Abstract
Rationale
Recommended medication prescribing hierarchies for adult attention-deficit hyperactivity disorder (ADHD) vary between different guideline committees. Few trials directly compare competing ADHD medications in adults and provide little insight for clinicians making treatment choices.
Objective
The objective of this study was to assess comparative benefits and harms of competing medications for adult ADHD using indirect comparison meta-analysis.
Materials and methods
Eligible studies were English-language publications of randomized controlled trials comparing ADHD drugs to placebo. Data sources were electronic bibliographic databases, Drugs@FDA, manufacturer data, and reference lists. Two reviewers independently abstracted data on design, internal validity, population, and results. Benefits and harms were compared between drug types using indirect comparison meta-regression (ratio of relative risks).
Results
Twenty-two placebo-controlled trials were included (n = 2,203). Relative benefit of clinical response for shorter-acting stimulants, primarily immediate release methylphenidate, was 3.26 times greater than for patients taking longer-acting stimulants (95% CI 2.03, 5.22) and 2.24 times greater than for patients taking longer-acting forms of bupropion (95% CI 1.23, 4.08). Immediate release methylphenidate is also the only drug shown to reduce ADHD symptoms in adults with substance abuse disorders. Neither non-stimulants nor longer-acting stimulants reduced adverse effects compared to shorter-acting stimulants. Key gaps in evidence were academic, occupational, social functioning, cardiovascular toxicity, and longer-term outcomes, influences of ADHD subtype and/or comorbidities, and misuse/diversion of the drugs.
Conclusions
Current best evidence supports using immediate release methylphenidate as first-line treatment for most adults with ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Attention-deficit hyperactivity disorder (ADHD) is no longer thought of as only a childhood problem. The notion of ADHD in adults was officially adopted more than 25 years ago when diagnostic criteria first appeared in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) in 1980 (Dodson 2005). Recent prevalence estimates indicate that up to 4.5% of adults suffer from ADHD(Kessler et al. 2005, 2006; Montano 2004).
In the context of adulthood, ADHD symptoms tend to manifest themselves into problems with follow-through, forgetting, organization, losing things, meeting deadlines, not completing tasks, not planning ahead, and having a poor sense of time (Riccio et al. 2005). Recent retrospective cohort studies suggest that adult ADHD is also associated with an increased risk of comorbid psychiatric conditions that include anxiety, depression, personality disorders, and drug and alcohol dependence (Asherson 2005; Secnik et al. 2005). Up to 87% of adults with ADHD will suffer from at least one of these comorbidities (McGough et al. 2005; Montano 2004).
The consequences and economic burden associated with the impairments of adult ADHD are vast and may outnumber those in children. Adults with ADHD can suffer from academic, occupational, and social dysfunction and problems with general medical health. One retrospective cohort study of a claims database estimated the total excess cost of ADHD patients (ages 7–44 years) in the USA in 2000 to be $31.6 billion. This includes work loss costs of $3.7 billion and non-ADHD-related healthcare utilization costs of $12.1 billion (Birnbaum et al. 2005). Other large observational studies suggest that adults with ADHD have increased risks of driving impairments leading to accident claims, higher days of lost work/human capital, criminality, and higher costs associated with annual medical care (Barkley 2004; Kessler et al. 2005; Matza et al. 2005; Secnik et al. 2005; Swensen et al. 2004).
Drug treatment has played an important role in the management of ADHD in children, and clinicians have used their pediatric care experiences to inform their clinical management of ADHD in adults (Dodson 2005). ADHD drug choices are numerous, the majority of which are psychostimulant agents with varying durations of action (immediate vs extended release rates) and delivery systems (oral tablets, capsules, chewables, solutions, and transdermal patch system). Other newer agents used to treat ADHD include atomoxetine and longer-acting forms of bupropion. Prescribing for adult ADHD is largely off-label, as only Adderall XR® and Strattera® are FDA-approved for this indication.
Choice of drug requires careful consideration by a skilled clinician and can be based on a variety of factors including efficacy, side effects, toxicity, cost, patient preference, potential for abuse and/or diversion, and the presence of coexisting serious medical and/or psychiatric illnesses. Uncertainty remains regarding optimum prescribing hierarchies. Trials directly comparing competing ADHD drugs in adults are few and provide little insight for clinicians making treatment choices. In addition, recommendations for prescribing hierarchies differ between recently released treatment guidelines. The British Association for Psychopharmacology (BAP) has recommended that all amphetamine- and methylphenidate-based agents and atomoxetine are effective and that choice of agent may depend on pharmacological factors other than efficacy. In contrast, the Canadian ADHD Resource Alliance (CADDRA) has specified the long-acting agents Adderall® XR, Concerta®, and Strattera® as first-line treatments. Adult ADHD treatment guidelines are not yet available in the USA.
There are numerous placebo-controlled trials of drug treatments in adults with ADHD. These studies were conducted in relatively similar populations using similar methods and could be used to further address questions related to prescribing hierarchies using indirect comparison meta-analyses. However, to our knowledge, no such analyses have been done. Thus, we undertook a systematic review with meta-analyses of placebo-controlled trials to assess the effectiveness and safety of the most commonly used newer ADHD drugs (non-stimulants and longer-acting stimulants) compared to conventional shorter-acting stimulants. The evaluation reported here is an update and expansion of work originally completed for the Drug Effectiveness Review Project (DERP; McDonagh and Peterson 2006).
Materials and methods
Data sources and searches
Included populations were adults with any subtype of ADHD, attention-deficit disorder (ADD), hyperkinetic disorder, or minimal brain dysfunction. Included stimulants were amphetamine mixture, dextroamphetamine sulfate, methylphenidate HCl, dexmethylphenidate HCl, and modafinil. Included non-stimulants were atomoxetine HCl (formerly tomoxetine) and bupropion HCl. We included English-language publications of randomized controlled trials that compared an included medication to placebo. We placed no restrictions on sample size or trial duration.
To identify relevant citations, we searched the Cochrane Central Register of Controlled Trials (first Quarter 2007), Cochrane Database of Systematic Reviews (first Quarter 2007), MEDLINE (1966 to March Week 3, 2007), EMBASE (second Quarter 2004), and PsycINFO (1974 to March Week 4, 2007). We used the following search terms: methylphenidate, Concerta, Metadate, Methylin, Ritalin, dexmethylphenidate, Focalin, amphetamine, Adderall, dextroamphetamine, Dexedrine, atomoxetine, Strattera, Wellbutrin, bupropion, modafinil, Provigil, attention deficit disorder with hyperactivity, attention deficit disorder, attention deficit, ADHD. Searches were limited by terms for humans, adults, and English language. We also searched reference lists of included studies, (Food and Drug Administration Drugs@FDA), and drug information materials submitted by drug manufacturers per request by DERP. All citations were imported into an electronic database (EndNote 9.0).
Study selection
Two reviewers independently assessed abstracts for inclusion using the criteria described above. Full-text articles of potentially relevant abstracts were retrieved, and a second review for inclusion was conducted by reapplying the inclusion criteria. All disagreements were resolved through consensus.
Data extraction and quality assessment
Two reviewers independently abstracted the following data from included trials: study design, setting, population characteristics (including sex, age, ethnicity, and diagnosis), eligibility, and exclusion criteria; interventions (dose and duration) and comparisons; numbers screened, eligible, enrolled, and lost to follow-up; method of outcome ascertainment; and results for each outcome. We recorded intention-to-treat results when reported. Disagreements were resolved through consensus.
We assessed the internal validity (quality) of trials using predefined criteria based on the US Preventive Services Task Force and the National Health Service Centre for Reviews and Dissemination (UK) criteria (Anonymous 2001; Harris et al. 2001). We rated the internal validity of each trial based on the methods used for randomization, allocation concealment, and blinding; the similarity of compared groups at baseline; maintenance of comparable groups; adequate reporting of dropouts, attrition, crossover, adherence, and contamination; loss to follow-up; and the use of intention-to-treat analysis.
Data synthesis and analysis
For effectiveness, we sought evidence of academic, occupational, social, and/or legal outcomes and of complete symptom remission. Efficacy outcomes were incidence of clinical response and change from baseline in ADHD symptom scores. The ADHD Rating Scale (ADHD-RS) was most commonly used to measure symptoms, and clinical response was most commonly defined as the proportion of patients with a 30% or greater improvement in ADHD-RS Total Score. We also sought evidence of ADHD drug misuse or diversion or impact of use on current or past substance use disorders. Adverse event outcomes included sleep or appetite disturbances, anxiety, and serious cardiovascular event outcomes. All-cause treatment discontinuations were evaluated as an overall measure of effectiveness and tolerability.
According to drug type, we grouped trials into four categories including atomoxetine, longer-acting forms of bupropion, shorter-acting stimulants, and longer-acting stimulants. For all outcomes, we combined data from placebo-controlled trials for each drug type to calculate pooled relative risks (RR) with 95% confidence intervals. As a way to test the sensitivity of our inferences to variation in statistical method, we also calculated risk differences (RD) for all outcomes. We planned to report only RR estimates, except when the results of RD analyses led to varying conclusions. As there were no such cases of this, however, only RR estimates are reported herein. RRs greater than 1.0 and positive (+) RD values were both interpreted as suggesting superiority of an agent over placebo for efficacy outcomes and indicators of inferiority for adverse effects. If not reported, we planned to calculate symptom change scores based on available data, conservatively assuming zero correlation between baseline and endpoints. For crossover trials, the data were conservatively treated as being from parallel trials, as no estimate based on appropriate analysis of crossover trials was reported (Elbourne et al. 2002).
Statistical heterogeneity between studies was evaluated by the Cochran’s Q test. We used a random effects model to combine the data to account for variation among studies. When there is no variation among studies, the random effects model yields the same results as a fixed effects model.
Indirect comparison was used to evaluate the difference between drug types in treatment effect or adverse event based on data from placebo-controlled trials using a meta-regression approach. The magnitude of difference was characterized using relative risk ratio (RRR) for RRs and difference of risk difference (DRD) for RDs. RRRs greater than 1.0 and positive (+) DRD values were interpreted as suggesting that drug A is associated with a higher relative benefit compared to drug B for efficacy outcomes and higher relative risk for adverse events. Meta-regression of RRs were planned to identify sources of variation between studies. The following study level variables were prespecified for inclusion in the meta-regression model for exploratory purposes: dosage level (“low”, “medium”, or “high”, e.g., methylphenidate: low ≤20 mg/day, medium 21–50 mg/day, high ≥60 mg/day), study duration, use of crossover or parallel design, single or multicenter, age, gender, race, ADHD subtype, presence of at least one lifetime comorbidity, ADHD diagnostic methods, mean Hollingshead socioeconomic score, site of conduct, substance abuse status, and method of clinical response assessment. Only one study level variable was evaluated at each time in most meta-regression due to the small number of trials. Publication bias was assessed using funnel plots and Egger’s linear regression method (Egger et al. 1997). Sensitivity analyses were also performed to further explore sources of significant variation. All analyses were performed with Stata V9.0 (StataCorp LP, College Station, Texas).
Results
Search results
Figure 1 details literature search and study selection results. Included were 22 placebo-controlled trials of 2,203 adults with ADHD (Biederman et al. 2006; Carpentier et al. 2005; Kooij et al. 2004; Levin et al. 2001, 2006, 2007; Michelson et al. 2003; Paterson et al. 1999; Reimherr et al. 2005, 2007; Schubiner et al. 2002; Spencer et al. 2005, 2001, 1998, 1995, 2007; Weisler et al. 2006; Weiss et al. 2006; Wender et al. 1985; Wilens et al. 2005, 2001). Trials were generally short-term, with follow-up durations ranging from 2 to 13 weeks.
Patient characteristics
Fifty-nine (59) percent of the overall patient population was male, with a mean age of 38 years. Only 45% of trials reported race composition, and those included predominantly white participants. Only 41% of trials reported important clinical characteristics. Among those, the predominant ADHD subtype was most commonly the combined subtype (Table 1), and affective and anxiety disorders were the most commonly reported psychiatric comorbidities. Most trials were carried out in the outpatient settings of specialty clinics or university-based hospitals.
Methodologic quality
The methodologic quality was generally consistent across trials, with the majority of shortcomings related to under-reporting of design characteristics (Table 1). All but one trial lacked sufficient information to properly assess randomization and allocation concealment methods (Michelson et al. 2003). The majority of trials lacked intent-to-treat analyses, excluding data from an average of 12% of patients (range 1 to 28%), and reasons for exclusions were often not reported. The potential for bias was greatest in the three trials that excluded the largest proportions of patients (20 to 28%), and results from these trials were interpreted with caution (Carpentier et al. 2005; Reimherr et al. 2005; Weisler et al. 2006).
Treatment was double-blinded in all trials. The majority of trials used a parallel design (Biederman et al. 2006; Levin et al. 2001, 2006, 2007; Michelson et al. 2003; Paterson et al. 1999; Reimherr et al. 2005; Schubiner et al. 2002; Spencer et al. 2005, 2007; Weisler et al. 2006; Weiss et al. 2006; Wilens et al. 2005, 2001). Risk of bias due to carry-over effects appeared low among the trials that used cross-over designs (Carpentier et al. 2005; Kooij et al. 2004; Reimherr et al. 2007; Spencer et al. 2001, 1998, 1995; Wender et al. 1985). Fifty-nine percent of trials were manufacturer-funded (Biederman et al. 2006; Michelson et al. 2003; Reimherr et al. 2005, 2007; Spencer et al. 2005, 2001, 1998, 2007; Weisler et al. 2006; Weiss et al. 2006; Wilens et al. 2005, 2001).
Effectiveness outcomes
No included trial evaluated effectiveness outcomes.
Efficacy outcomes
Clinical response was the most common and consistently reported outcome, and meta-analyses were based on trials of longer-acting forms of bupropion (Reimherr et al. 2005; Wilens et al. 2005, 2001), shorter-acting stimulants (Carpentier et al. 2005; Kooij et al. 2004; Schubiner et al. 2002; Spencer et al. 2005, 2001, 1995; Weiss et al. 2006; Wender et al. 1985), and longer-acting stimulants (Biederman et al. 2006; Levin et al. 2006, 2007; Reimherr et al. 2007; Spencer et al. 2007). Clinical response rate data from one trial of tomoxetine was insufficient for inclusion in these efficacy meta-analyses (Spencer et al. 1998).
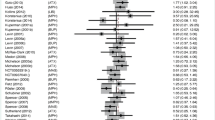
Figure 2 shows meta-analyses of trials comparing an ADHD drug vs placebo, grouped by drug type, for the outcome of clinical response and summarizes the pooled results. Overall, the outcome of clinical response was more likely for all the drug treatment groups compared to placebo. The RR of clinical response was 4.32 for shorter-acting stimulants (95% CI 3.03, 6.16), 1.87 for longer-acting forms of bupropion (95% CI 1.36, 2.58), and 1.35 for longer-acting stimulants (95% CI 0.997, 1.84). Indirect comparison of the estimated RRs showed significant differences among drug types (χ 2 = 24.15; p = 0.0001), with shorter-acting stimulants favored over longer-acting forms of bupropion (p = 0.008) and longer-acting stimulants (p < 0.001) for the outcome of clinical response. The size of the RR for clinical response for shorter-acting stimulants was 2.24 times greater (RRR) than for patients taking longer-acting forms of bupropion (95% CI 1.23, 4.08) and 3.26 times greater (RRR) than for patients taking longer-acting stimulants (95% CI 2.03, 5.22). Eggar’s test and funnel plots showed no obvious indications of publication bias; however, interpretation of these findings were limited by the relatively small number of trials (Terrin and Lau 2005).
Meta-regression was also performed to investigate whether study level characteristics affected the comparison among drug types. Results of a regression model with substance abuse status and drug types found less clinical response in trials of adults with comorbid substance abuse compared to those without (RRR = 0.53; 95% CI 0.38, 0.74). However, in adults with ADHD and substance abuse disorders, immediate release methylphenidate was still found effective in treating ADHD symptoms (RR 2.72; 1.36, 5.42), whereas the sustained release form was not (RR 0.83; 95% CI 0.60, 1.14; Carpentier et al. 2005; Levin et al. 2006, 2007; Schubiner et al. 2002). Adjustment for comorbid substance abuse did not impact our overall finding that shorter-acting stimulants lead to greater benefits than longer-acting stimulants (RRR 3.36; 95% CI 2.28, 4.95) or bupropion (RRR 2.79; 95% CI 1.71, 4.55). Other study level characteristics including clinical response assessment methods (assessment based on ‘30% or greater reduction in ADHD-RS Total Score’ vs other methods, assessment based on patient self-report vs rating from clinician), or other clinical or patient characteristics were not found to affect the comparison among drug types, either.
Regarding clinical response assessment methods, ADHD drugs were found to be of less benefit relative to placebo when clinical response was assessed based on the criteria ‘30% or greater reduction in ADHD-RS Total Score’ compared to when any other response criteria was used (RRR 0.65; 95% CI 0.44, 0.96). Additionally, when clinical response assessment was based primarily on investigator ratings, ADHD drugs were found to be of greater benefit relative to placebo compared to when clinical response assessment was based primarily on patient self-report (RRR 1.72; 95% CI 1.20, 2.45). However, when substance abuse status was also included in the regression model, neither of these variables remained statistically significant.
ADHD symptom change score data were too few and heterogeneous to permit meaningful meta-analyses.
Treatment discontinuations
Indirect comparison meta-analyses found that all ADHD drug types had similar risks of all-cause early treatment discontinuation relative to placebo even after adjusting for differences in trial durations (χ 2 = 2.08; p = 0.5559; Table 2; Biederman et al. 2006; Levin et al. 2001, 2006, 2007; Reimherr et al. 2005; Spencer et al. 2005, 2001, 2007; Weisler et al. 2006; Wilens et al. 2005, 2001). Dosage, setting (multicenter or single center), age, gender, and substance abuse status also were not shown to be significant sources of variation for this outcome.
Tolerability
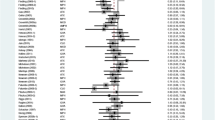
Adverse event reporting was sparse in placebo-controlled trials. Overall, shorter-acting stimulants, longer-acting stimulants, and atomoxetine groups had significantly higher risk of appetite loss relative to placebo groups (Table 2), but indirect comparisons suggested no significant differences between different drug types (χ 2 = 0.78; p = 0.68; Biederman et al. 2006; Kooij et al. 2004; Reimherr et al. 2007; Schubiner et al. 2002; Spencer et al. 2005, 2001, 2007; Weisler et al. 2006). However, these indirect meta-analyses were sensitive to inclusion of outlier appetite loss data from a trial of extended release dexmethylphenidate (Spencer et al. 2007). While we could not account for the source of variance, when we eliminated the outlier from the analyses, risk of appetite loss appeared significantly greater for longer-acting stimulants than shorter-acting stimulants (RRR 4.14; 95% CI 1.41, 12.11) and atomoxetine (RRR 3.38, 95% CI 1.04, 10.997). Appetite loss data were not available in longer-acting bupropion trials (Reimherr et al. 2005; Wilens et al. 2005, 2001).
Regarding sleep disturbances, RR was significantly higher in ADHD drug-treated groups than in placebo groups (Table 2), but no significant differences were detected among different drug types (χ 2 = 2.62; p = 0.45; Biederman et al. 2006; Kooij et al. 2004; Levin et al. 2007; Michelson et al. 2003; Paterson et al. 1999; Reimherr et al. 2007; Schubiner et al. 2002; Spencer et al. 2005, 2001, 1998, 2007; Weisler et al. 2006; Wilens et al. 2005).
Upon meta-regression, no study-level variable was found to have significant effects on RRs of adverse events. Reports of anxiety outcomes were deemed too few to permit meaningful meta-analyses (Biederman et al. 2006; Reimherr et al. 2007; Schubiner et al. 2002; Spencer et al. 2001, 1998, 1995; Weisler et al. 2006).
Cardiovascular toxicity
No reports of sudden death were found in any included trial. The only reports of withdrawals due to adverse cardiovascular effects came from a trial of Adderall XL® (Weisler et al. 2006). Two patients taking Adderall XL® were withdrawn due to tachycardia, and an additional patient was withdrawn due to development of hypertension. No information was provided about which dosage level of Adderall XL® (20, 40, or 60 mg) the patients were taking when they experienced these potentially serious cardiovascular events.
Discussion
To our knowledge, this is the first indirect comparison meta-analysis of placebo-controlled trials of competing drugs for treatment of ADHD in adults. Given that very few randomized controlled trials have directly compared competing ADHD drugs in adults, we considered that indirect comparison meta-analysis could be a useful tool in evaluating the larger body of evidence from placebo-controlled trials. Additionally, in light of disagreement among recently released prescribing guideline recommendations, we recognized that careful consideration of our findings could provide useful insights for the practicing clinician faced with choosing among numerous effective drugs for their adult patients with ADHD.
Overall, immediate release methylphenidate is the most well-studied drug for treatment of ADHD in adults. And, although it could be expected that the differing mechanisms of action, pharmacokinetics related to half-life or delivery system, and convenience of non-stimulants and longer-acting stimulants would lead to improvements in effectiveness or adverse event profiles over immediate release methylphenidate, these were not apparent in our analyses. In randomized controlled trials in adults with ADHD, immediate release methylphenidate is distinguished by a favorable balance of benefits and harms, and these findings deserve attention. With regard to efficacy, chances of clinically significant improvement were 2.7 to 3.3 times greater in trials of primarily immediate release methylphenidate than in trials with longer-acting forms of bupropion or longer-acting stimulants, respectively. Immediate release methylphenidate was the only drug shown to reduce ADHD symptoms in adults with substance abuse disorders without worsening substance use. Furthermore, there was no evidence of reduced risks of appetite loss, insomnia, or duration-adjusted early treatment discontinuation for non-stimulants or longer-acting stimulants over immediate release methylphenidate.
Regardless of the implications of our findings concerning immediate release methylphenidate, there is still much uncertainty about all drugs used in adults with ADHD with regard to the possibility of variable treatment effects across different subgroup populations based on ADHD subtype and/or comorbidities, benefits and risks over longer-term treatment durations, potential misuse/diversion of the drugs, and in cardiovascular toxicity. We found no randomized controlled trials that evaluated any of these important treatment aspects, and the longest-term trial included in this review, involving a shorter-acting stimulant, was only 20 weeks in duration. We also found no trials that evaluated the effects of ADHD drugs on academic, occupational, or social functioning, or on engagement in risky behavior. These types of “real-life” outcomes may be more important to clinicians and patients than changes in subjective ADHD rating scale scores, but there is currently a lack of available evidence in these areas.
The lack of trials evaluating the cardiovascular safety of ADHD drugs may be the most concerning gap in the evidence and necessarily warrants further attention. Recent prioritization of cardiovascular safety research was triggered by identification of cases of sudden death or serious cardiovascular events associated with amphetamine or methylphenidate products and atomoxetine that were voluntarily submitted to the FDA adverse event reporting system. Two FDA advisory committees were convened to formulate a research agenda to study the potential risks of cardiovascular or cerebrovascular morbidity and mortality and to discuss methods for informing the public about research findings (Food and Drug Administration 2006a,b). The proceedings of one of those sessions led to the widely publicized recommendation by the Drug Safety and Risk Management Advisory Committee to add black box warnings to ADHD drug labels regarding the potential cardiovascular risks. In follow-up, the FDA has recently directed manufacturers to revise their ADHD drug labeling to strengthen warnings about serious cardiovascular events. It is anticipated that findings from research of the cardiovascular safety of ADHD drugs will soon be forthcoming.
In addition to the obvious gaps in the available evidence, our indirect comparison meta-analysis has a number of limitations. Chiefly, we acknowledge that indirect comparison meta-analysis may be an inherently imperfect approach for generating firm conclusions about the differential effects among various types of drugs used for treatment of adult ADHD. Indirect comparison methods have been validated (Song et al. 2003), although conflicting results between indirect comparison meta-analysis and direct meta-analysis of head-to-head trials could occur (Chou et al. 2006). Direct comparisons from good quality head-to-head trials are the preferred method for determining differences in drug effects. Until such trials emerge, however, our indirect comparison meta-analysis provides clinicians with the most current unbiased summary of evidence for use in treatment decision making for their adult ADHD patients.
Additionally, the findings from our indirect comparison meta-analysis may be confounded by study-level variability in important clinical characteristics such as ADHD subtype or presence of psychiatric comorbidities. Although meta-regression of the available data did not reveal that any patient characteristics had confounding effects on estimates of relative risk, we recognize that under-reporting of ADHD subtype and comorbidity information may have limited our ability to detect such differences.
Finally, we note that our main findings are based only on efficacy outcome data from a modest number of short-term randomized controlled trials, especially with regard to longer-acting forms of bupropion and atomoxetine. The extent to which our findings may be used to guide treatment decisions across all care settings is uncertain, as it is unclear as to how well the patients selected for these trials represent the broader target population of adults with ADHD.
Although immediate release methylphenidate presented as having the most favorable benefit and risk profile, our findings also support the value of atomoxetine, long-acting forms of bupropion, and longer-acting stimulants as viable treatment alternatives. Choice of a non-stimulant drug may depend largely on other factors such as individual patient preferences and/or contraindications for stimulant treatments, as was proposed by the BAP guideline committee.
Concerta®, Adderall XR®, and Focalin ER® are a few of the latest additions to the armamentarium of longer-acting psychostimulants available for ADHD treatment which are being marketed as having unique advantages over shorter-acting stimulants, including lower risk of abuse and diversion, improved medication adherence, and superior tolerability profiles. Despite recommendations from the CADDRA CAP Guidelines Committee for use of Adderall XR® and Concerta® as first-line treatments, as well as Strattera®, the findings from our indirect comparison meta-analyses do not support these recommendations. The small amount of existing evidence for Concerta® and Adderall XR® has shown that both longer-acting stimulants are effective in reducing ADHD symptoms in adults, but findings from our indirect comparison meta-analyses suggest that the chances for clinically significant improvements in adults using longer-acting stimulants were no better, if not somewhat more modest, than with the shorter-acting stimulants. Furthermore, we are not aware of any evidence from randomized controlled trials that addresses the issues of potential improvements in adherence or in decreased risk of abuse or diversion with the longer-acting stimulant preparations.
In conclusion, this systematic review and indirect comparison meta-analysis of placebo-controlled trials found that conventional shorter-acting stimulants are currently distinguished as having a more favorable balance of benefits and harms compared to newer non-stimulants and longer-acting stimulants in adults. In indirect comparison meta-analyses, shorter-acting stimulants proved superior in reducing ADHD symptoms in adults and had comparable tolerability profiles relative to longer-acting stimulants and non-stimulants. High-quality, head-to-head trials are desperately needed to confirm our findings, however, and would provide the best assessment of comparative effectiveness and safety among these drugs.
Another important finding is the identification of key gaps in the current evidence in the areas of academic, occupational, and social functioning, the potential roles of ADHD subtype and/or comorbidities in treatment, misuse/diversion of the drugs, cardiovascular toxicity, and effects over longer-term treatment durations. Evolving evidence regarding the comparative effectiveness and safety of competing ADHD drugs on these key parameters will be of great value in guiding future treatment recommendations.
References
Anonymous (2001) Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews CRD Report Number 4 (2nd edition). York, UK, NHS Centre for Reviews and Dissemination
Asherson P (2005) Clinical assessment and treatment of attention deficit hyperactivity disorder in adults. Expert Rev Neurother 5:525–539
Barkley RA (2004) Driving impairments in teens and adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 27:233–260
Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T et al (2006) A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 59:829–835
Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, Swensen AR (2005) Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin 21:195–206
Carpentier PJ, de Jong CA, Dijkstra BA, Verbrugge CA, Krabbe PF (2005) A controlled trial of methylphenidate in adults with attention deficit/hyperactivity disorder and substance use disorders. Addiction 100:1868–1874
Chou R, Fu R, Huffman LH, Korthuis PT (2006) Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta-analyses [see comment]. Lancet 368:1503–1515
Dodson WW (2005) Pharmacotherapy of adult ADHD. J Clin Psychol 61:589–606
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test [see comment]. BMJ 315:629–634
Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A (2002) Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 31:140–149
Food and Drug Administration (2006a) Drug Safety and Risk Management Advisory Committee Meeting, vol 2006
Food and Drug Administration (2006b) Pediatric Advisory Committee, vol 2006
Food and Drug Administration Drugs@FDA, http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D (2001) Current methods of the third U.S. Preventive Services Task Force. Am J Prev Med 20:21–35
Kessler RC, Adler L, Ames M, Barkley RA, Birnbaum H, Greenberg P et al (2005) The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med 47:565–572
Kessler RC, Adler L, Barkley RA, Biederman J, Conners CK, Demler O et al (2006) The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry 163:716–723
Kooij JJ, Burger H, Boonstra AM, Van der Linden PD, Kalma LE, Buitelaar JK (2004) Efficacy and safety of methylphenidate in 45 adults with attention-deficit/hyperactivity disorder. A randomized placebo-controlled double-blind cross-over trial. Psychol Med 34:973–982
Levin ED, Conners CK, Silva D, Canu W, March J (2001) Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol 9:83–90
Levin F, Evans S, Brooks D, Kalbag A, Garawi F, Nunes E (2006) Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend 81:137–148
Levin FR, Evans SM, Brooks DJ, Garawi F (2007) Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend 87:20–29
Matza LS, Paramore C, Prasad M (2005) A review of the economic burden of ADHD. Cost Eff Resour Alloc 3:5
McDonagh MS, Peterson K (2006) Drug class review of pharmacologic treatment of ADHD, vol 2006
McGough JJ, Smalley SL, McCracken JT, Yang M, Del’Homme M, Lynn DE, Loo S (2005) Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 162:1621–1627
Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ et al (2003) Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 53:112–120
Montano B (2004) Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 65(Suppl 3):18–21
Paterson R, Douglas C, Hallmayer J, Hagan M, Krupenia Z (1999) A randomised, double-blind, placebo-controlled trial of dexamphetamine in adults with attention deficit hyperactivity disorder [comment]. Aust N Z J Psychiatry 33:494–502
Reimherr FW, Hedges DW, Strong RE, Marchant BK, Williams ED (2005) Bupropion SR in adults with ADHD: A short-term, placebo-controlled trial. Neuropsychiatric Disease and Treatment 1:245–251
Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK (2007) A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 68:93–101
Riccio CA, Wolfe M, Davis B, Romine C, George C, Lee D (2005) Attention deficit hyperactivity disorder: manifestation in adulthood. Arch Clin Neuropsychol 20:249–269
Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N et al (2002) Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol 10:286–294
Secnik K, Swensen A, Lage MJ (2005) Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. Pharmacoeconomics 23:93–102
Song F, Altman DG, Glenny A-M, Deeks JJ (2003) Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. Br Med J 326:472
Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K (1995) A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 52:434–443
Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J et al (1998) Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 155:693–695
Spencer T, Biederman J, Wilens T, Faraone S, Prince J, Gerard K et al (2001) Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder [comment]. Arch Gen Psychiatry 58:775–782
Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J et al (2005) A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 57:456–463
Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L, Adult ARG (2007) Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 61:1380–1387
Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux P-Y, Secnik K (2004) Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health 35:346.e341–349
Terrin NSCH, Lau J (2005) In an empirical evaluation of funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol 58:894–901
Weisler RH, Biederman J, Spencer TJ, Wilens TE, Faraone SV, Chrisman AK et al (2006) Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr 11:625–639
Weiss M, Hechtman L, The Adult ARG (2006) A randomized double-blind trial of paroxetine and/or dextroamphetamine and problem-focused therapy for attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry 67:611–619
Wender PH, Reimherr FW, Wood D, Ward M (1985) A controlled study of methylphenidate in the treatment of attention deficit disorder, residual type, in adults. Am J Psychiatry 142:547–552
Wilens TE, Spencer TJ, Biederman J, Girard K, Doyle R, Prince J et al (2001) A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 158:282–288
Wilens TE, Haight BR, Horrigan JP et al (2005) Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 57:793–801
Acknowledgments
The authors thank Sujata G. Thakurta, MPA:HA, Research Assistant 2 for her substantial contribution to this work in the areas of administrative, technical, and material support.
Funding for this systematic review and meta-analysis was supported by the Drug Effectiveness Review Project (DERP). The authors retained full and independent control of the design of this meta-analysis; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the final manuscript. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no financial interest in any company that makes or distributes any of the products reviewed in this report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial disclosures
None of the authors or the person named in the Acknowledgments section have any financial interest in any company that makes or distributes the products reviewed in this report.
Rights and permissions
About this article
Cite this article
Peterson, K., McDonagh, M.S. & Fu, R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology 197, 1–11 (2008). https://doi.org/10.1007/s00213-007-0996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0996-4