Abstract
Rationale and objectives
Flinders sensitive line (FSL) rats, an animal model of depression, display a different pattern of maternal behavior compared to Sprague-Dawley (SD) controls. In this study, we examined the rewarding value of mother–infant interaction for FSL dams.
Materials and methods
In the main study, we measured monoamine levels in the nucleus accumbens (NAc) of early postpartum FSL and SD dams during an interaction with pups, using the microdialysis technique. In addition, we compared the preference patterns of FSL and SD rats using the conditioned place preference paradigm, with pups as the unconditioned stimuli.
Results
Dopamine (DA) levels in dialysates from the NAc of SD dams but not FSL dams were elevated while interacting with pups but the metabolism of DA to dihydroxyphenylacetic acid was greater in FSL than in SD dams. While SD dams showed a conditioned preference for a region that was associated with SD pups, FSL dams did not show a preference for regions associated either with SD or FSL pups, but water deprived FSL rats demonstrated a preference to a region associated with water, eliminating an alternative explanation of learning deficit in FSL rats.
Conclusions
Taken together, these results suggest that FSL dams are less rewarded by pups, compared to control dams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Flinders sensitive line (FSL) rat was derived from Sprague-Dawley (SD) rats, and was found to meet the various validity criteria for an animal model of depression (Overstreet 1993, 2002; Overstreet et al. 2005; Yadid et al. 2000a,b). Among other characteristics, it was found that FSL male rats show exaggerated immobility in a 5-min variation of the forced swim test (Overstreet 1986, 1993, 2002; Yadid et al. 2001), which is a commonly used paradigm for screening antidepressant drugs (Overstreet et al. 2005; Porsolt et al. 1977, 1978). Chronic treatment with various antidepressants counteracted the exaggerated immobility of FSL rats (Schiller et al. 1992; Zangen et al. 1997, 1999, 2001). When examined after acute or chronic exposure to stressors, FSL male rats exhibited a greater decrease in saccharin intake compared to controls (Ayensu et al. 1995; Pucilowski et al. 1993) and were considered to have stress-induced anhedonia (Pucilowski et al. 1993; see also Overstreet 1993, 2002; Overstreet et al. 2005).
A recent study (Lavi-Avnon et al. 2005a) found that FSL postpartum females also show exaggerated immobility in the swim test in the first and third postpartum weeks. Moreover, FSL female rats were found to express different patterns of maternal behaviors towards their infants in these two postpartum periods, compared to Sprague-Dawley (SD) controls (Lavi-Avnon et al. 2005a,b). Specifically, FSL dams are less motivated when monitored for licking and non-nutritive contact with pups and show less nutritive contact. The latter finding is most prominent in the third week postpartum. These findings extended the face validity of the FSL model into the realm of maternal behavior because human mother–infant interactions are different in depressed and nondepressed dyads (Field 1984, 1992; Field et al. 1985, 1988; Murray et al. 1996).
Dopamine (DA) levels in the nucleus accumbens (NAc)—a brain region involved in reward, motivation, and hedonia (Ikemoto and Panksepp 1999)—were studied in male FSL rats. Tissue DA levels are higher in the NAc and in the striatum of FSL rats compared to SD controls (Dremencov et al. 2004; Zangen et al. 1999) but basal extracellular DA levels in the NAc of FSL rats are lower than in control SD rats (Dremencov et al. 2004, 2005; Zangen et al. 2001). These findings led to the suggestion that FSL male rats have either decreased neuronal release of DA, or increased neuronal reuptake, in dopaminergic neurons of the mesolimbic pathway (Dremencov et al. 2004, 2005; Yadid et al. 2000a,b; Zangen et al. 2001). This is in accordance with the growing recognition of the role of the mesolimbic DA reward circuit in depression (Nestler and Carlezon 2006).
In the current study, we focused on the involvement of the reward system of FSL rats and functioning of their dopaminergic system in maternal behavior. This issue is of great interest, considering that maternal behavior is largely regulated by the reinforcing value of the pups to the dam. Research has demonstrated the reinforcement of pups to the rat dam; however, these studies examined dams from normal, nondepressed strains. Using the conditioned place preference (CPP) paradigm to demonstrate reinforcement, maternal-behaving rats have been shown to prefer a pup-associated box over a nonpup-associated box, as long as they are not experimentally deprived from the pups’ olfactory and somatosensory cues during the exposure phases (Fleming et al. 1994; Magnusson and Fleming 1995). This tendency was found to be dopamine dependent (Fleming et al. 1994). In addition, on day 8 postpartum, dams prefer a pup-associated box over a cocaine-associated box, while later in lactation, this pattern of preference is reversed (Mattson et al. 2001).
Maternal behaviors of normal rats are also associated with different levels of dopamine, which have a crucial role in regulating reinforcement and reward. Pharmacological approaches have found that systemic and central NAc injections of DA agonists and antagonists affect different aspects of maternal behavior in rats (Giordano et al. 1990; Keer and Stern 1999; Silva et al. 2001; Stern and Keer 1999; Stern and Protomastro 2000; Vernotica et al. 1999). In vivo experiments revealed changes in extracellular DA levels in the NAc shell of lactating rats while interacting with pups as a function of the amount of time spent licking/grooming the pups (Champagne et al. 2004). In addition, a microdialysis study (Hansen et al. 1993) has shown that maternal behavior enhances DA release in the NAc. Finally, a fMRI study revealed an increase in the activity of the mesolimbic dopaminergic system while SD dams were interacting with pups but not while they were exposed to cocaine (Ferris et al. 2005).
Recently increasing interest is aimed toward the mechanisms underlying the relationship between maternal behavior and depression (Newport et al. 2002; Smith et al. 2004) because depression is one of the most widespread health disorders in women, and it frequently occurs after childbirth (Hopkins et al. 1984; Marcus et al. 2001; Ohara et al. 1984; Stowe and Nemeroff 1995). To examine whether an abnormal brain reward system has a role in the abnormal profile of maternal behavior seen in FSL dams and to elucidate the involvement of the dopaminergic system in the maternal behavior of the FSL dams, we performed a microdialysis study in which microdialysate from the NAc shell was collected from both FSL and SD dams while interacting with their pups. Because recent data suggest an involvement of both dopaminergic and serotonergic systems in depression (Dremencov et al. 2004, 2005; Esposito 2006; Bonhomme and Esposito 1998), we examined the levels of both neurotransmitters and their metabolites in dialysates. We hypothesized that FSL dams will show lower accumbal DA levels and turnover compared to controls, while interacting with pups. Moreover, strain differences in maternal care (examined in terms of nutritive [nursing] and non-nutritive behaviors) were predicted to be associated with alterations in the pup-care-induced response of the dopaminergic system.
We also conducted a behavioral CPP test with pups as the rewarding, unconditioned stimulus. In this test, FSL and control SD dams interacted with pups, and then were tested for preference to the pup-associated box. A follow-up experiment was conducted with water as the unconditioned stimulus, to examine learning and memory processes or physical disabilities. If reward underlies maternal behavior, then FSL dams will be expected to show less preference to the region associated with pups compared to controls when tested for CPP.
Materials and methods
General method
Virgin SD and FSL female rats were mated with males from the same strain in their breeding colonies, in the Developmental Psychobiology laboratory at Bar-Ilan University. Both lines were likely to be inbred because of the relatively small number of original parents. Pregnant rats were housed individually in a clean polycarbonate cage (18.5 cm height × 26.5 cm width × 43 cm length), with a stainless steel wire lid and wood shavings as bedding material. Food and water were freely available (except in experiment 2b). The colony room was on a 14:10 h light/dark cycle, with lights on at 05:00. Room temperature was maintained at 22 ± 2°C. The isolated females were checked daily for parturition. Newborn litters that were found until 12:00 h each day were designated as born on that day (day 0). The study was approved by the Institutional Animal Care and Experimentation Committee, in accordance with Israeli law requirements. Animal care adhered to the “Principles of laboratory animal care”, as well as the guidelines of the Society for Neuroscience. In all tests, a deprivation from the stimulus was conducted to make rewarding effects more prominent.
Experiment 1—microdialysis
The procedure was based on the work of Hansen et al. (1993) and performed as described previously (Zangen et al. 1999). Subjects were six primiparous FSL and seven primiparous SD dams raising their own pups. Litters were culled to six at postpartum day 1. All microdialysis tests were performed on days 5–7 postpartum. Immediately after separation from pups, dams were anesthesized i.p. with a 100 mg/kg ketamine + 10 mg/kg xylazine solution. Pups were removed to a warm and humid incubator and stayed there overnight until they were reunited with their mother, approximately 20 h after separation.
Surgery
A guide cannula was surgically implanted (1.4 mm anterior, 1.2 mm lateral from bregma and 5.6 mm ventral to dura, (Paxinos and Watson 1998)), using a stereotactic device (David-Kopf Instruments, Tujunga, CA, USA) and then cemented to the skull. A microdialysis probe (2 mm in length, 20 kDa cutoff value, CMA/12; Carnegie Medicine, Stockholm, Sweden) was immediately inserted through the guide cannula directly to the NAc shell. The animal was then placed into a circular transparent cage (37 cm in diameter), the floor being covered with wood shavings as bedding material. Food and water were available ad libitum.
Microdialysate collections
Experiments were initiated 18–20 h after probe implantation, in awake freely moving rats. Artificial CSF (145 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, and 1.0 mM MgCl2, pH 7.4) was continuously pumped (1 μl/min) through the dialysis probe using a microinjection pump (CMA/100, Carnegie Medicine, Sweden). Samples were collected at 20 min intervals into polyethylene tubes with 15 μl of a 0.02% EDTA and 1% ethanol solution for preservation, and were immediately stored at −70°C until assayed. After five baseline sample collections, the pups were returned to their mother for 2 h. Over this period, six samples were collected. Then, pups were removed from the dam and four more samples were collected. After this sample collection, dams were overdosed with chloral hydrate and the brains were removed to verify location of probe implantation. During the mother–pups interaction, the durations of nutritive contact, non-nutritive contact and licking were continuously recorded.
Analysis of extracellular monoamine levels in dialysates
Thawed dialysates were injected into the solvent stream of a high pressure liquid chromatography/electrochemical detection apparatus equipped with a 50 × 4.6 mm; 3 μ particle Spherisorb S30DS2 reversed phase column (Regis, IL, USA) at ambient temperature. The mobile phase (1 ml min−1) consisted of 6.9 g NaH2PO4, 200 mg heptane-1-sulfonic acid (sodium salt), 80 mg disodium ethylene-diaminetetra-acetic acid (Na2EDTA), and 30 ml methanol per liter and was adjusted to pH 2.6 using orthophosphoric acid. Catecholamines and metabolites in the eluate were detected using a model 5100A Coulochem detector (ESA, MA, USA) with model 5011 analytical cell and model 5020 guard cell (ESA, USA). The first electrode of the dual electrode analytical cell was set at +0.06 V and compounds were detected at the second electrode, set at −0.26 V reduction potential. Output of the detector was analyzed using Borwin software, and quantitation of peaks was carried out by comparing peak heights of samples with those of standard solutions. The detection limits for the various compounds are as follows: DA 1 nM, dihydroxyphenylacetic acid (DOPAC) 1 nM, HVA 2 nM, 5-HT 1 nM, and 5-HIAA 2 nM. Metabolism of DA to DOPAC was expressed as DOPAC/DA ratio.
Experiment 2a—CPP for pups
Only synchronized parturitions (i.e., no more than 24 h interval between them) were used in this experiment. Each dam was designated to be an experimental dam or a donor dam. Experimental dams were nine SD and nine FSL dams exposed to SD and FSL foster litters (from the donor dams), respectively. A third group was added, in which nine FSL dams were exposed to SD foster litters. This group was added to receive, at least partially, an answer to the question of whether the pattern of results shown by FSL dams is because of an abnormal reward system of the dam or because of abnormal, nonrewarding characteristics of FSL pups. All pups were fostered to control for the fostering effect that was unavoidable in the third group. We had no a priori reason to study a potential fourth group in which SD dams would have been exposed to FSL foster litters, as this study was not focused on examining modification of the pup-preference of SD dams. Moreover, because FSL litters are usually very small, there would have been great difficulties in finding synchronized parturitions that fit this condition. The experimental dams were separated from their litters on postnatal day 1, and were moved to a clean cage with no pup cues. Litters of the donor dams were culled to six pups, with sex distribution kept as equal as possible in each litter. A CPP procedure was conducted through days 2–6 postpartum, as described in previous literature (Fleming et al. 1994; Magnusson and Fleming 1995).
Apparatus
The CPP apparatus consisted of a white plastic box, 22 cm w × 90 cm l × 30 cm h, which was divided into two compartments (22 cm w × 40 cm l × 30 cm h), and a separation section between them (22 cm w ×10 cm l ×30 cm h). The two compartments were visually and texturally different from each other: one contained vertical black and white stripes on its walls and a smooth texture floor. The other contained gray walls (with an intermediate hue) and equally spaced perforations on its floor. The lid of the latter compartment was decorated with four black stripes to further distinguish between the two compartments. The separation section was neutral, containing white walls and smooth floor. On exposure days, the dividers between the compartments were closed. On test days, a 10-cm gate was opened between each compartment and the separation section, allowing the dam to freely access both compartments. Pilot data revealed that there was no difference in the baseline preference of postpartum rats to any of the compartments. Moreover, the design was counterbalanced and each rat was exposed to pups in only one of the compartments.
Procedure
Twenty-three hours after separation from pups, the CPP procedure began. For each dam, one compartment was used as the pup-exposure compartment, and the other was used as the no-pup compartment.
Exposure phase
On days 2 and 4 postpartum, dams were moved to the pup exposure compartment and after 60 s, 6 pups (1–5 days old) from a donor mother were introduced into one corner of that compartment (at the far corner away from the separation section). A 59-min interaction then began, and maternal observations (spot checks) were made every 10 min, using a 1/0 sampling of the behaviors defined below. On days 3 and 5 postpartum, dams were moved to the no-pup compartment, and were left in that compartment for a 60-min period. On these days, 1 h after returning the dams to their home cages, six pups (1–5 days old) were introduced to them for a 1-h period. In this way, pup deprivation was kept between 21–23 h.
Maternal observations
A total of six spot checks were made in each dam–pup interaction session. Frequencies of the behaviors were recorded according to these definitions:
- Nutritive contact:
-
the dam is immobile/passive while crouching over the pups, with at least half of them under her ventrum
- Non-nutritive contact:
-
the dam is over or near the pups, in contact with at least half of them, while active and engaging in other behaviors directed towards either pups or herself
- Licking:
-
the dam performs repetitive tongue and head movements over the pup’s body or anogenital region
- Carrying:
-
the dam picks up the pup, with or without moving it to another location in the cage
The mean weight of pups was recorded after each exposure phase because SD rats are usually found to be heavier than FSL rats and weight may play a role in the reinforcing properties of pups to the dam. All dams were weighed after the CPP procedure was finished to detect the common weight differences between lines.
Test phase
The test phase occurred in the absence of pups. On day 6 postpartum, the dam was moved to the separation section in the middle of the CPP apparatus. The separation gates were then removed and the dam was allowed to move freely in the apparatus for 10 min. This phase was recorded on videotape and analyzed off-line, by an experimenter that was blind to both the dam’s line and the line of pups that were introduced during the exposure phase. Using a computer-based event recorder, the time spent in each of the compartments was recorded.
Experiment 2b—CPP for water
To make sure that the pattern of results seen in experiment 2a, was not a byproduct of a basic deficit of FSL female rats in learning or memory, we further examined whether FSL female rats could distinguish between the two CPP compartments, when water was used as a reward. Subjects were 10 FSL and 11 SD adult female rats. Because water is considered a potent natural reinforcer, two changes were made from our initial design, to avoid a ceiling effect in preference. These changes followed previous literature on CPP conditioning with water (Agmo et al. 1993): (1) exposure phases were 30 min long (resulting a deprivation period of 22–23.5 h), and (2) a 10-min pretest was conducted for each animal before the CPP procedure began; the water was introduced during the exposure phases in the initially less preferred compartment (as in Agmo et al. 1993).
Data analysis and statistics
Independent t tests were performed to compare the basal levels of amines and metabolites between the two animal groups. For each amine and metabolite, a two-way analysis of variance (ANOVA) was also performed, with animal group as the independent measure and collection number as the repeated measure. Wherever a significant interaction effect was found, simple contrasts were computed to reveal the differences in specific collection periods. To examine the pattern of passive and active maternal behaviors in each animal group, passive nutritive and active non-nutritive contact were compared using an ANOVA test with the collection number as the repeated measure.
In the CPP procedures, the measure of preference was calculated as the proportion of time spent in the US-associated compartment, relative to the total time spent in the two compartments, and independent samples t tests were performed on this measure between groups. Frequencies of maternal behaviors were summed for each dam and then compared between groups using the multivariate analysis of variance.
Results
Experiment 1—microdialysis
The basal levels of the amines and their metabolites in NAc microdialysate are presented in Table 1. As seen in the table, there were no significant differences between groups.
Analysis of DA levels throughout the 15 collection periods (20 min each) revealed a strain main effect, with higher DA levels expressed by SD dams compared to FSL dams, F(1,11) = 14.06, p < 0.01. In addition, a significant strain × collection time interaction was found, F(15,165) = 2.34, p < 0.01. This latter finding indicated differences between strains in the pattern of change in DA levels upon exposure to pups. As can be seen from Fig. 1, the strains were significantly different from each other throughout periods 8–11 and 14. One should note that pups were introduced to dams at the beginning of period 6, and were taken out at the beginning of period 12.
Analysis of DOPAC and HVA microdialysate levels did not reveal significant effects. However, compared to the basal levels, upon pup exposure, there was a nonsignificant increase of these metabolites in FSL dams (percentage of increase of the highest peaks from baseline, 135.1 ± 32.1% and 133.1 ± 20.2%, respectively), which was more than in SD dams (percentage of increase of the highest peaks from baseline, 107.5 ± 22.5% and 121.3 ± 18.7%, respectively).
When analyzing the metabolism of DA to DOPAC, a significant strain main effect (F(1,11) = 8.3, p < 0.05) and strain × collection time interaction (F(15,165) = 1.88, p < 0.05) were found. As can be seen from Fig. 2, at periods 8, 9, 14, and 15, FSL dams expressed a higher metabolism of DA to DOPAC compared to SD dams, indicating a higher rate of intraneuronal DA metabolism (Eisenhofer et al. 2004). Changes in (DOPAC+HVA)/DA during the pup exposure period were not significant, because of a high degree of variability in the HVA levels.
Levels of 5HT, 5HIAA, and 5HIAA/5HT did not reveal any significant effects. However, in both strains, there was a nonsignificant increase in 5HT levels at the time of pup exposure, although not at the same collection time (percentage of increase of the highest peaks from baseline: 249 ± 53.86% in FSL dams and 232 ± 87.61% in SD dams).
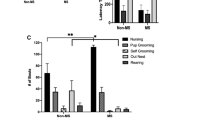
Differences were found between strains also in the pattern of maternal behaviors that were expressed throughout the dam–pups interaction: A significant interaction between behavior type (active/passive) and collection period was found in SD dams (Fig. 3a; F(5, 30) = 3.39, p < 0.05) but not in FSL dams (Fig. 3b). Thus, SD dams showed a gradual increase (of 287%) in nutritive (passive) contact and gradual decrease (of 475%) in non-nutritive (active) contact, across periods 6–9. This pattern was reversed between periods 9–11, in which a decrease (of 197%) in nutritive contact and an increase (of 237%) in non-nutritive contact were observed. In contrast, FSL dams showed a continuous, nonsignificant tendency to increase nutritive and decrease non-nutritive contacts. Licking was a central component of the non-nutritive contact episodes. Therefore, although it was not analyzed statistically, its pattern of change is presented in Fig. 3 to enable an estimation of its relative part in the dam–pups interaction.
Durations in seconds (mean±SE) of passive and active maternal behaviors shown by SD dams (a) and FSL dams (b) in the six fractions (6–11) in which dam–pups interaction was allowed. Patterns of passive nutritive and active non-nutritive contact were compared using an ANOVA test with the collection number as the repeated measure. Licking was a central component of the non-nutritive contact episodes. Therefore, although it was not analyzed statistically, its pattern of change is presented here to enable an estimation of its relative part in the dam–pups interaction
Finally, when comparing the maternal behavior of FSL and SD dams, a significant strain effect was found in the duration of non-nutritive contact, F(1,11) = 5.22, p < 0.05, with FSL dams engaging more in non-nutritive contact than SD dams (466.41 ± 50.45 vs 309.314 ± 46.7). This fits with the pattern above, where SD dams spend more time nursing, while FSL dams show a less differentiated pattern of maternal behavior.
Experiment 2a—CPP for pups
As shown in Fig. 4, while SD dams showed a significant preference for the SD-pup-associated compartment (59.15%), t(16) = 2.2, p < 0.05, FSL dams did not show a preference for the FSL-pup-associated compartment (49.6%). In addition, when comparing the preference of FSL dams to the compartment paired with FSL or SD pups, no significant differences were found (49.57 vs 49.63%, respectively, p > 0.1). In all groups, average duration spent in the center compartment was shorter (M = 165.44, 168.89, and 187.89), though not significantly so, than the duration spent in the other two compartments (M = 215.22 and 219, 256.66 and 176.33, 204.22 and 207.77, respectively), thus eliminating the possibility of a preference for the novel environment.
A few differences were found in the maternal behaviors observed. Our first comparison, between FSL and SD dams that interacted with pups from their same strain revealed an overall significant strain main effect, F(4,13) = 4.48, p < 0.05. Specifically, FSL compared to SD dams, showed greater frequency of carrying pups in their mouth F(1,16) = 14.55, p < 0.01. In addition, there was a nonsignificant tendency of FSL dams to show less nutritive contact compared to SD dams F(1,16) = 4.26, p = 0.055. It is interesting to note that FSL dams did not show any differences in maternal behavior when interacting with FSL compared to SD pups (all values of p > 0.1). Mother’s weight was significantly lower in FSL dams compared to SD dams, t(26) = 5.53, p < 0.001, but the overall pups’ weight was not different between groups (all values of p > 0.1). The latter finding may be a result of the large range of pups’ ages.
Experiment 2b—CPP for water
FSL rats showed a greater preference (62.32%) for the water-associated compartment compared to SD rats (50.52%), t(19) = 2.35, p < 0.05, indicating no general deficit of FSL rats in learning to differentiate between the apparatus compartments.
Discussion
In SD but not FSL dams, maternal behavior was associated with an increase in NAc DA while the dam was interacting with her pups. Changes in the Nac serotonergic system during mother–pup interaction were not significant (differences in other areas are possible), suggesting a specific role for the Nac dopaminergic system in this situation.
In the SD dams, initial reunion with the pups did not produce an immediate DA increase. Instead, DA levels in the first hour gradually increased in parallel with increasing nursing (r = 0.93) and decreasing non-nutritive contact (r = −0.92). DA levels peaked approximately 1 h after pups were introduced to the dam and remained at high levels until pups were removed (Fig. 1).
These findings replicate, extend and elaborate on the previous NAc microdialysis report of Hansen et al. (1993), in which DA was elevated by 23% in a 1-h dam–pups reunion. The dams in that study spent over 1/2 of the time nursing and 1/6 of the time pup-licking/sniffing. The current findings show a similar DA increase over the first hour of reunion (24.4%), a general replication of the average maternal behavior levels (nursing M = 589 s per collection period (approximately 1/2 of the time), non-nutritive M = 308 s per fraction (approximately 1/4 of the time)), and interesting contrasting patterns over time of DA with nutritive and non-nutritive behaviors, as described above.
In the second hour of observation, DA levels peaked and remained at this high level (70–80% increase) for the duration. Remarkably, in the first assessment after pup removal, DA levels dropped completely back to baseline. During this second hour, nursing levels first peaked then decreased, while non-nutritive contact slightly decreased and then moderately increased.
Previous studies showed that DA-1 and DA-2 (D1/D2) receptor agonists increased non-nutritive and decreased nutritive contact, while D1/2 antagonists produced the opposite pattern (Giordano et al. 1990; Keer and Stern 1999; Silva et al. 2001; Stern and Protomastro 2000). Administration of a D1/2 antagonist to the NAc and striatum decreased pup licking and increased suckling-induced kyphosis, effects attributed to a cataleptic impact of the D1/2 blockade (Keer and Stern 1999). Our results in the second hour of pup exposure, when DA levels are at peak levels, support these studies.
Our current microdialysis study and that of Hansen et al. (1993) suggest that DA levels gradually increase in the NAc while there is a parallel increase in nursing. Thus, DA may promote nutritive care through its action on other receptors, possibly D3 type, which are expressed primarily in limbic brain areas, and their effects are mostly inhibitory (e.g., Bouthenet et al. 1991; Diaz et al. 2000; Menalled et al. 1999; Xu et al. 1997; Richtand 2006). More specifically, activation of D3 receptors in the NAc has been shown to decrease exploratory locomotion dose-dependently (Pritchard et al. 2003). In addition, Xu et al (1997) suggested that D3’s function is to modulate motor and reward-related behaviors by inhibiting the cooperative effects of postsynaptic D1 and other D2 class receptors. It is interesting to note that this inhibitory effect can be overcome with sufficient levels of synaptic DA. Therefore, we speculate that the relations between DA and nursing may be related to the different action of DA via its different receptors (Richtand 2006).
In FSL dams, in contrast, there was no significant change in DA levels during the course of mother–infant interaction. These dams did not show evidence of prolonged nursing periods; instead in each 20-min observation period, throughout the 2-h study, they nursed about 1/2 of the time (M = 633 s for each fraction), and spent an almost similar time performing non-nutritive contact in four out of the six observation periods. Thus, while SD dams showed a clear differentiation between the expression of nursing vs non-nutritive behaviors, devoting most of their maternal behavior to nursing after initial contact with the pups, FSL dams did not appear selectively goal-oriented towards nursing in most of the observation periods.
It has been suggested that responses to rewards and reward-predicting stimuli activate mesolimbic DA neurons (e.g., Wightman and Robinson 2002). It would appear that reunion with the pups is not rewarding for FSL dams, according to this measure. Nevertheless, even without a DA response in the NAc, these dams still perform general maternal behaviors, and raise their pups (though in abnormal patterns; see Lavi-Avnon et al. 2005a,b). This shows the additional importance of other neural systems in mediating maternal care (Gammie 2005; Numan 2006).
Previous data have established that increase of accumbal DA is associated with reward and reward-predicting stimuli (cf., Schultz 2004). In this sense, initial contact of the dam with the pups after reunion could be viewed as either type of stimulus, either intrinsically rewarding or predicting future nursing-related reward. Accordingly, we have observed DA increases in association with dam–pup reunion in our current experiment. However, microdialysis studies such as ours, with intervals between sampling of several minutes, do not have an adequate temporal resolution to answer the question of whether the increased dopamine preceded and possibly triggered nursing or vice versa. We note that the new technique of fast-scan cyclic voltammetry (Carelli 2004) may have the potential to resolve this issue, as it resolved the dopamine responses to contingent (response dependent) and noncontingent cocaine administration, and dopamine release time-locked to reinforced responses, in rats self-administering cocaine (Stuber et al. 2005). In general accordance, we speculate that in nondepressed/normal dams separated and then reunited with their pups-Nac DA may increase when they smell/see the pups (Fleming et al. 1996; Fleming and Walsh 1994, found c-fos increases in the Nac), even before the actual rewarding contact and nursing events.
In the CPP procedure, FSL dams did not prefer the pup-associated compartment, regardless of the strain of pups they were exposed to. In contrast, SD dams did show a preference for the compartment that was associated with SD pups. SD rats did not show a preference to the water-associated box, for unknown reasons. In contrast, FSL female rats demonstrated their ability to make such CPP associations to the water-associated region, ruling out the possibility of physical abnormalities as well as learning and memory deficits in FSL dams, and rather indicating that FSL dams may be selectively unrewarded (or “maternally anhedonic”) by pups. These results correspond to the “stress-induced anhedonia” characteristic of FSL rats (Pucilowski et al. 1993; see also Overstreet 1993, 2002), considering that the early postpartum period and demands of lactation and protection of the newborn pup may represent a stress-laden situation. Furthermore, the absence of pup-related preference shown by FSL rats, and the previous literature that reported an abnormal reward system (Dremencov et al. 2004, 2005; Yadid et al. 2000a,b; Zangen et al. 1999, 2001) suggest deficient DA neurotransmission in FSL rats, as was shown also in the microdialysis study.
When examining the maternal behavior frequencies in the CPP apparatus, FSL dams tended to be seen less in nursing postures, and showed greater frequencies of carrying pups. This pattern may reflect a stressful reaction of the FSL dams, preventing them from being in the “relaxed” posture of nursing. Accordingly, our previous study that examined maternal behavior in FSL and SD dams in their home cages revealed a tendency for FSL rats to express less nursing and more carrying of pups, especially after exposure to stress (Lavi-Avnon et al. 2005b).
It appears that FSL dams have a higher metabolism of DA to DOPAC compared to SD dams, during exposure to the pups. One possibility is that the reuptake mechanism of FSL dams is overactivated. Such overactivity was previously suggested by Yadid et al. (2000a,b). This in turn can shorten the postsynaptic effects of DA and thus disrupt the intensity of the reinforcement (Feldman et al. 1997). Nevertheless, no significant differences were found in the density of [3H]GBR-12935 [a dopamine transporter (DAT) ligand] in the NAc shell of FSL and SD male rats (unpublished data). Also, clinical studies have shown that human subjects with major depression episodes have significantly lower DAT within all striatal regions (Meyer et al. 2001, 2002). Another possibility is based on a study by Schwartz et al. (2003), in which FSL male rats were characterized by lowered expression of vesicular monoamine transporters, compared to SD rats. This may indicate a deficit in DA recycling into the vesicles and thus higher levels of DOPAC in the terminal button and less DA spillover (Yadid et al. 2000a,b).
The finding that SD dams showed no increase either in DA metabolites (DOPAC and HVA) or in metabolism of DA to DOPAC after pup exposure was surprising. Nevertheless, some studies showed that the proportions between neurotransmitter synthesis and turnover rate, accompanied by the secretion of metabolites to the extracellular fluid, are not necessarily constant (Cumming et al. 1992; Hansen et al. 1993; Westerink and Kikkert 1986; Yadid et al. 2001).
This research has a number of limitations that should be mentioned. In the microdialysis study, dams were exposed to their own pups, but not in the CPP study. In addition, the dam–pup separation durations were different in the two studies. This practice was chosen in accordance with previously published protocols. Nevertheless, it could be that with their own pups, FSL dams would have shown place preference, although not showing pups-induced dopamine release. These procedural differences could also account for differences observed in patterns of maternal behavior in the two studies. We note also that while there is a clear difference in dopamine release between FSL and SD rats, differences in maternal behavior are not so clear-cut. Although not a sufficient explanation, it is our experience that the variability in behavioral variables, and their sensitivity to external influences is often greater than in physiological variables. In addition, the selectivity of the “maternal anhedonia” evident in this study by FSL dams towards pups can be questioned. Would these dams be similarly less interested/rewarded in any object put into their cage? To answer that, we performed a follow-up study, in which a novel object (a plastic doll, about 5-cm tall) was the target. The preliminary data showed that FSL rats showed significantly less (mean = 3 ± 0.75 s) physical contact with the novel object than control, SD rats (17 ± 2.5 s). Although we did not study DA release, this suggests that FSL rats are not selective in their curiosity/exploration of novel objects, and they may avoid both pups and other objects, as part of their general avoidant/anhedonic phenotype. We plan to test DA release in future experiments.
In total, the experiments described above support the hypothesis that the mother–infant interactions of FSL dyads are accompanied by abnormal limbic dopaminergic activity of the dam, which reflects and/or affects her deficient pup-reward mechanism. Consequently, lower DA response was found in the NAc of FSL dams while interacting with pups and when tested behaviorally, FSL dams did not express a preference to a pup-associated region. Finding the reward mechanisms involved in maternal behavior may highlight a possible brain mechanism for the maternal aspects of depression.
Abbreviations
- FSL:
-
Flinders sensitive line
- SD:
-
Sprague-Dawley
- Nac:
-
nucleus accumbens
- CPP:
-
conditioned place preference
- DA:
-
dopamine
- DOPAC:
-
dihydroxyphenylacetic acid
- HVA:
-
homovanilic acid
- 5HT:
-
serotonin
- 5HIAA:
-
5-hydroxyindoleacetic acid
References
Agmo A, Federman I, Navarro V, Pauda M, Velazquez G (1993) Reward and reinforcement produced by drinking water: role of opioids and dopamine receptor subtypes. Pharmacol Biochem Behav 46:183–194
Ayensu WK, Pucilowsky O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS (1995) Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav 57:165–169
Bonhomme N, Esposito E (1998) Involvement of serotonin and dopamine in the mechanism of action of novel antidepressant drugs: a review. J Clin Psychopharmacol 18:447–454
Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Carelli RM (2004) Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology 47(Suppl 1):180–189
Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ (2004) Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24:4113–4123
Cumming P, Brown E, Damsma G, Fibiger H (1992) Formation and clearance of interstitial metabolites of dopamine and serotonin in the rat striatum: an in vivo microdialysis study. J Neurochem 59:1905–1914
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P (2000) Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20:8677–8684
Dremencov E, Gispan-Herman I, Rosenstein M, Mendelman A, Overstreet DH, Zohar J, Yadid G (2004) The serotonin-dopamine interaction is critical for fast onset action of antidepressant treatment: in vivo studies in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry 28:141–147
Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, Yadid G (2005) Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 48:34–42
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349
Esposito E (2006) Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets 7:177–185
Feldman RS, Meyer JS, Quenzer LF (1997) Principles of neuropsychopharmacology. Sinauer Associates, Massachusetts
Ferris CF, Kulkarni P, Sullivan JM, Harder JA, Messenger TL, Febo M (2005) Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25:149–156
Field T (1984) Early interactions between infants and their postpartum depressed mothers. Infant Behav Dev 7:517–522
Field T (1992) Infants of depressed mothers. Dev Psychopathol 4:49–66
Field T, Sandberg D, Garcia R, Vega-Lahr N, Goldstein S, Guy L (1985) Pregnancy problems, postpartum depression and early mother–infant interactions. Dev Psychol 21:1152–1156
Field T, Healy B, Goldstein S, Perry S, Bendell D, Schanberg S, Zimmerman EA, Kuhn C (1988) Infants of depressed mothers show depressed behavior even with non depressed adults. Child Dev 59:1569–1579
Fleming AS, Walsh C (1994) Neuropsychology of maternal behavior in the rat: c-fos expression during mother-litter interactions. Psychoneuroendocrinology 19:429–443
Fleming AS, Korsmit M, Deller M (1994) Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology 22:44–53
Fleming AS, Morgan HD, Walsh C (1996) Experimental factors in postpartum regulation of maternal care. In: Rosenblatt JS, Snowdon CT (eds) Parental care: evolution, mechanisms and adaptive significance. Advances in the Studies of Behavior, 25. Academic, San Diego, CA, pp 215–242
Gammie SC (2005) Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev 4:119–135
Giordano AL, Johnson AE, Rosenblatt JS (1990) Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav 48:211–214
Hansen S, Bergvall AH, Nyiredi S (1993) Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav 45:673–676
Hopkins J, Marcus M, Campbell SB (1984) Postpartum depression: a critical review. Psychol Bull 95:498–515
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev 31:6–41
Keer SE, Stern JM (1999) Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav 67:659–669
Lavi-Avnon Y, Shayit M, Yadid G, Overstreet HD, Weller A (2005a) Immobility in the swim test and observations of maternal behavior in lactating Flinders sensitive line rats. Behav Brain Res 161:155–163
Lavi-Avnon Y, Yadid G, Overstreet DH, Weller A (2005b) Abnormal maternal behavior in a genetic animal model of depression. Physiol Behav 84:607–615
Magnusson JE, Fleming AS (1995) Rat pups are reinforcing to the maternal rat: role of sensory cues. Psychobiology 23:69–75
Marcus SM, Barry KL, Flynn HA, Tandon R, Greden JF (2001) Treatment guidelines for depression in pregnancy. Int J Gynaecol Obstet 72:61–70
Mattson BJ, Williams SE, Rosenblatt JS, Morell JI (2001) Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 115:683–694
Menalled LB, Dziewczapolski G, Garcia MC, Rubinstein M, Gershanik OS (1999) D3 receptor knockdown through antisense oligonucleotide administration supports its inhibitory role in locomotion. Neuroreport 10:3131–3136
Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH (2001) Lower dopamine transporter binding potential in striatum during depression. Neuroreport 12:4121–4125
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S (2002) Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berlin) 163:102–105
Murray L, Stanley C, Hooper R, King F, Fiori-Cowley A (1996) The role of infant factors in postnatal depression and mother–infant interactions. Dev Med Child Neurol 38:109–119
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159
Newport DJ, Stowe ZN, Nemeroff CB (2002) Parental depression: animal models of an adverse life events. Am J Psychiatry 159:1265–1283
Numan M (2006) Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev 5:163–190
Ohara MW, Neunaber DJ, Zekoski EM (1984) Prospective study of postpartum depression: prevalence, course, and predictive factors. J Abnorm Psychol 93:158–171
Overstreet DH (1986) Selective breeding for increased cholinergic function: development of a new animal model of depression. Biol Psychiatry 21:49–58
Overstreet DH (1993) The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17:51–68
Overstreet DH (2002) Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behav Genet 32:335–348
Overstreet DH, Friedman E, Mathe AA, Yadid G (2005) The Flinders sensitive line rat: a selective bred putative animal model of depression. Neurosci Biobehav Rev 29:739–759
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, New York
Porsolt RD, Pichon ML, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioral despair: a new model sensitive to antodepressant treatments. Eur J Pharmacol 47:379–391
Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM (2003) 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology 28:100–107
Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS (1993) Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav 54:1215–1220
Richtand NM (2006) Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology 31:2368–2375
Schiller GD, Pucilowski O, Wienicke C, Overstreet DH (1992) Immobility reducing effects of antidepressants in a genetic animal model of depression. Brain Res Bull 28:821–823
Schultz W (2004) Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol 14:139–147
Schwartz K, Yadid G, Weizman A, Rehavi M (2003) Decreased limbic vesicular monoamine transporter 2 in a genetic rat model of depression. Brain Res 965:174–179
Silva MRP, Bernardi MM, Felicio LF (2001) Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav 68:461–468
Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW (2004) Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29:227–244
Stern JM, Keer SE (1999) Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res 99:231–239
Stern JM, Protomastro M (2000) Effects of low dosages of apomorphine on maternal responsiveness in lactating rats. Pharmacol Biochem Behav 66:353–359
Stowe ZN, Nemeroff CB (1995) Women at risk for postpartum onset major depression. Am J Obstet Gynecol 173:639–645
Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863
Vernotica EM, Rosenblatt JS, Morell JI (1999) Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci 113:377–390
Westerink BH, Kikkert RJ (1986) Effects of various centrally acting drugs on the efflux of dopamine metabolites from the rat brain. J Neurochem 46:1145–1152
Wightman RM, Robinson DL (2002) Transient changes in mesolimbic dopamine and their association with “reward”. J Neurochem 82:721–735
Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S (1997) Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19:837–848
Yadid G, Harvey-White JD, Kopin IJ, Goldstein DS (2000a) Estimation of striatal dopamine spillover and metabolism in vivo. Neuroreport 11:3367–3373
Yadid G, Nakash R, Deri I, Grin T, Kinor N, Gispan I, Zangen A (2000b) Elucidation of the neurobiology of depression: insights from a novel genetic animal model. Prog Neurobiol 62:353–378
Yadid G, Overstreet DH, Zangen A (2001) Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res 896:43–47
Zangen A, Overstreet DH, Yadid G (1997) High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J Neurochem 69:2477–2483
Zangen A, Overstreet DH, Yadid G (1999) Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Res 824:243–250
Zangen A, Nakash R, Overstreet DH, Yadid G (2001) Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology 155:434–439
Acknowledgements
All authors deny any involvement, financial or otherwise, that might potentially bias this work. The research reported in this paper was completed as part of the first author’s Ph.D. dissertation, in the Department of Psychology, Bar-Ilan University, Ramat-Gan, Israel. YLA was supported by Bar-Ilan University’s President’s Fellowship for Outstanding Doctoral Students. Experiment 1 (the microdyalisis study) was supported by a predoctoral research grant from the Israel Foundation Trustees to YLA. Experiment 2 (the CPP study) was supported by a grant from the Israel Foundation Trustees to AW. A portion of this research was presented at the annual meeting of the International Society for Developmental Psychobiology, in June 2004, Aix en Provance, France. The experiments reported in this manuscript comply with the current laws of the State of Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavi-Avnon, Y., Weller, A., Finberg, J.P.M. et al. The reward system and maternal behavior in an animal model of depression: a microdialysis study. Psychopharmacology 196, 281–291 (2008). https://doi.org/10.1007/s00213-007-0961-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0961-2