expect all animals similarly treated with the same amount of d-amphetamine to demonstrate similar decreases in the reinforcing strength of the same dose of cocaine. In “Experiment 2b”, animals that self-administered cocaine during the treatment period showed decreased breakpoints but animals that were not permitted to self-administer cocaine during the treatment period did not show a change in cocaine-reinforced responding when subsequently assessed. Therefore, the explanation must necessarily involve a combination between d-amphetamine and cocaine. Intuitively, one would expect that the combination of d-amphetamine plus self-administered cocaine would create more tolerance than d-amphetamine alone. While this explanation may apply to “Experiment 2b”, one would expect to see the greatest amount of tolerance (i.e., greatest decrease in breakpoints) for animals self-administering the most cocaine, which would be at the highest dose. The results from “Experiment 2a” show that this was not the case.
A cocaine injection can be thought of as a compound stimulus having both positive and aversive properties, and thus, its reinforcing strength reflects the net effect of these competing features (Ettenberg 2004; Wheeler et al. 2008). Therefore, the observed reductions in breakpoints in the present study might be explained by either a decrease in the positive reinforcing effects (as mentioned above) or an augmentation of anxiogenic or other limiting/aversive effects, or a combination of the two. The gradual diminishment of breakpoints observed during the d-amphetamine treatment period appears to reflect the process by which animals learn of a change in the net effect of cocaine. If d-amphetamine treatment primarily enhanced the aversive properties of self-administered cocaine in the present study, one would expect the greatest enhancement for the highest dose of cocaine. However, breakpoints for 1.5 mg/kg/inj cocaine did not decrease during the treatment period. As with the concept of decreased reinforcing strength mentioned above, this idea cannot fully explain the treatment effect.
It is important to note that breakpoints for 0.75 mg/kg/inj cocaine recovered only gradually after d-amphetamine treatment and never fully reached baseline levels. This could have important therapeutic implications. It is not clear what factors control this recovery process, although it is unlikely that it is simply the clearance of d-amphetamine, which would occur in a few days. We speculate that the long recovery process may have to do with negative associative effects that diminish only slowly after d-amphetamine is cleared.
Food-maintained responding has typically been assessed in many studies investigating the therapeutic potential of d-amphetamine as a pharmacotherapy for cocaine abuse (Barrett et al. 2004; Negus 2003; Negus and Mello 2003a, 2003b). It was included in the present study to evaluate the possibility that d-amphetamine might non-specifically disrupt responding or perhaps produce stereotyped behavior directed toward the lever. We chose to use 45 mg sucrose pellets as a food reinforcer because in previous studies, we have shown that they support similar baseline breakpoints as the moderately high dose of cocaine reported here (Roberts et al. 1996). Even though animals reached similar baseline breakpoints for food and cocaine (0.75 mg/kg/inj) in the present study, d-amphetamine treatment was found to have opposite effects on food- and cocaine-reinforced responding. These data, in combination with the body weight data, add useful information on the effects of extended treatment with d-amphetamine and suggest that the present dose delivered via mini-pump is not debilitating or overtly toxic. However, much has been written about the problems associated with comparing cocaine- and food-reinforced responding as a way to assess selectivity of a potential pharmacotherapy (Barrett et al. 2004). While the present food data is generally in agreement with the observations of Negus and Mello (Negus 2003; Negus and Mello 2003a, 2003b), we are reluctant to draw strong conclusions about specificity. In light of the unanticipated results of “Experiment 2b”, which imply the necessity of the combination of d-amphetamine and cocaine self-administration in reducing the reinforcing effects of cocaine, it appears that the effect of d-amphetamine treatment alone on food-reinforced responding is tangential to the issue.
Further work is necessary to distinguish between the contribution of a learned component versus other pharmacological interactions that might occur with simultaneous cocaine and d-amphetamine administration (see Jayanthi and Ramamoorthy 2005; Scarponi et al. 1999; Ukairo et al. 2007). Whatever the mechanism, cocaine self-administration was shown here to be suppressed for up to 14 days after mini-pump removal. To the degree that decreases in cocaine-reinforced responding in rats apply to the development of medications for cocaine addiction, it appears that there are interactions between cocaine and d-amphetamine that could be exploited.
References
Barrett AC, Miller JR, Dohrmann JM, Caine SB (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47(Suppl 1):256–273
Ettenberg A (2004) Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 27:721–728
Fiore MC (2000) US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care 45:1200–1262
Foltin RW, Evans SM (1999) The effects of d-amphetamine on intake of food and a sweet fluid containing cocaine. Pharmacol Biochem Behav 62:457–464
Glowa JR, Wojnicki FHE, Matecka D, Rice KC, Rothman RB (1995) Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: II. Comparisons with other drugs and repeated administrations. Exp Clin Psychopharmacol 3:232–239
Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG (2001) Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol 21:522–526
Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J (2004a) Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology 29:969–981
Grabowski J, Shearer J, Merrill J, Negus SS (2004b) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464
Jayanthi LD, Ramamoorthy S (2005) Regulation of monoamine transporters: influence of psychostimulants and therapeutic antidepressants. AAPS J 7:E728–738
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244
Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP (2008) New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol 11:425–438
Kreek MJ, Vocci FJ (2002) History and current status of opioid maintenance treatments: blending conference session. J Subst Abuse Treat 23:93–105
Li SM, Campbell BL, Katz JL (2006) Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther 317:1088–1096
Lorrain DS, Arnold GM, Vezina P (2000) Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res 107:9–19
Lynch WJ, Heaser WA, Carroll ME (1998) Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp Clin Psychopharmacol 6:255–263
Mansbach RS, Balster RL (1993) Effects of mazindol on behavior maintained or occasioned by cocaine. Drug Alcohol Depend 31:183–191
Negus SS (2003) Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology 28:919–931
Negus SS, Mello NK (2003a) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology 167:324–332
Negus SS, Mello NK (2003b) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend 70:39–52
O’Brien CP (2005) Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiat 162:1423–1431
Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW (1996) Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther 277:212–218
Preti A (2007) New developments in the pharmacotherapy of cocaine abuse. Addict Biol 12:133–151
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Roberts DC, Andrews MM, Vickers GJ (1996) Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15:417–423
Roberts DCS, Bennett SA, Vickers GJ (1989a) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology 98:408–411
Roberts DCS, Loh EA, Vickers GJ (1989b) Self-administration of cocaine on a progressive ratio schedule in rats: dose–response relationship and effect of haloperidol pretreatment. Psychopharmacology 97:535–538
Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Scarponi M, Bernardi G, Mercuri NB (1999) Electrophysiological evidence for a reciprocal interaction between amphetamine and cocaine-related drugs on rat midbrain dopaminergic neurons. Eur J Neurosci 11:593–598
Schenk S, Partridge B (1999) Cocaine-seeking produced by experimenter-administered drug injections: dose–effect relationships in rats. Psychopharmacology 147:285–290
Shearer J (2008) The principles of agonist pharmacotherapy for psychostimulant dependence. Drug Alcohol Rev 27:301–308
Shearer J, Wodak A, van BI, Mattick RP, Lewis J (2003) Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction 98:1137–1141
Theeuwes F, Yum SI (1976) Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann Biomed Eng 4(4):343–353
Ukairo OT, Ramanujapuram S, Surratt CK (2007) Fluctuation of the dopamine uptake inhibition potency of cocaine, but not amphetamine, at mammalian cells expressing the dopamine transporter. Brain Res 1131:68–76
Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N (2002) Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci 22:4654–4662
Vocci FJ, Acri J, Elkashef A (2005) Medication development for addictive disorders: the state of the science. Am J Psychiatry 162:1432–1440
Vocci FJ, Elkashef A (2005) Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry 18:265–270
Ward SJ, Morgan D, Roberts DC (2005) Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology 30:286–295
Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM (2008) Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron 57:774–785
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding provided by Grants F30DA18014 (CML), R01DA14030 (DCSR), and P50DA06634-14 (DCSR).
Keri A. Chiodo* and Christopher M. Läck* contributed equally to this project and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Chiodo, K.A., Läck, C.M. & Roberts, D.C.S. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous d-amphetamine treatment in rats. Psychopharmacology 200, 465–473 (2008). https://doi.org/10.1007/s00213-008-1222-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1222-8