Abstract
Rationale
Many antipsychotics cause orthostatic hypotension possibly due to antagonist action on resistance vessel α1A-adrenoceptors (α1A-AR).
Objective
We have tested this possibility by determining in Wistar rats how the orthostatic hypotensive effect of several antipsychotic drugs compares with their affinity for adrenoceptors in mesenteric small arteries (MSA with mainly α1A-AR) and aorta (mainly α1D-AR).
Materials and methods
Using a tilt setup, orthostatic hypotension was measured in anaesthetized rats for prazosin and the antipsychotics haloperidol, sertindole, risperidone, clozapine, ziprasidone, domperidone, olanzapine, and aripiprazole. For in vitro studies, segments of MSA and aorta were mounted on a wire myograph for isometric tension recording. Cumulative concentration-response curves were constructed to phenylephrine (PE) in the absence and presence of the drugs. Apparent affinity (pA 2) was calculated by Schild analysis.
Results
Prazosin antagonized tilt-induced and PE responses in both studies (threshold 4 ng/ml, pA 2 9.52 MSA, 10.1 aorta). The rank order of the potency of the antipsychotics in the tilt experiments correlated (r 2 = 0.69, P = 0.01) with the pA 2-values in MSA: Risperidone and sertindole had the highest potency in the tilt test (threshold 159 and 97 ng/ml) and the highest apparent affinity in MSA (pA 2 8.92 and 8.78), in contrast with aripiprazole and domperidone, which had the lowest in each case (threshold 4.1 and 3.0 μg/ml, pA 2 7.17 and 6.99). In aorta, the pA 2 values did not correlate with the in vivo potencies; in particular, sertindole had no functional affinity in aorta.
Conclusion
We conclude that the orthostatic hypotensive effect in rats of the antipsychotic drugs investigated is mediated through α1A-ARs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antipsychotic drugs are used in the treatment of schizophrenia, a common psychotic disease that affects around 0.7–1% of the world population (Williams et al. 2004). Almost all available antipsychotic drugs are dopamine D2-receptor antagonists, and it is generally accepted that antipsychotic effects are mediated through blockade of mesolimbic D2 dopaminergic receptors (Lewis and Lieberman 2000). Nevertheless, most antipsychotic drugs have effects on other receptors such as serotonin 5-HT2 receptors, muscarinic (M2) receptors, and α-adrenoceptors, particularly α1-adrenoceptors (Buckley and Sanders 2000; Svensson 2003). Extrapyramidal side effects (EPS) in patients under treatment with typical antipsychotic drugs such as haloperidol are mediated via striatal dopamine D2 receptor blockade (Lidow et al. 1998). These side effects are reduced for the atypical drugs such as clozapine, possibly because of lower affinity of atypical antipsychotics for D2-receptors and greater affinity for 5HT2-receptors (Strange 2001).
In addition to EPS, the atypical antipsychotics like the typical ones frequently have cardiovascular side effects such as increase in the QT interval of the electrocardiogram and orthostatic hypotension (Buckley and Sanders 2000). The latter may be caused by their antagonist action against α1-adrenoceptors (α1-AR). The importance of the α1 component of these drugs as regards their antipsychotic effects has been studied intensively (Lane et al. 1988; Prinssen et al. 1994; Wadenberg et al. 2000). However, the role of α1-AR subtype with respect to their cardiovascular side effects, especially orthostatic hypotension, has received little attention, and to our knowledge, there are no data to show a direct comparison between in vivo and in vitro effects of antipsychotics. We have therefore investigated the hypothesis that the ability of antipsychotic drugs to cause orthostatic hypotension is correlated to their α1-AR antagonist affinity (especially α1A-AR) on resistance vessels.
The hypothesis has been tested by comparing the rank order of a range of antipsychotic drugs both in vivo in an animal tilt model of orthostatic hypotension (Take et al. 1998; Hashimoto et al. 1999; Hieble et al. 1999; Akiyama et al. 2002; de Moura et al. 2005), and in vitro using isolated rat mesenteric small arteries (MSA) studied in a myograph (Mulvany and Halpern 1977). These latter vessels are important in the control of blood pressure (Fenger-Gron et al. 1995) and contain mainly α1A-AR (Ipsen et al. 1997). In the in vivo experiments, the rank order of the drugs was based on the lowest concentration to give a detectable orthostatic hypotension response. In the in vitro experiments, rank order was based on the apparent affinity (pA 2) of the drugs for α-AR, determined using Schild analysis of the contractile responses to PE. For comparison, we also studied the α-AR affinities in rat thoracic aorta, a vessel containing predominantly α1D-AR (Hussain and Marshall 1997; Marti et al. 2005).
Materials and methods
This study was conducted in accordance with the current Danish Animal Testing Act.
In vivo experiments
Tilt setup
Male normotensive Wistar rats (220–250 g) were anesthetized with administration of pentobarbital (1%, 50 mg/kg) intraperitoneally, in a volume of 5 ml/kg. During the surgical implantation of the catheters, the anesthesia was supplemented with isoflurane. The left common carotid artery was cannulated with a polyethylene tube to monitor the systemic arterial pressure and for final blood sampling. Systemic arterial pressure was monitored by a pressure transducer (SensoNor 844, SensoNor, Norway) placed at the heart level so that the tilting itself did not influence blood pressure measurement. The left jugular vein was cannulated for administration of test drugs or vehicle. Then, the rats were fixed in the supine position with the fulcrum of the customized tilt table at the level of the heart.
In vivo experimental procedure
Observation of the tilt-induced blood pressure response in rats has previously been described (Take et al. 1998; Hashimoto et al. 1999; Hieble et al. 1999; Akiyama et al. 2002; de Moura et al. 2005). In this study, the method has been customized as follows. Rats were rapidly (within 1–2 s) subjected to a consistent 60° head-up tilt from the horizontal position. This was considered as the pre-dose tilt. If a normal blood pressure response was not observed (immediate tilt-induced blood pressure <85% of pre-tilt blood pressure and compensatory blood pressure response >90% of pre-tilt blood pressure) within three pre-dose tilts (10 min apart), the rat was omitted from the study. If more than one pre-dose tilt was performed, the final tilt was used in the analysis. Vehicle or test drugs were administered 5 min after the pre-dose tilt. A similar post-dose tilt was initiated 10 min after the test drug had been administered. Each tilt was timed to last 60 s.
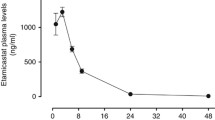
Each rat was injected once intravenously (i.v.) with one of ten different compounds: vehicle (10% hydroxypropyl-β-cyclodextrin, HPβC), prazosin (0.0011 to 0.044 mg/kg), clozapine (0.1 to 3 mg/kg), ziprasidone (0.01 to 1 mg/kg), sertindole (0.1 to 5 mg/kg), aripirazol (1 to 30 mg/kg), haloperidol (0.3 to 10 mg/kg), risperidone (0.01 to 1 mg/kg), olanzapine (0.01 to 3 mg/kg), and domperidone (0.3 to 10 mg/kg). Each drug was tested at three to six dose levels (two to five animals per dose level). Blood samples were collected immediately after the end of the post-dose tilt for measurement of drug plasma concentrations.
Analysis of drug plasma concentration
Analysis of plasma concentration for all tested drugs was performed using turbulent flow chromatography followed by detection using mass spectrometry (API 3000, MDS Sciex, Canada) according to a method described early (Sanchez and Kreilgaard 2004). The concentration of compounds in plasma was determined by standard calibration curves (1–1,000 ng/ml) for each analysis prepared using ethylene diamine tetra-acetic acid (EDTA)-treated plasma from untreated animals.
In vivo data analysis
Continuous recording of blood pressure and mean arterial blood pressure (MAP) was performed. Exact values were measured immediately before tilt (baseline at 0 s) and at 2, 5, 10, 20, 30, 40, 50 and 60 s after the start of tilt. Due to marked fall in MAP caused by i.v. injections of the test drugs, the tilt response was expressed with reference to the baseline value before the tilt (100%) plotted against time after the start of tilt, from which the area under the curve from 0 to 60 s (AUC0–60) was calculated. The effect of the dose of test drug on the tilt response was taken as ΔAUC0–60 = AUC0–60 (pre-dose tilt)-AUC0–60 (post-dose tilt). All values are presented as mean ± SEM.
Statistical analyses of data were performed using a computer program (GraphPad Prism, San Diego, CA, USA). A nonparametric test (Kruskal-Wallis) was used for each drug to simultaneously determine significance over all dosing levels. If there was overall significance, individual comparisons were made to determine the possible significance of differences at each dosing level, compared to vehicle (Dunn’s test). Statistical significance was set at p < 0.05. In the vehicle-administered animals, the pre-dose tilt was compared to the post-dose tilt by a Student’s t test. For each drug, the plasma concentration of the lowest dose that was found to be significantly different from vehicle has been selected as an estimate of the potency of each drug and, thereby, to generate the rank order between the drugs.
In vitro experiments
Rat isolated small mesenteric artery and aorta preparation
Male Wistar rats (300–350 g) were killed by cervical dislocation, after which the mesentery or the aorta was removed immediately and placed in cold physiological salt solution (PSS 4°C) of the following composition (mM): NaCl 119, KCl 4.7, CaCl2 1.6, MgSO4.7H2O 1.17, NaHCO3 25, glucose 5.5, KH2PO4 1.18, and EDTA 0.026. The solution was gassed with 5% CO2 in air. Segments of thoracic aorta and mesenteric small arteries (approximately 2 mm) cleaned of surrounding adipose tissue were mounted as ring preparations on two 100- or 40-μm wires, respectively, in a wire myograph (J.P. Trading, Aarhus, Denmark). The vessels were allowed to equilibrate in PSS (thermostatically controlled at 37 ± 0.5°C for at least 30 min).
After the stabilization period, the internal diameter of each vessel was set to an extension equivalent to 0.9 times the estimated diameter at 100 mmHg effective transmural pressure (l100 = 200–250 μm) according to the standard procedure of Mulvany and Halpern (1977). After examination of viability of vessels, as described below, the endothelium was removed by gently rubbing the lumen with a hair to confirm that the main function of the antipsychotic drugs was the result of their action on smooth muscle cells not on the endothelium. Removal of the endothelium was confirmed by the presence and absence of relaxation to acetylcholine (ACh, 10−5 M), before and after removal, in mesenteric arteries precontracted with noradrenaline (NA, 10−5 M). The presence of endothelium in rat thoracic aorta, before and after removal, was tested using phenylephrine (PE) 10−6 M as preconstrictor then ACh (3 × 10−6 M). Experiments were only performed on vessels that showed less than 10% relaxation after endothelial removal. In some experiments, where indicated, the endothelium was not removed.
Experimental procedure
Mesenteric small arteries
After standardization of the internal diameter, the viability of vessels was examined by exposing them to NA 10−5 M three times, 2 min per activation, with washouts after each exposure. As described above, the function of the endothelium was assessed after the third exposure to NA. After removal of endothelium, the vessels were incubated with propranolol (10−6 M) and cocaine (3 × 10−6 M) for at least 10 min to block β-AR and neuronal uptake of NA, respectively.
Initially, a PE concentration-response curve was obtained by adding PE from 0.02 to 640 μM in twofold increments to the bath every 2 min. The vessels were then washed to baseline and incubated with test drug for 30 min. A new concentration–response curve for PE was constructed in the presence of the drug. Only one concentration of the drug was used for each vessel.
Aorta
The standard start for testing the viability of aorta preparations was made by exposing them to NA (10−6 M) three times for 3 min per activation. After testing the function of endothelium and its removal, as described above, a curve for PE (3 × 10−9 M to 3 × 10−5 M) was constructed in control condition (with vehicle) and in the presence of propranolol (10−6 M), and yohimbine (10−7 M), cocaine (6 × 10−6 M), and corticosterone 21-acetate (CCA, 10−6 M) to block β-AR, α2-AR, neuronal, and extra neuronal uptake of NA, respectively. After the washing and incubation of the vessels with antagonist for 30 min, the second concentration–response curve for PE was constructed as described above.
In vitro data analysis
The mechanical responses of the vessels were measured as force and expressed as active wall tension, which is the increase in measured force divided by twice the segment length (Mulvany and Halpern 1977). Responses are plotted graphically as means from at least four separate experiments (one vessel per animal in each experiment), with vertical lines representing SEM. When error bars do not appear on the figures, this is because they are small and fall within the dimension of the symbols. Curves were fitted (GraphPad Prism, San Diego, CA, USA) to all the data by nonlinear regression to determine Hill slopes for the agonist concentration–response curves and to calculate pD 2 values (−log of the EC50 values). The EC50 value in the presence and absence of test drug (antagonist) was used to determine the concentration-ratio (CR). pA 2 values were calculated by linear regression by use of the same computer program and were obtained from the x-intercept of the plot of log (CR-1) against log molar antagonist concentration (Arunlakshano and Schild 1959). Slope values were evaluated for statistical difference from unity by t test. Statistical analysis of concentration-response curves was performed using two-way analysis of variance followed by Bonferroni post-test.
Drugs and solutions
The following drugs were used. Noradrenalinehydrochloride (HCl), l-phenylephrine (HCl) acetylcholine chloride, cocaine (HCl), dl-propranolol (HCl), yohimbine (HCl), corticosterone 21-acetate (CCA), and prazosin (HCl) were all obtained from Sigma (St. Louis, USA). Sertindole, risperidone, clozapine, ziprasidone, aripiprazole, and olanzapine were all synthesized and provided by H. Lundbeck A/S, Denmark. Haloperidol was purchased from Sigma, and domperidone was purchased from Research Biochemical International (RBI, Natick, MA, USA). All drugs for in vitro experiments, unless otherwise stated, were dissolved in double-distilled, deionized water. CCA was dissolved in absolute ethanol and diluted further with 50% ethanol. Prazosin HCl was dissolved initially in 50% ethanol to give a 1 mM stock solution and subsequently diluted in distilled water. Sertindole was dissolved in water including a few drops of 0.1 M HCl, heated to 40°C, then diluted in distilled water. Clozapine, ziprasidone, aripiprazole, and domperidone were dissolved initially in dimethylsulfoxide to a stock of 10 mM, and then diluted in distilled water. Ziprasidone, aripiprazole, and domperidone were diluted in 50% ethanol to a concentration of 1 mM and then diluted with distilled water. Risperidone, haloperidol, and olanzapine were dissolved in absolute ethanol to make a stock solution of 10 mM. Further dilution for risperidone and olanzapine was in distilled water and for haloperidol in 50% ethanol to a concentration of 1 mM, and then further diluted in distilled water. All stock solutions for in vitro experiments were stored frozen in aliquots and thawed and diluted fresh daily. All test drugs for in vivo experiments were dissolved in 10% hydroxypropyl-ß-cyclodextrin (HPβC) on the day of use and administered in a volume of 1 ml/kg.
Results
In vivo study
The combined use of pentobarbital and isoflurane gave a satisfactory anesthesia that did not suppress the tilt responses. Thus, an almost complete compensation of reflex responses was found to the initial tilt-induced drop in blood pressure during the pre-dose tilt periods in anesthetized rats (pre-dose tilt responses in Fig. 1a). There were no significant differences in the pressure responses between pre-dose and post-dose tilt in the control (vehicle administered) animals (Table 1). As seen in Fig. 1b, administration of risperidone (1 mg/kg, i.v.) inhibited the reflex response, which did not reestablish during the 60-s post-dose tilt. ΔAUC0–60 values and the mean of plasma concentrations of drugs at the end of tilt procedure are indicated in Table 1. Dose-dependent increases in the ΔAUC0–60 values were observed for all drugs except aripiprazole and domperidone. Haloperidol showed a dose-dependent increase in ΔAUC0–60, but the effect was just not statistically significant (p = 0.07; Tables 1 and 2).
Effect of risperidone on head-up tilt-induced orthostatic hypotension. Representative tracing of tilt-induced change on blood pressure (BP, upper traces) and mean arterial blood pressure (MAP, lower traces) in a pre-dose tilt and b post-dose tilt after administration of risperidone at 1 mg/kg, i.v. Time from start to end of each tilt was 60 s
To estimate the drugs’ potencies to cause orthostatic hypotension on the tilt setup, we have measured for each drug investigated the plasma concentrations for each dose (Table 1). These measurements were then used to rank the tested drugs based on the plasma concentration of the first dose of drugs having a significant increase in ΔAUC0–60 compared with vehicle (see Table 1). As shown in Table 2, prazosin (used as a positive control) has the highest rank followed by sertindole, and thereafter in order risperidone, ziprasidone, olazapine, clozapine, haloperidol, domperidone, and aripiprazole. Figure 2 shows the changes in blood pressure during the 60° head-up tilt for 60 s after administration of various doses of prazosin and risperidone. Prazosin at more than 0.022 mg/kg, i.v., completely depressed the tilt induced blood pressure responses (Fig. 2a, Table 1). Although 0.3 and 1 mg/kg, i.v. administration of risperidone completely depressed the responses, partial compensation was observed at 0.03 and 0.1 mg/kg (Fig. 2b).
Effect of a prazosin and b risperidone on tilt-induced blood pressure response in the anesthetized rats. Mean arterial blood pressure (MAP) was taken immediately before tilt (as 0 s) and 5, 10, 20, 30, 40, 50, and 60 s after the start of tilt. Values are given as percentage values compared to baseline values (100%). Changes from the baseline in MAP value before tilt (ΔMAP) were plotted against time after the start of tilt. Each point shows the mean ± SEM of five rats
In vitro study
Antagonism of PE responses in rat small mesenteric arteries in vitro
In the rat mesenteric small artery, PE produced isometric contraction in a concentration-dependent manner (pD 2 = 6.24 ± 0.02, n = 11). Prazosin shifted the concentration–response curve to PE markedly to the right (Fig. 3a, Table 3). The responses to PE were antagonized by domperidone, risperidone, and sertindole in a concentration-dependent manner with no depression in maximum responses (Figs. 3 and 4, Table 3) and pA 2 values 6.99, 8.92, and 8.78, respectively. None of the Schild slopes differed significantly from unity (Table 3). For sertindole and risperidone, exactly the same results were obtained in vessels where the endothelium had not been removed (n = 12, data not shown).
Phenylephrine concentration–response curves in the presence of prazosin (a and b) and domperidone (d and e) on rat endothelial-denuded mesenteric small arteries (a, d) and rat aorta (b, e). c, f show corresponding Schild plots comparing the Schild lines for small mesenteric arteries (MSA) and aorta. Each symbol represents the mean, and the vertical lines show the SEM of at least four separate experiments. For other details, see Table 3
Phenylephrine-concentration–response curves in the presence of risperidone (a and b) and sertindole (d and e) on rat endothelial-denuded mesenteric small arteries (a, d) and rat aorta (b, e). c, f show corresponding Schild plots comparing the Schild lines for small mesenteric arteries (MSA) and aorta. Each symbol represents the mean, and the vertical lines show the SEM of at least four separate experiments. For characteristics, see Table 3
The responses to PE were also antagonized by the presence of ziprasidone and aripiprazole (Fig. 5), and clozapine, haloperidol, and olanzapine (data not shown). Schild regression analysis carried out for these drugs against PE gave pA 2 values of 7.98, 7.17, 7.64, 7.64, and 7.35, respectively. The slopes of the Schild plots were not significantly different from unity, respectively (Table 3). The antagonists in Table 3 are arranged in order of their pA 2 values found in MSA, with prazosin having the greatest affinity and domperidone having the least.
Phenylephrine concentration–response curves in the presence of aripiprazole (a and b) and ziprasidone (d and e) on rat endothelial-denuded mesenteric small arteries (a, d) and rat aorta (b, e). c and f show corresponding Schild plots comparing the Schild lines for mesenteric small arteries (MSA) and aorta. Each symbol represents the mean, and the vertical lines show the SEM of at least four separate experiments. For characteristics, see Table 3
Antagonism of phenylephrine responses in rat aorta in vitro
Phenylephrine produced concentration-dependent contractions of rat aorta (pD 2 = 7.33 ± 0.04, n = 9) with a greater potency than that seen in the mesenteric small arteries (pD 2 = 6.24, see above). The PE concentration–response curves were markedly right-shifted by prazosin (Fig. 3b and Table 3). Risperidone antagonized the PE-concentration–response curves in rat aorta with high affinity (pA 2 value, 8.36) and no depression of the maximum response at the higher concentration (Fig. 4b, Table 3). Sertindole produced rightward shifts of the concentration–response curves to PE (Fig. 4e), and gave a Schild slope that was greater than unity (1.99, Table 3). Clozapine and haloperidol antagonized PE responses in rat aorta with the same pA 2 values (7.3) but different Schild slopes (Table 3).
We performed Schild analysis for ziprasidone on rat aorta using six different concentration of ziprasidone (Fig. 5e). As ziprasidone gave similar rightward shifts of the PE-concentration–response curves at concentrations 3 × 10−7 and 5 × 10−7 M and very small rightward shift for 10−7 M (Fig. 5e), we exposed the vessels with higher concentrations of ziprasidone (10−6, 3 × 10−6, and 10−5 M), and based on these, a low pA 2 value was calculated (6.93) with a Schild slope that was not significantly different from unity (Table 3). Calculated pA 2 value for domperidone was 6.11, with significant depression in maximum response at 10−5 M but a Schild slope not significantly different from unity (Fig. 3e and Table 3).
There was no rightward shift of the PE-concentration–response curves in the presence of 10−6 M aripiprazole. From the rightward shifts at two higher concentrations (3 × 10−6 and 10−5 M, Fig. 5b) pA 2 value equal to 5.96 was calculated. For olanzapine, pA 2 values equal to 6.37 was calculated with a Schild slope that was not significantly different from unity (Table 3).
Comparison of tilt test potencies and pA 2 values in MSA and aorta
The calculated pA 2 values for MSA and aorta were similar for prazosin, risperidone, haloperidol, and clozapine. Markedly lower pA 2 values were, however, seen in aorta vs. MSA for sertindole (pA 2 6.31 vs. 8.78) and to a lesser extent for domperidone, olanzapine, and ziprasidone (pA 2 6.11, 6.37, and 6.93 vs. 6.99, 7.35, and 7.98, respectively). Lowest affinity in aorta was found for aripiprazole, which was also considerably lower than in MSA (pA 2 5.96 vs.7.17, Table 3). As shown in Fig. 6, the potencies of the antipsychotic drugs in the tilt test correlated with the pA 2 values in MSA (r 2 = 0.69, P = 0.01) but not with the pA 2 values in the aorta (P = 0.21)
Comparison of pA 2 values for the investigated antipsychotic drugs determined in mesenteric small arteries (MSA) and in aorta with potencies of these drugs in the tilt test (expressed as log [threshold dose]). The data are taken from Tables 2 and 3. Significant correlation was seen for MSA (r 2 = 0.69, p = 0.01, regression line). No correlation was seen for aorta
Discussion
The present study appears to be the first where the in vivo potency of a wide range of clinically available typical and atypical antipsychotic drugs in an animal model of orthostatic hypotension has been compared with the functional affinity of these drugs for α1-AR in separate vascular subtype models in the same species. Eight antipsychotic drugs, plus prazosin as a positive control, were evaluated for their orthostatic hypotensive effect in vivo, and their α1A- and α1D-AR antagonist apparent affinities (pA 2) have been determined in vitro in rat mesenteric small arteries (MSA) and rat thoracic aorta, respectively. In the following, we refer to responses of MSA and aorta as α1A-AR and α1D-AR responses, respectively. The results show a good agreement in the rank order of the in vivo potencies and in vitro affinities for α1A-AR but not for α1D-AR (Table 3).
Investigations employing functional, radioligand binding, and molecular methods have demonstrated the existence of a heterogeneous population of α1-AR subtypes throughout the vascular system (Kenny et al. 1995; Hussain and Marshall 1997; Docherty 1998; Hrometz et al. 1999). Although the messenger RNA (mRNA) encoding the three α1-AR subtypes is expressed in many arteries (Piascik and Perez 2001), Marti et al. (2005) found in a molecular and functional study on MSA and rat aorta that the α1A subtype is the dominant subtype in MSA (with 73% of mRNA level) compared with 79% of mRNA level for the α1D subtype in the rat aorta. A major functional role of α1A in MSA has also been proposed by other authors (Ipsen et al. 1997; Stam et al. 1999), whereas several lines of evidence show that the α1D-subtype is the main functional α1-AR subtype in rat aorta (Hussain and Marshall 1997; Hrometz et al. 1999; Gisbert et al. 2003). Although there are some functional studies on spleen and liver indicating the presence of α1B-AR in these tissues (Sleight et al. 1993; Eltze 1996), as yet there is little direct evidence for the role of the α1B-AR as a mediator of contractile function in blood vessels (Daly et al. 2002). This subtype has only been identified at a low level (1.7–11.1%) of mRNA in peripheral vessels (Marti et al. 2005), confirming previous observations of the lack of α1B-AR in the vasculature (Piascik and Perez 2001).
It is well known that peripheral vasodilators, specifically α1-AR blockers, such as prazosin and terazosin, have the potential to cause orthostatic hypotension (Andros et al. 1996; Poon and Braun 2005). Consistent with this, in the present study, we found that prazosin had a high potency to cause orthostatic hypotension in our animal model, as well as a high apparent affinity of prazosin for α1-AR in both MSA and rat aorta (pA 2 9.5 and 10.1, respectively), in agreement with previous studies (Kenny et al. 1995; Hussain and Marshall 1997; Testa et al. 1997; Stam et al. 1999). Antipsychotic drugs also cause blockade of α1-AR (Schotte et al. 1996; Ipsen et al. 1997; Richelson and Souder 2000; Wadenberg et al. 2000; Schmidt et al. 2001) and also cause postural hypotension; for example, postural hypotension was seen in 77% of the people receiving antipsychotic drugs versus 15% receiving placebo (Silver et al. 1990). The side effect of postural hypotension with antipsychotic drugs is thus believed to be mediated by their α1-AR antagonist affinity (Casey 1996, 1997).
Among the eight antipsychotic drugs investigated in this study, there was a remarkable agreement (Table 3) in the rank orders of potency as regards the induction of orthostatic hypotension and the apparent affinity of the drugs for α1A-AR (as determined in MSA). Thus, risperidone and sertindole had the highest potency concerning orthostatic hypotension and apparent affinity for α1A-AR, in contrast to aripiprazole and domperidone, which show the lowest potencies and affinities in these models. On the other hand, the rank order of apparent affinity for α1D-AR (as determined in aorta) showed little correlation with the rank order of the other two assays (Table 3). Thus, for example, the pA 2 values for both risperidone and sertindole for the α1A-AR were high (pA 2 = 8.92 and 8.78, respectively), but their affinity for α1D-AR was very different (pA 2 = 8.36 and 6.31, respectively).
Richelson and Sounder (2000) showed that both risperidone and sertindole had high affinity for α1-AR using radioligand binding assay and post-morten normal human brain tissue, but the subtypes involved were not investigated. In subtype studies, a high affinity of risperidone for α1A-AR has been found in isolated rat vas deferens (Eltze 1996), although this was not the case for rat hippocampus (Sleight et al. 1993). To our knowledge, there are no data on risperidone affinity for α1D-AR, but low (Eltze 1996) and high (Sleight et al. 1993) affinity for α1B-AR has been reported in guinea pig/mouse spleen and rat hippocampus, respectively. As regards sertindole, the present data confirm previous data from our laboratory, showing that this drug is approximately 300-fold selective for α1A-AR compared to α1D-AR; indeed, the high Schild slope seen here in the aorta experiments (1.99) suggests that sertindole has little functional affinity for the α1D-AR. The significance of the low affinity for α1D-AR is not known. The similarity between studies on MSA with and without endothelium confirms that the action of the tested compounds (risperidone and sertindole) is being mediated through effects on the vascular smooth muscle.
Consistent with the high α1A-AR affinity of risperidone and sertindole, and the high potency of these drugs in the tilt test, the incidence of dizziness in patients taking sertindole (Sramek et al. 1997) and risperidone (Barnes and McPhillips 1999) appears to be quite high. For risperidone, the high affinity of risperidone for α1-AR compared to the low affinity for dopamine D2 receptors can explain the high risk (48%) of orthostatic hypotension (Poon and Braun 2005). In a case report study, postural hypotension, tachycardia, and syncope have been reported in a patient taking a relatively small overdose of risperidone (Kopala et al. 1998).
Aripiprazole is the first atypical antipsychotic that has potent partial agonist activity at dopamine D2 and 5-HT1A receptors (Burris et al. 2002; Jordan et al. 2002). In the present in vivo investigation, no significant effects were found at the highest doses of aripiprazole (Table 2). The pA 2 value for aripiprazole on α1A-AR (7.17) was also relatively low, which is in concordance with the lack of reported orthostatic hypotension in clinical studies (Keck and McElroy 2003), a potential benefit of this drug.
Clinical studies have confirmed that domperidone, as a D2-antagonist that does not cross the blood–brain barrier, is an effective treatment for preventing early orthostatic hypotension in Parkinson’s patients under dopamine agonist therapy (Lang et al. 1990; Sigurdardottir et al. 2001). Such orthostatic hypotension is often found with initiation of dopamine agonist therapy, particularly in Parkinson’s disease, and is thought to be caused by venous and arterial dilation through inhibition of the sympathetic nervous system (Kujawa et al. 2000). Our finding of a low potency of domperidone for orthostatic hypotension in the tilt model and a relatively low affinity of domperidone on MSA (pA 2 = 6.99) is consistent with the relatively low incidence of orthostatic hypotension when used as a peripheral dopamine antagonist (Lopes et al. 1988).
The relatively low affinity of olanzapine for both investigated α1-AR subtypes in the present study compared with ziprasidone is in agreement with the study of Schmidt et al. (2001). It was therefore unexpected that olanzapine showed a relatively high potency in the tilt test, and the reason for this discrepancy is not clear. Clinically, olanzapine has a low orthostatic effect (Beasley et al. 1997), but this is thought to be due to the relatively high affinity of olanzapine for dopamine D2 receptors compared to its affinity for α1-AR (Schmidt et al. 2001). Radioligand binding studies with ziprasidone by Schmidt et al. (2001) also identified a lower affinity of ziprasidone for human α1-AR compared to its dopamine D2 receptors, suggesting that also ziprasidone may have a lower potential to produce orthostatic hypotension in the clinic, but clinical data are lacking.
The anomalous Schild analysis for ziprasidone on rat aorta was unexpected. Here, despite repeated tests, a large gap between phenylephrine concentration–response curves at 5 × 10−7 and 10−6 M ziprasidone with depression of the maximum responses at higher concentrations compared to small right shifts without depression in maximum responses by lower concentrations 10−7, 3 × 10−7, and 5 × 10–7 M of ziprasidone. This behaviour of ziprasidone might be due to partial agonist activity at α1D-AR, but this possibility is not supported by our finding that the Schild slope was unity; thus, further experiments are required to elucidate this anomaly.
The similar potencies in the tilt test for clozapine and haloperidol, and the similar pA 2 values for α1A-AR found in the present investigation are consistent with the similar affinity of clozapine and haloperidol for human α1-AR reported previously (Schmidt et al. 2001). Clinical data on the orthostatic effects of clozapine are limited, but there are several reports that these are found in some patients (Tuunainen et al. 2002). The findings of the present study are concerned with the acute effects of the drugs investigated. It is, however, well known that the orthostatic hypotensive action of antipsychotics is strongest at the start of treatment and that the effect diminishes over the following days and weeks. The reason for this tolerability is not clear (Stanniland and Taylor 2000). The present study should therefore be considered as providing information that may be relevant for the initial stages of antipsychotic treatment. On this basis, the ability of the antipsychotic drugs, at the higher doses, to prevent recovery of blood pressure during the tilt test (Fig. 2) is consistent with the drug-induced orthostatic hypotension being mediated through the peripheral vascular adrenoceptors. However, as with the exception of domperidone, all the drugs pass the blood–brain barrier, a central contribution to their action cannot be entirely excluded.
In summary, for most of the antipsychotics investigated in the present study, there was a good correlation between the in vivo potency to induce orthostatic hypotension and the affinity for α1A-AR, except olanzapine, which showed slightly higher in vivo potency than expected from the in vitro affinity results. No obvious correlation was found between in vivo effects and affinity toward α1D-AR. Therefore, based on the present comparative functional study, we conclude that α1A-AR are mainly responsible for the orthostatic hypotensive effect of treatment of the rats. Whether this is also the case in man remains to be determined.
References
Akiyama K, Hora M, Yamagishi R, Kitazawa M (2002) Effects of KMD-3213, a uroselective alpha 1A-adrenoceptor antagonist, on the tilt-induced blood pressure response in normotensive rats. Jpn J Pharmacol 90:131–137
Andros E, Detmar-Hanna D, Suteparuk S, Gal J, Gerber JG (1996) The effect of aging on the pharmacokinetics and pharmacodynamics of prazosin. Eur J Clin Pharmacol 50:41–46
Arunlakshano O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58
Barnes TR, McPhillips MA (1999) Critical analysis and comparison of the side-effect and safety profiles of the new antipsychotics. Br J Psychiatry Suppl 38:34–43
Beasley CM Jr., Hamilton SH, Crawford AM, Dellva MA, Tollefson GD, Tran PV, Blin O, Beuzen JN (1997) Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol 7:125–137
Buckley NA, Sanders P (2000) Cardiovascular adverse effects of antipsychotic drugs. Drug Safety 23:215–228
Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389
Casey DE (1996) Side effect profiles of new antipsychotic agents. J Clin Psychiatry 57(Suppl 11):40–45
Casey DE (1997) The relationship of pharmacology to side effects. J Clin Psychiatry 58(Suppl 10):55–62
Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, McGrath JC (2002) A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics 9:85–91
de Moura MM, dos Santos RA, Fontes MA (2005) Evidence for a functional cardiac interaction between losartan and angiotensin-(1–7) receptors revealed by orthostatic tilting test in rats. Br J Pharmacol 144:755–760
Docherty JR (1998) Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol 361:1–15
Eltze M (1996) In functional experiments, risperidone is selective, not for the B, but for the A subtype of alpha 1-adrenoceptors. Eur J Pharmacol 295:69–73
Fenger-Gron J, Mulvany MJ, Christensen KL (1995) Mesenteric blood pressure profile of conscious, freely moving rats. J Physiol 488(Pt 3):753–760
Gisbert R, Madrero Y, Sabino V, Noguera MA, Ivorra MD, D’Ocon P (2003) Functional characterization of alpha 1-adrenoceptor subtypes in vascular tissues using different experimental approaches: a comparative study. Br J Pharmacol 138:359–368
Hashimoto Y, Ohashi R, Minami K, Narita H (1999) Comparative study of TA-606, a novel angiotensin II receptor antagonist, with losartan in terms of species difference and orthostatic hypotension. Jpn J Pharmacol 81:63–72
Hieble JP, Kolpak DC, McCafferty GP, Ruffolo RR Jr., Testa R, Leonardi A (1999) Effects of alpha1-adrenoceptor antagonists on agonist and tilt-induced changes in blood pressure: relationships to uroselectivity. Eur J Pharmacol 373:51–62
Hrometz SL, Edelmann SE, McCune DF, Olges JR, Hadley RW, Perez DM, Piascik MT (1999) Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J Pharmacol Exp Ther 290:452–463
Hussain MB, Marshall I (1997) Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol 122:849–858
Ipsen M, Zhang Y, Dragsted N, Han C, Mulvany MJ (1997) The antipsychotic drug sertindole is a specific inhibitor of alpha1A-adrenoceptors in rat mesenteric small arteries. Eur J Pharmacol 336:29–35
Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA (2002) The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol 441:137–140
Keck PE Jr., McElroy SL (2003) Aripiprazole: a partial dopamine D2 receptor agonist antipsychotic. Expert Opin Investig Drugs 12:655–662
Kenny BA, Chalmers DH, Philpott PC, Naylor AM (1995) Characterization of an alpha 1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol 115:981–986
Kopala LC, Day C, Dillman B, Gardner D (1998) A case of risperidone overdose in early schizophrenia: a review of potential complications. J Psychiatry Neurosci. 23:305–308
Kujawa K, Leurgans S, Raman R, Blasucci L, Goetz CG (2000) Acute orthostatic hypotension when starting dopamine agonists in Parkinson’s disease. Arch Neurol 57:1461–1463
Lane RF, Blaha CD, Rivet JM (1988) Selective inhibition of mesolimbic dopamine release following chronic administration of clozapine: involvement of alpha 1-noradrenergic receptors demonstrated by in vivo voltammetry. Brain Res 460:398–401
Lang AE, Riley DE, Vachon L, Lataste X (1990) CQA 206–291 in Parkinson’s disease: an acute single escalating dosage study. Can J Neurol Sci 17:416–419
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334
Lidow MS, Williams GV, Goldman-Rakic PS (1998) The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 19:136–140
Lopes dF Sr., Zanella MT, Andriolo A, Ribeiro AB, Chacra AR (1988) Peripheral dopaminergic blockade for the treatment of diabetic orthostatic hypotension. Clin Pharmacol Ther 44:670–674
Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, Moreno L, Villagrasa V, Barettino D, D’Ocon P (2005) Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol 289:H1923–H1932
Mulvany MJ, Halpern W (1977) Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41:19–26
Piascik MT, Perez DM (2001) Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther 298:403–410
Poon IO, Braun U (2005) High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 30:173–178
Prinssen EP, Ellenbroek BA, Cools AR (1994) Combined antagonism of adrenoceptors and dopamine and 5-HT receptors underlies the atypical profile of clozapine. Eur J Pharmacol 262:167–170
Richelson E, Souder T (2000) Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 68:29–39
Sanchez C, Kreilgaard M (2004) R-Citalopram inhibits functional and 5-HTP-evoked behavioural responses to the SSRI, escitalopram. Pharmacol Biochem Behav 77:391–398
Schmidt AW, Lebel LA, Howard HR Jr., Zorn SH (2001) Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol 425:197–201
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van GP, Lesage AS, De LK, Leysen JE (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124:57–73
Sigurdardottir GR, Nilsson C, Odin P, Grabowski M (2001) Cardiovascular effects of domperidone in patients with Parkinson’s disease treated with apomorphine. Acta Neurol Scand 104:92–96
Silver H, Kogan H, Zlotogorski D (1990) Postural hypotension in chronically medicated schizophrenics. J Clin Psychiatry 51:459–462
Sleight AJ, Koek W, Bigg DC (1993) Binding of antipsychotic drugs at alpha 1A- and alpha 1B-adrenoceptors: risperidone is selective for the alpha 1B-adrenoceptors. Eur J Pharmacol 238:407–410
Sramek JJ, Mack RJ, Awni W, Hourani J, Jhee SS, Barto S, Cutler NR (1997) Two rapid-dose titrations of sertindole in patients with schizophrenia. J Clin Psychopharmacol 17:419–422
Stam WB, Van der Graaf PH, Saxena PR (1999) Analysis of alpha 1L-adrenoceptor pharmacology in rat small mesenteric artery. Br J Pharmacol 127:661–670
Stanniland C, Taylor D (2000) Tolerability of atypical antipsychotics. Drug Safety 22:195–214
Strange PG (2001) Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev 53:119–133
Svensson TH (2003) Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 27:1145–1158
Take H, Shibata K, Awaji T, Hirasawa A, Ikegaki I, Asano T, Takada T, Tsujimoto G (1998) Vascular alpha1-adrenoceptor subtype selectivity and alpha1-blocker-induced orthostatic hypotension. Jpn J Pharmacol 77:61–70
Testa R, Guarneri L, Angelico P, Poggesi E, Taddei C, Sironi G, Colombo D, Sulpizio AC, Naselsky DP, Hieble JP, Leonardi A (1997) Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part II. J Pharmacol Exp Ther 281:1284–1293
Tuunainen A, Wahlbeck K, Gilbody S (2002) Newer atypical antipsychotic medication in comparison to clozapine: a systematic review of randomized trials. Schizophr Res 56:1–10
Wadenberg ML, Hertel P, Fernholm R, Hygge BK, Ahlenius S, Svensson TH (2000) Enhancement of antipsychotic-like effects by combined treatment with the alpha1-adrenoceptor antagonist prazosin and the dopamine D2 receptor antagonist raclopride in rats. J Neural Transm 107:1229–1238
Williams JB, Mallorga PJ, Jeffrey CP, Pettibone DJ, Sur C (2004) Effects of typical and atypical antipsychotics on human glycine transporters. Schizophr Res 71:103–112
Acknowledgment
This work was supported by an unrestricted research grant from H. Lundbeck A/S (Copenhagen, Denmark). We thank H. Lundbeck A/S for gifts of all antipsychotic drugs. The authors also gratefully acknowledge the technical assistance of Ms. Henriette Johanson and Ms. Rikke Bregnhardt Jørgensen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nourian, Z., Mow, T., Muftic, D. et al. Orthostatic hypotensive effect of antipsychotic drugs in Wistar rats by in vivo and in vitro studies of α1-adrenoceptor function. Psychopharmacology 199, 15–27 (2008). https://doi.org/10.1007/s00213-007-1064-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-1064-9