Abstract
Rationale
Partial or complete ablation of serotonin transporter (SERT) expression in mice leads to altered responses to serotonin receptor agonists and other classes of drugs.
Objectives
In the current report, we review and integrate many of the major behavioral, physiological, and neurochemical findings in the current literature regarding pharmacological assessments made in SERT mutant mice.
Results
The absence of normal responses to serotonin reuptake inhibiting (SRI) antidepressants in SERT knockout (−/−) mice demonstrates that actions on SERT are a critical principle mechanism of action of members of this class of antidepressants. Drugs transported by SERT, (+)-3,4-methylenedioxymethamphetamine (MDMA) and 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP), are also inactive in SERT −/− mice. Temperature, locomotor, and electrophysiological responses to various serotonin receptor agonists, including 8-hydroxy-2-(di-n-propylamino)-tetraline (8-OH-DPAT), ipsapirone, and RU24969, are reduced in SERT −/− mice, despite comparatively lesser reductions in Htr1a and Htr1b binding sites, G-proteins, and other signaling molecules. SERT −/− mice exhibit an ∼90% reduction in head twitches in response to the Htr2a/2c agonist (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), associated with a profound reduction in arachidonic acid signaling, yet only modest changes in Htr2a and Htr2c binding sites. SERT −/− mice also exhibit altered behavioral responses to cocaine and ethanol, related to abnormal serotonin, and possibly dopamine and norepinephrine, homeostasis.
Conclusions
Together, these studies demonstrate a complex and varied array of modified drug responses after constitutive deletion of SERT and provide insight into the role of serotonin, and in particular, its transporter, in the modulation of complex behavior and in the pharmacological actions of therapeutic agents and drugs of abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The serotonin transporter (SERT, 5-HTT) is a major regulator of synaptic serotonin availability, and therefore of downstream signaling via the 14-plus pre- and postsynaptic serotonin receptors. Drugs that inhibit SERT are currently the most widely prescribed class of antidepressants and anxiolytics (Heydorn 1999; Jones and Blackburn 2002; Murphy et al. 1998). The biochemical consequences of chronic administration of SERT inhibitors [referred to as serotonin reuptake inhibitors (SRIs), including the more selective serotonin reuptake inhibitors (SSRIs)] are thought to include, foremost, an increase in extracellular levels of serotonin followed by neuroadaptive alterations in serotonin receptors and intracellular signaling pathways, as well as time-dependent effects on neurogenesis. Together, these changes are thought to contribute to the clinical effects of these drugs. Evidence in support of this comes from rodent models of depressive behaviors, typically based on learned helplessness, including the tail suspension test, forced swim test, and anhedonia measures, which are all sensitive to SRIs (Cryan and Holmes 2005; Perrault et al. 1992; Strekalova et al. 2006).
Mice with a homozygous targeted disruption of the SERT gene [SERT knockout (−/−) mice], and heterozygote littermate mice with 50% reductions in SERT (SERT +/− mice), have a superficially unremarkable behavioral phenotype, at least early in adulthood (Bengel et al. 1998). This is in contrast to dopamine transporter (DAT) knockout mice, which have an ∼25% reduction in overall body weight and marked hyperactivity, particularly in novel environments, as well as reduced survival rates 10 weeks after birth (Giros et al. 1996). The general neurochemical phenotype of SERT +/− and −/− mice somewhat resembles that of mice given SRIs in that they have elevated extracellular serotonin concentrations (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004) and downregulated or otherwise altered serotonin receptor binding sites and receptor signaling (Li et al. 1999, 2000, 2003; Qu et al. 2005), although SERT −/− mice differ in having reductions in basal tissue serotonin content in the brain and periphery, which are not compensated for despite increased serotonin synthesis (Bengel et al. 1998; Kim et al. 2005).
Further understanding of the consequences of the neurochemical and other changes in SERT mutant mice has been sought using drugs to probe various behavioral (e.g., anxiety-like behaviors and stress responses) or physiological (e.g., neuron firing rates and hormonal changes) functions. In this report, we review and integrate the current literature involving pharmacological assessments made in SERT mutant mice. We present the major behavioral (Table 1), physiological (Table 2), and neurochemical (Table 3) findings to date, organized throughout the paper according to individual drugs and drug classes. The primary goals of this review are to evaluate our current understanding of the effects of a constitutive loss of SERT and of the phenotype of SERT mutant mice, to gain clearer insight into the mechanistic contribution of SERT to the actions of a given drug or class of drugs, and finally, to improve our knowledge of the neural basis of various drugs and classes of drugs.
Exogenous serotonin application and administration of drugs that alter serotonin levels
SERT binding
Several studies have examined levels of SERT protein expression in SERT mutant mice, including quantitative autoradiography studies using ligands such as [125I]3β-(4′-iodophenyl)-tropane-2β-carboxylic acid methyl ester ([125I]-RTI-55; Bengel et al. 1998; Perez et al. 2006), [3H]-cyanoimipramine (Montañez et al. 2003), 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylamine (AFM), and 3-amino-4-[2-(dimethylaminomethylphenylthio)]-benzonitrile (DASB; Li et al. 2004b), in addition to tissue homogenate receptor binding studies using [3H]-paroxetine (Sora et al. 2001). Together, these reports indicate that SERT +/− mice have an ∼50% decrease in the number of SERT binding sites and that SERT −/− mice lack SERT expression in all brain regions examined, including frontal cortex, brainstem, hippocampus, and striatum, as well as many other brain regions (Fig. 1a). By contrast, no significant differences in the binding of [3H]-nisoxetine to the norepinephrine transporter (NET) in the CA3 region of the hippocampus across genotypes have been shown (Fig. 1b; Montañez et al. 2003) nor in [3H]-2h-carbomethoxy-3h-(4-fluorophenyl)-tropane ([3H]-CFT) binding to DAT (Sora et al. 2001), suggesting that major adaptive changes in the expression of other monoamine neurotransmitter transporters do not occur in mice deficient in SERT. Interestingly, while DAT levels appear to be unaltered, dopamine cell bodies in the substantia nigra show ectopic serotonin immunoreactivity in SERT −/− mice, suggesting that DAT is capable of taking up serotonin under certain circumstances (see below; Zhou et al. 2002). Furthermore, pretreatment of SERT −/− mice with the DAT inhibitor GBR12935 prevented accumulation of serotonin in nigral dopamine neurons, whereas the SERT inhibitor paroxetine failed to have similar effects (Zhou et al. 2002).
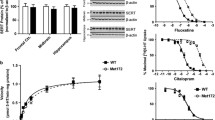
SERT and NET binding and function. SERT +/− and −/− mice showed an ∼50% decrease and a complete lack in density, respectively, of [3H]CN-IMI (cyanoimipramine) binding to SERT in the CA3 region of the hippocampus (a), whereas there were no differences between the three genotypes of [3H]NISOX (nisoxetine) binding to NET (b) (adapted from Montañez et al. 2003). At baseline, serotonin clearance in the CA3 region of the hippocampus was slower in SERT −/− mice; application of the SRI fluvoxamine prolonged serotonin clearance in SERT +/+ mice, and this effect was exaggerated in SERT +/− mice. However, fluvoxamine had no effect in SERT −/− mice (c) (adapted from Montañez et al. 2003). On the tail suspension test, SERT −/− mice showed no change in immobility after the SRI fluoxetine, unlike their SERT +/+ and +/− littermates (d) (estimated based on the Holmes et al. 2002b). However, after desipramine, which acts on NET, SERT −/− mice showed decreased immobility similar to SERT +/+ and +/− mice (e) (estimated based on Holmes et al. 2002b). * Significant difference from SERT +/+ mice, + significant difference from SERT +/− mice, # significant difference from vehicle/baseline
Uptake and clearance of serotonin
Bengel et al. (1998), as well as Pan et al. (2001), used measurements of [3H]-serotonin uptake by brain synaptosomes and primary cultures of midbrain neurons, respectively, to establish a loss of serotonin uptake in SERT −/− mice. However, Bengel et al. (1998) also reported that uptake rates of [3H]-serotonin into synaptosomes prepared from brainstem and frontal cortex were not different between SERT +/+ and +/− mice. This study was conducted using traditional radiochemical methods that determine the rate of [3H]-serotonin uptake using six concentrations of serotonin after an incubation period of several minutes; this method was later shown to be relatively insensitive to the more modest changes in uptake rates occurring in SERT +/− mice (Perez and Andrews 2005; Perez et al. 2006).
Using high-speed chronoamperometry to examine uptake rates, Perez and Andrews (2005) found that synaptosomes prepared from frontal cortex, striatum, and brainstem from SERT +/− mice showed an approximate 50% reduction in the rates of serotonin uptake compared to SERT +/+ mice and confirmed a lack of detectable uptake in synaptosomes from SERT −/− mice. Chronoamperometric recordings in vivo similarly demonstrated that clearance of serotonin pressure ejected into the CA3 region of the hippocampus was decreased by 50% in SERT +/− mice (Montañez et al. 2003). In vivo clearance of exogenously applied serotonin in SERT −/− mice was reduced to a greater extent than that observed in SERT +/− mice, and residual clearance was attributed to diffusion in the tissue. As shown in Fig. 1c, the T80 value (time for the current due to serotonin to decay to 80% of its maximal amplitude) was significantly greater in SERT −/− mice than in both SERT +/+ and +/− mice after serotonin application (Daws et al. 2006; Montañez et al. 2003). In striatal synaptosomes prepared from SERT +/+ mice incubated with the SRI paroxetine, uptake rates were similar to those observed in mice lacking both copies of the SERT gene (Perez and Andrews 2005)
Firing rates after serotonin application
The decrease in and complete lack of SERT expression in SERT +/− and −/− mice, respectively, are also evident in electrophysiological studies examining the effects of serotonin application on cell firing rates. For example, Gobbi et al. (2001) examined the effects of microiontophoretic application of serotonin on the firing rates of cells in the CA3 region of the hippocampus, as well as the time it takes for firing rates to recover from this effect of serotonin. Serotonin application induced a current-dependent inhibition of CA3 pyramidal cell firing activity in mice of all three genotypes, with a trend toward a lesser effect in SERT +/− and −/− mice compared to SERT +/+ mice. Moreover, the duration of the suppression of firing after serotonin application was prolonged by 200% in SERT −/− mice compared to SERT +/+ mice. Unlike their SERT −/− counterparts, however, the duration of the suppression of firing of serotonin neurons in SERT +/− mice was not significantly different from that observed in SERT +/+ mice (Gobbi et al. 2001). It has been suggested that SERT +/− mice may have developed an as yet unidentified compensatory mechanism in that, although they have differing levels of SERT expression and uptake/clearance of serotonin, they do not have differences in their responses to serotonin-induced suppression of firing rates (Montañez et al. 2003).
Early postnatal administration of the serotonin synthesis inhibitor PCPA
SERT −/− mice have marked increases in the concentration of serotonin in the extracellular space (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004). A number of studies suggest that some of the alterations observed in SERT −/− mice might be consequences of the presence of increased extracellular serotonin present during early development. For example, SERT +/− and −/− mice have reduced cortex layer IV somatosensory barrel fields, in agreement with findings from several other studies demonstrating that an excess of extracellular serotonin during early postnatal development prevents normal formation of whisker barrels (Persico et al. 2001; Salichon et al. 2001). Similar alterations were also observed after postnatal administration of the SRI paroxetine (Xu et al. 2004), and in mice with a genetic deletion of monoamine oxidase type-A (MAO-A; Persico et al. 2001; Salichon et al. 2001). As expected from prior data demonstrating that serotonin is essential to the formation of normal whisker barrels during the postnatal period (D’Amato et al. 1987), treatment of SERT +/− and −/− mice with the serotonin synthesis inhibitor p-chlorophenylalanine (PCPA, a selective tryptophan hydroxylase inhibitor) on postnatal days 1 and 2 rescued development of somatosensory barrelfields in these mice (Persico et al. 2001). These observations were extended by showing that early postnatal PCPA treatment restored abnormal glucose utilization responses to whisker stimulation found in the entire trigeminal-thalamo-somatosensory cortex barrel pathway in adult SERT −/− mice (Esaki et al. 2005). This PCPA rescue study supports the hypothesis that functional barrelfield deficits in SERT −/− mice reflect the presence of excessive postnatal serotonin.
SERT −/− mice have been shown to exhibit enhanced rapid eye movement (REM) sleep over 24 h compared to their SERT +/+ counterparts (Alexandre et al. 2006; Wisor et al. 2003). Alexandre et al. (2006) reversed this increase in REM sleep observed in SERT −/− mice via daily administration of PCPA [100 mg/kg subcutaneous (sc)] beginning on postnatal day 5 for a total of 2 weeks. This treatment resulted in a total recovery of normal REM sleep in both male and female adult SERT −/− mice to levels observed in their SERT +/+ counterparts.
These findings show that the effects of PCPA are not dependent on the presence of SERT and help to clarify the overall state of altered serotonin homeostasis in SERT −/− mice (Kim et al. 2005), and point to the role of excessive extracellular serotonin levels during early development in the phenotype of SERT −/− mice.
PCPA administration in adult SERT mutant mice
Studies have also assessed the effects of PCPA administered to adult SERT mutant mice. In contrast to SERT +/− mice, which show normal basal tissue serotonin levels in all brain regions, SERT −/− mice have an ∼60–80% decrease in basal tissue concentrations of serotonin, as well as significant (but relatively smaller) decreases in the concentration of serotonin’s major metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in frontal cortex, hippocampus, striatum, brainstem, and hypothalamus relative to SERT +/+ mice (Bengel et al. 1998). Kim et al. (2005) and Sheridan et al. (1999) confirmed these findings, showing that baseline serotonin was reduced by ∼55–75% in these same brain regions in SERT −/− mice, except in frontal cortex, where serotonin concentrations were reduced by only 40%, compared to SERT +/+ mice (Kim et al. 2005). 5-HIAA was decreased by ∼25–50% in these same brain regions in SERT −/− mice, whereas SERT +/− mice showed normal levels of 5-HIAA in all brain areas examined (Kim et al. 2005; Sheridan et al. 1999).
In SERT +/+ mice, administration of PCPA [200 mg/kg intraperitoneal (ip)] for 3 days resulted in 20–35% depletions of serotonin in frontal cortex, hippocampus, striatum, brainstem, and hypothalamus 3 days posttreatment (Sheridan et al. 1999). SERT −/− mice sustained somewhat greater proportional decrements in serotonin of approximately 45–70% compared to vehicle-treated SERT −/− mice. However, the absolute magnitude of the PCPA-induced depletion was greater on average in SERT +/+ mice than in SERT −/− mice (2.0 vs 1.3 ng/mg protein, respectively). PCPA treatment had no effect on norepinephrine concentrations in any of the three genotypes (Sheridan et al. 1999). These results are congruent with studies assessing serotonin synthesis, in that the somewhat lesser reductions in serotonin observed in SERT −/− mice after PCPA might reflect greater synthesis rates found in SERT −/− mice (Kim et al. 2005).
The serotonin-depleting and hypothermic effects of 2′-NH2-MPTP
1-Methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP) is a close structural analog of the Parkinson’s disease-producing dopamine neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). However, unlike MPTP, 2′-NH2-MPTP depletes serotonin, 5-HIAA, and norepinephrine, without decreasing dopamine, in mice and rats (Andrews and Murphy 1993c; Unger et al. 2002).
Acutely, administration of 2′-NH2-MPTP (4 × 15 mg/kg ip) resulted in an initial increase in core body temperature in SERT +/− and −/− mice, followed by hypothermia in SERT +/+ and +/− mice, whereas this hypothermic response did not occur in SERT −/− mice (Numis et al. 2004). One week after 2′-NH2-MPTP administration (4 × 15 mg/kg ip at 2-h intervals), serotonin (in frontal cortex and hippocampus) and 5-HIAA (in frontal cortex, hippocampus, and brainstem) levels were significantly depleted in SERT +/+ and +/− mice compared to vehicle-treated controls, whereas no depletion occurred in these brain areas in SERT −/− mice (Numis et al. 2004). In fact, 2′-NH2-MPTP-treated SERT −/− mice displayed significantly higher levels of serotonin and 5-HIAA than did 2′-NH2-MPTP-treated SERT +/+ mice in these same brain areas, although at baseline, SERT −/− mice had 30–60% reductions in serotonin and 5-HIAA concentrations, as described above (Bengel et al. 1998; Kim et al. 2005). In the brainstem, mice were less vulnerable to the effects of 2′-NH2-MPTP, as serotonin levels were not as severely depleted (Numis et al. 2004). However, SERT −/− mice were relatively less protected from 2′-NH2-MPTP in the brainstem and showed decreased levels of serotonin similar to that seen in SERT +/+ and +/− mice. In contrast to serotonin, potentiated reductions in norepinephrine levels in 2′-NH2-MPTP-treated SERT −/− mice were found in frontal cortex, hippocampus, and brainstem compared to vehicle-treated SERT −/− mice and 2′-NH2-MPTP-treated SERT +/+ mice in hippocampus and brainstem. There were no differences between the three genotypes in striatal or cortical dopamine levels or homovanillic acid concentrations in response to 2′-NH2-MPTP administration. These findings further substantiate that SERT is necessary for 2′-NH2-MPTP-induced neurotoxicity in frontal cortex and hippocampus, but not for norepinephrine neurotoxicity (Numis et al. 2004). Further, these findings are in agreement with prior data showing that SRIs, but not other monoamine transport inhibitors, protected against serotonin depletion induced by 2′-NH2-MPTP, thus validating the conclusion that transport via SERT is a component of the neurotoxic mechanism of 2′-NH2-MPTP (Andrews and Murphy 1993a, b), but leave unexplained the brainstem effects.
Behavioral and hypothermic responses to the serotonin precursor 5-HTP
SERT −/− mice have been shown to display some baseline serotonin syndrome-like behaviors, including low body posture, tremor, and backward movement (Kalueff et al. 2007). Administration of the serotonin precursor 5-hydroxy-l-tryptophan (5-HTP) induced exaggerated serotonin syndrome-like behaviors in SERT −/− mice compared to SERT +/+ mice (Fox et al. 2006; Fox and Murphy 2006). SERT −/− mice also showed exaggerated hypothermic responses to the serotonin precursor 5-HTP (Fox et al. 2006; Fox and Murphy 2006), despite decreased Htr1a-mediated hypothermic responses (described below; Bouali et al. 2003; Holmes et al. 2003a; Li et al. 1999, 2000). Studies are currently underway to investigate the receptor(s) mediating these exaggerated responses observed in SERT −/− mice.
SRIs and other antidepressants
Behavioral responses to SRIs and non-SRI antidepressants
One study investigated the anti-immobility effects of the SRI fluoxetine and two other non-SRIs in the tail suspension test (Holmes et al. 2002b), a behavioral task that has been shown to be sensitive to most clinically effective antidepressants (Cryan and Holmes 2005; Porsolt 2000; Porsolt et al. 1977; Steru et al. 1985). In this study, fluoxetine was effective in decreasing immobility in SERT +/+ and +/− mice, but this response was absent in SERT −/− mice (Fig. 1d; Holmes et al. 2002b). For non-SRI antidepressants, SERT +/− and −/− mice both displayed responses similar to those observed in SERT +/+ mice. Mice of all three genotypes were sensitive to the anti-immobility effects of imipramine, although SERT −/− mice showed the weakest effects, as might be expected due to the actions of imiprimine via NET and SERT. SERT +/− and −/− mice were also sensitive to the anti-immobility effects of desipramine, which primarily acts on NET, and in fact, the strongest responses to desipramine were seen in SERT −/− mice (Fig. 1e; Holmes et al. 2002b).
Neurochemical and neurophysiological responses to SRIs
In neurochemical studies, SERT −/− mice have also failed to show the responses to SRIs observed in their SERT +/+ counterparts. For example, Montañez et al. (2003) investigated the rate of clearance of serotonin pressure ejected into the CA3 region of the hippocampus in anesthetized mice utilizing high-speed chronoamperometry. When fluvoxamine was applied locally by pressure ejection, there was a significant prolongation in the T80 value for serotonin clearance in SERT +/+ mice, whereas SERT −/− mice were unresponsive to fluvoxamine (Montañez et al. 2003). Interestingly, in SERT +/− mice, the effect of fluvoxamine on clearance of serotonin from the CA3 region of the hippocampus was significantly exaggerated (Fig. 1c), an effect possibly reflecting altered adaptive regulation of the remaining single SERT (Montañez et al. 2003). Desipramine had no effect on serotonin clearance in SERT +/+, +/−, or −/− mice, in keeping with its primary NET inhibitory effects (Montañez et al. 2003) and the normal NET binding described above (Fig. 1b; Montañez et al. 2003).
Fabre et al. (2000) examined the effects of citalopram on serotonin outflow in the substantia nigra of anesthetized SERT +/+ and −/− mice using microdialysis. At baseline, serotonin outflow was approximately six times higher in SERT −/− mice than in SERT +/+ mice. In SERT +/+ mice, the addition of citalopram to the perfusing fluid resulted in a progressive increase in serotonin outflow, whereas the addition of citalopram had no effect on serotonin outflow in SERT −/− mice (Fabre et al. 2000). In a study examining the specificity of fluoxetine in altering brain concentrations of serotonin, systemic administration of fluoxetine did not alter dopamine levels in the caudate putamen or the nucleus accumbens in either SERT +/+ or −/− mice, demonstrating the selectivity of SRIs for SERT (Shen et al. 2004).
Application of the SRI paroxetine to brainstem slices from SERT +/+ and +/− mice resulted in a concentration-dependent inhibition of the firing rates of dorsal raphe nucleus (DRN) neurons (Mannoury la Cour et al. 2001), supporting an in vivo study in which microiontophoretic application of paroxetine significantly prolonged the recovery time of the firing rate of DRN neurons in SERT +/+ and +/− mice, but not in SERT −/− mice, whose recovery time was already prolonged (Gobbi et al. 2001). Similarly, citalopram potently inhibited the firing rate of DRN neurons in SERT +/+ mice in a concentration-dependent manner, but essentially had no effect in SERT −/− mice (Mannoury la Cour et al. 2001).
Postnatal administration of fluoxetine
To examine the developmental effects of SERT blockade, Ansorge et al. (2004) investigated the effects of postnatal inhibition of SERT via daily administration of fluoxetine (10 mg/kg ip, postnatal days 4–21). By adulthood, fluoxetine-treated SERT +/+ and +/− mice showed decreased exploratory behavior (decreased total distance traveled), decreased time spent ambulating and rearing in the open field test, and a decrease in the total number of arm entries in the elevated plus maze. There were no differences in locomotion in the home cage, suggesting that these findings were specific to exploratory behavior, rather than differences in locomotor activity. These behaviors observed in SERT +/+ and +/− mice treated with postnatal fluoxetine resembled the behavior of saline-treated SERT −/− mice (Ansorge et al. 2004).
In the novelty-suppressed feeding paradigm, thought to reflect anxiety- and depression-related behaviors, untreated SERT −/− mice showed longer latencies to begin feeding compared to SERT +/+ and +/− mice (Ansorge et al. 2004; Lira et al. 2003). Postnatal treatment with fluoxetine increased the latency to feed in SERT +/− mice to that observed in SERT −/− mice. SERT −/− mice also showed an impairment in shock avoidance, used to assess behavioral responses to stress, and postnatal fluoxetine treatment altered shock avoidance in SERT +/+ and +/− mice to levels observed in SERT −/− mice (Ansorge et al. 2004). Fluoxetine had no effect on the behaviors of SERT −/− mice in any of these tests, confirming that its effects were selective for SERT (Ansorge et al. 2004). These findings show that the effects of constitutive loss of SERT resemble in some ways those produced by postnatal inhibition of SERT (Hariri and Holmes 2006).
Drugs of abuse
MDMA
(+)-3,4-Methylenedioxymethamphetamine (MDMA), which releases serotonin via a SERT-dependent mechanism (Rudnick and Wall 1993), has altered effects in SERT mutant mice. Bengel et al. (1998) reported a lack of locomotor-stimulating effects of MDMA (5 mg/kg ip) in SERT −/− mice relative to the marked stimulation seen in SERT +/+ mice, with intermediate responses occuring in SERT +/− mice (Fig. 2b; Bengel et al. 1998). MDMA self-administration is also absent in SERT −/− mice (Trigo et al. 2007). Although SERT +/+ mice acquired and maintained responding for MDMA at each dose tested (0.03–0.25 mg/kg per infusion), SERT −/− mice did not respond to any dose of MDMA, despite similar levels of responding for food and water on the final training day. This suggests a role for SERT in the rewarding effects of MDMA (Trigo et al. 2007). Using microdialysis, administration of MDMA (10 mg/kg ip) increased levels of extracellular dopamine in the nucleus accumbens in both SERT +/+ and −/− mice, whereas extracellular levels of serotonin in the prefrontal cortex were increased in SERT +/+ mice only (Trigo et al. 2007). In addition, the ability of MDMA administration to induce expression of the gamma-amino butyric acid (GABA) transporter 1 in frontal cortex and midbrain was reduced in SERT −/− mice (Peng and Simantov 2003).
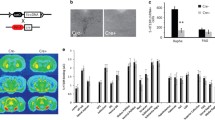
Locomotor activity. SERT −/− mice showed decreased baseline locomotor activity, including in the open field test (a) (Kalueff et al. 2007). SERT −/− mice showed no locomotor response to drugs acting on the serotonin system, including MDMA (b) (estimated based on Bengel et al. 1998) and the Htr1a/1b agonist RU24969 (c) (estimated based on Holmes et al. 2002a). However, SERT −/− mice showed essentially normal locomotor responses to drugs that act on other neurotransmitter systems, including normal increases in locomotion after +-amphetamine (d) (estimated based on Bengel et al. 1998), cocaine (e) (Wichems et al. 1998) and the dopamine D1 agonist SKF 38939 (f) (Wendland and Murphy, unpublished data). * Significant difference from SERT +/+ mice, + significant difference from SERT +/− mice, # significant difference from vehicle/baseline
Psychostimulants
SERT +/− and −/− mice displayed largely normal locomotor-stimulant responses to +-amphetamine (5 mg/kg ip; Fig. 2d; Bengel et al. 1998), although SERT −/− mice displayed somewhat lesser vertical activity (rears) and somewhat increased time in the center of an open field (Fig. 3a,b; Wichems et al. 1998). Along similar lines, cocaine increased locomotion in mice of all three genotypes (Fig. 2e), but with a lesser and delayed peak of activity in SERT −/− mice (Fig. 3c; Sora et al. 2001; Wichems et al. 1998). The ability of psychostimulants to induce a significant conditioned place preference (CPP), an index of reward-related behaviors (Tzschentke 1998; van der Kooy 1987), has also been examined in SERT mice. Cocaine-induced CPP was significantly enhanced in SERT −/− mice compared to SERT +/+ mice (Sora et al. 1998, 2001), although another study found no difference between the genotypes for cocaine-induced CPP (Hall et al. 2002). In contrast, mice lacking either one or two SERT alleles plus both DAT alleles exhibited a total ablation of cocaine-induced CPP (Sora et al. 1998, 2001). Methylphenidate also produced an intact or even “enhanced” CPP in SERT +/− and −/− mice compared to SERT +/+ mice (Sora et al. 1998). Finally, cocaine increased concentrations of extracellular dopamine in the caudate putamen, nucleus accumbens, and prefrontal cortex in mice of all SERT genotypes, although increased extracellular levels of serotonin in the caudate putamen were relatively lower in SERT −/− mice and only minimally changed in the nucleus accumbens and prefrontal cortex of these mice (Shen et al. 2004).
Locomotor activity over time. Following +-amphetamine, SERT −/− mice showed attenuated increases in vertical activity (rears) (a) and somewhat increased time in the center of an open field (b) (Wichems et al. 1998). SERT −/− mice showed a somewhat smaller and delayed peak of horizontal activity after cocaine administration (c) (Wichems et al. 1998)
Ethanol
There have been several assessments of the behavioral and neurochemical effects of ethanol in SERT mutant mice. After administration of 4 g/kg ethanol, mice of all three SERT genotypes showed a significant decrease in horizontal locomotor activity (Fig. 4; Engel and Murphy, unpublished data). After administration of a lower ethanol dose (2 g/kg), SERT −/− mice displayed a significant increase in horizontal activity compared to both SERT +/+ and +/− mice, and compared to the activity of SERT −/− mice after administration of either 1 or 4 g/kg ethanol.
Locomotor effects of ethanol. After administration of 4 g/kg ethanol, mice of all three SERT genotypes showed a significant decrease in horizontal locomotor activity (percent baseline). However, after administration of a lower ethanol dose (2 g/kg), SERT −/− mice displayed a significant increase in horizontal activity compared to both SERT +/+ and +/− mice, and compared to the activity of SERT −/− mice after administration of either 1 or 4 g/kg ethanol (Engel and Murphy, unpublished data). Inlay is horizontal activity (beam breaks) at baseline and after ethanol. * Significant difference from SERT +/+ mice, + significant difference from SERT +/− mice, # significant difference from 1 g/kg, ## significant difference from 2 g/kg
Using a two-bottle choice paradigm of progressive ethanol self-administration, SERT −/− mice consumed less ethanol than SERT +/+ mice, with significant differences at the highest concentrations (15 and 20% ethanol; Këlai et al. 2003). Ethanol consumption was significantly lower in SERT −/− mice (∼4 g/kg vs ∼9 g/kg per day in SERT +/+ mice), and total ethanol consumption over the 40-day study period was decreased by 55% in SERT −/− mice. Administration of fluoxetine (10 mg/kg ip per day) over 10 days decreased ethanol consumption by 38% in SERT +/+ mice, whereas fluoxetine had no effect in SERT −/− mice (Këlai et al. 2003). In another study examining lower ethanol concentrations, SERT −/− mice consumed significantly less ethanol than SERT +/+ controls at a concentration of 7%, suggesting a “modest” reduction in ethanol consumption in SERT −/− mice (Boyce-Rustay et al. 2006).
The sedative-hypnotic effects of ethanol were exaggerated in SERT −/− mice, as reflected in increased sleep time (Boyce-Rustay et al. 2006; Daws et al. 2006). SERT −/− mice showed significantly longer sleep times in response to ethanol (3 or 4 g/kg ip) than SERT +/+ mice (Boyce-Rustay et al. 2006; Daws et al. 2006). Similarly, fluoxetine (30 mg/kg ip) increased sleep time in SERT +/+ mice as well as, unexpectedly, in SERT +/− and −/− mice (Daws et al. 2006). On the accelerating rotarod test for ethanol-induced ataxia, SERT −/− were more impaired than SERT +/+ mice both before and after treatment with 1.5 g/kg ethanol (Boyce-Rustay et al. 2006). By contrast, mice of all three genotypes showed a similar, dose-dependent hypothermic response to 2 or 4 g/kg ethanol, similar increases in immobility on the tail suspension test after administration of 1 or 1.5 g/kg ethanol and equivalent ethanol-induced CPP (Boyce-Rustay et al. 2006).
As noted, clearance of exogenously applied serotonin is decreased in the hippocampus of SERT −/− mice (Daws et al. 2006; Montañez et al. 2003). Either systemic or local (into the hippocampus) administration of ethanol decreased the rate of serotonin clearance in a genotype- and ethanol-concentration-dependent manner (Daws et al. 2006). Specifically, clearance rates were reduced in ethanol-treated SERT +/+ mice, and to a greater extent in SERT +/− and SERT −/− mice (Daws et al. 2006). It is interesting to note that although the inhibition of serotonin clearance was significantly enhanced in SERT +/− mice, SERT +/− mice exhibited normal behavioral responses to ethanol (see above), indicating that the impaired serotonin clearance observed in SERT +/− mice is not manifested behaviorally (Daws et al. 2006). In SERT +/+ mice, administration of fluvoxamine and ethanol resulted in a greater inhibition of serotonin clearance than for either drug alone, showing again that either genetic or pharmacological blockade of SERT increases the effects of ethanol on this measure (Daws et al. 2006).
Drugs acting on serotonin receptors
Htr1 receptors
Neuroendocrine responses to the Htr1a agonist 8-OH-DPAT
Administration of the Htr1a agonist 8-hydroxy-2-(di-n-propylamino)-tetraline (8-OH-DPAT) dose-dependently increased concentrations of plasma oxytocin, corticosterone, and ACTH in SERT +/+ and +/− mice, whereas SERT −/− mice only showed an increase in plasma ACTH (Li et al. 1999). Pretreatment with the selective Htr1a antagonist N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]-ethyl]-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride (WAY 100635) blocked the neuroendocrine response induced by 8-OH-DPAT in SERT +/+ and +/− mice, confirming mediation by Htr1a receptors (Li et al. 1999). Given the known role of Htr1a receptors located in the paraventricular nucleus of the hypothalamus as mediators of these responses (Bagdy 1996), these data suggest desensitization of these receptors in SERT −/− mice. These findings are similar to the effects of chronic administration of the SRI fluoxetine, as rats administered fluoxetine daily evidenced a similar desensitization of Htr1a receptors, as assessed by decreased neuroendocrine responses to the Htr1a agonist 8-OH-DPAT (Raap et al. 1999).
In contrast to the attenuated responses to Htr1a stimulation, saline injection by itself produced significant increases in plasma ACTH and oxytocin in SERT +/− and −/− mice, but not in SERT +/+ mice, consistent with an exaggerated hypothalamic–pituitary–adrenal axis response to a mild stressor (Li et al. 1999, 2000; Tjurmina et al. 2002). Demonstrating that this phenotypic abnormality in SERT −/− mice was also Htr1a-mediated, the neuroendocrine response was effectively normalized by adenoviral vector-mediated expression of medial hypothalamic Htr1a receptors (Li et al. 2004a).
Hypothermic response to 8-OH-DPAT
The hypothermic effects of 8-OH-DPAT are mediated by Htr1a receptors located presynaptically on raphe neurons in mice (Bill et al. 1991; Goodwin et al. 1985; Martin et al. 1992). The effects of 8-OH-DPAT (0.1 mg/kg sc) were significantly attenuated in SERT +/− and −/− mice (Holmes et al. 2003a; Li et al. 1999, 2000), consistent with significant reductions in Htr1a binding sites in the raphe region in SERT +/− and −/− mice (Li et al. 1999, 2000). In SERT +/− mice, this attenuated effect was restricted to female mice (Fig. 5c,d). Using a higher dose of 8-OH-DPAT (0.4 mg/kg sc), this gender difference was also apparent in SERT −/− mice (Bouali et al. 2003), and these researchers demonstrated that sex hormones modulate these genotype effects. Castrated male SERT −/− mice showed evidence of Htr1a downregulation, while ovariectomized female SERT −/− mice showed Htr1a upregulation, as assessed by the hypothermic response to 8-OH-DPAT. Treatment of gonadectomized SERT −/− mice with testosterone or estradiol restored the hypothermic responses to 8-OH-DPAT to that of sham control levels in males and females, respectively (Bouali et al. 2003). Administration of the Htr1a antagonist WAY 100635 (1 mg/kg sc) increased the core body temperature of both male and female SERT −/− mice, while having no effect in SERT +/+ or +/− mice (Holmes et al. 2003a).
Htr1a binding and function. Male and female SERT −/− mice showed decreased densities of [125I]-MPPI-labeled Htr1a receptors in the DRN (a) (estimated based on Li et al. 2000). SERT +/− and −/− mice also showed decreased 8-OH-DPAT-induced inhibition of firing of serotonin DRN neurons (b) (estimated based on Gobbi et al. 2001). The hypothermic response to the Htr1a agonist 8-OH-DPAT was absent in male (c) and female (d) SERT −/− mice (estimated based on Li et al. 1999, 2000). For SERT +/− mice, there was a significant effect of gender, as male SERT +/− mice displayed 8-OH-DPAT-induced hypothermia similar to their SERT +/+ counterparts (c), whereas female SERT +/− mice had no hypothermic response to 8-OH-DPAT, like their SERT −/− littermates (d). * Significant difference from SERT +/+ mice, # significant difference from respective baseline (time 0)
Intracellular and extracellular recordings of DRN and hippocampal neurons
Baseline rates of spontaneous firing of DRN neurons are reduced 36% in SERT +/− mice and 66% in SERT −/− mice relative to SERT +/+ mice (Bouali et al. 2003; Gobbi et al. 2001). Administration of 8-OH-DPAT, either systemically or microiontophoretically, inhibited DRN firing in SERT +/+ mice, but did so to a lesser extent in SERT +/− mice, and even less so in SERT −/− mice (Fig. 5b; Bouali et al. 2003; Gobbi et al. 2001). In vitro brainstem slices showed similar gene dose-dependent alterations in response to the Htr1a agonists ipsapirone or 5-carboxamidotryptamine (5-CT; Mannoury la Cour et al. 2001). Indeed, there were ∼55- and ∼6-fold reductions in the potency of ipsapirone and 5-CT, respectively, in SERT −/− mice compared to SERT +/+ mice. These inhibitory effects were reversed by WAY 100635, validating actions at Htr1a receptors (Mannoury la Cour et al. 2001).
Similar to the findings for 8-OH-DPAT-induced hypothermia, there was a gender difference in 8-OH-DPAT-induced inhibition of the firing rates of DRN neurons in SERT −/− mice (Bouali et al. 2003). 8-OH-DPAT-induced inhibition of DRN neural firing was lesser in male SERT −/− mice compared to male SERT +/+ mice. Inhibition of firing approached 60% of baseline for very high doses (∼4,000 μg/kg) of this Htr1a agonist in SERT −/− mice, whereas complete inhibition was achieved by only 20 μg/kg in SERT +/+ mice. DRN firing was inhibited in SERT +/+ females in a fashion similar to that observed in male SERT +/+ mice, whereas no inhibitory response was observed in female SERT −/− mice. This gender difference was also reversed by gonadectomy and was reinstated by administration of testosterone or estradiol in gonadectomized male and female SERT −/− mice, respectively, again demonstrating a direct role for sex hormones in the gender-based Htr1a agonist response differences (Bouali et al. 2003).
Studies using intracellular recording techniques to assess membrane potential and input resistance (R in) also provide evidence of altered DRN sensitivity to Htr1a agonists in SERT −/− mice. Bath-applied ipsapirone induced a concentration-dependent membrane hyperpolarization and a decrease in R in of DRN neurons in SERT +/+ mice but not in SERT −/− mice (Mannoury la Cour et al. 2001). While 5-CT produced similar maximal hyperpolarization across genotypes, the potency of 5-CT was lower in SERT −/− mice. The effects of 5-CT were blocked by administration of an otherwise “silent” dose of WAY 100635, confirming a Htr1a receptor specific effect (Mannoury la Cour et al. 2001). However, application of 5-CT onto hippocampal slices evoked a (WAY 100635-reversible) membrane hyperpolarization and decrease in R in that was similar across genotypes (Mannoury la Cour et al. 2001).
Htr1a- and Htr1b/1d-mediated changes in serotonin synthesis
Ipsapirone significantly decreased accumulation of the serotonin precursor 5-HTP and 5-HIAA levels in SERT +/+ mice, indicative of reduced serotonin synthesis (Fabre et al. 2000). These effects were absent in SERT −/− mice. However, ipsapirone failed to alter tissue serotonin levels in mice of both genotypes. SERT −/− mice also failed to exhibit the enhancement of serotonin outflow in substantia nigra seen in SERT +/+ mice in response to the SRI citalopram and/or the Htr1b/1d antagonist GR 127935 (Fabre et al. 2000).
Htr1a/1b-mediated locomotor stimulation
In a behavioral assessment, systemic administration of the Htr1a/1b agonist RU 24969 increased locomotor activity in SERT +/+ and +/− mice, but not in SERT −/− mice (Fig. 2c; Holmes et al. 2002a). These data are consistent with the reduction, albeit modest, reported in Htr1b receptor binding in SERT −/− mice (Fabre et al. 2000). Interestingly, Htr1b knockout mice also show no locomotor response to RU24969 (Bonaventure et al. 1999), suggesting that despite only modest reductions evident in Htr1b binding sites in SERT −/− mice, Htr1b receptors mediating locomotor activity might be fully desensitized in SERT −/− mice.
Anxiety- and depression-related phenotypes
SERT −/− mice have also been shown to have heightened anxiety-like behaviors as compared to SERT +/+ mice (Fig. 6a; Ansorge et al. 2004; Holmes et al. 2003a; Kalueff et al. 2007), and altered Htr1a receptors are likely to contribute to the abnormal anxiety-like behaviors seen in SERT −/− mice (Holmes et al. 2003c). Systemic treatment with WAY 100635 (0.05–0.3 mg/kg sc) normalized increased anxiety-like behavior on the elevated plus maze in SERT −/− mice to levels observed in SERT +/+ mice, and had no effect in SERT +/+ or +/− mice. This effect appeared to be specific to anxiety behaviors, as vertical activity was abnormally low in SERT −/− regardless of treatment (Holmes et al. 2003c).
Anxiety-like behavior. SERT −/− mice displayed elevated baseline levels of anxiety-like behaviors, shown here as decreased time in the center of an open field (a) (Kalueff et al. 2007). The benzodiazepine diazepam “normalized” increased baseline anxiety in SERT −/− mice in the zero maze (b) and in the light-dark exploration test (c) (Wichems et al. 2000). * Significant difference from SERT +/+ mice, # significant difference from vehicle/baseline
REM sleep abnormalities are a major symptom of depression (Adrien 2002). As discussed above, postnatal PCPA treatment reversed the abnormally high REM sleep phenotype in adult SERT −/− mice (Alexandre et al. 2006). Treatment with WAY 100635 (1 mg/kg sc twice daily) for 4, but not 2, weeks commencing on postnatal day 5 had a similar effect in male SERT −/− mice, with a partial recovery in female SERT −/− mice. Four-week treatment during the second month of life failed to rescue the phenotype, suggesting that these effects occurred during a critical period of development (Alexandre et al. 2006). On the tail suspension test of depression-like behavior, SERT −/− mice showed heightened levels of “depression-related” immobility (Holmes et al. 2002b; Zhao et al. 2006). The same postnatal treatment with WAY 100635 that rescued REM abnormalities in SERT −/− mice also decreased immobility to levels observed in SERT +/+ mice, without affecting this behavior in SERT +/+ mice, and without affecting locomotor activity in SERT −/− mice (Alexandre et al. 2006).
These behavioral consequences of postnatal WAY 100635 treatment were paralleled by a partial recovery of reduced Htr1a binding in DRN, but not forebrain regions, of SERT −/− mice, and increased the hypothermic response to 8-OH-DPAT in female, but not male, SERT −/− mice (Alexandre et al. 2006). Moreover, while spontaneous DRN firing was unaltered, there was a partial rescue of the loss of 8-OH-DPAT-mediated inhibition of DRN firing in WAY 100635-treated female, but not male, SERT −/− mice.
Htr2 and Htr3 receptors
Treatment with the Htr2a/2c agonist (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) produced increases in plasma ACTH, corticosterone, and oxytocin in mice of all three genotypes, and the increase in oxytocin was significantly greater in SERT −/− mice (Li et al. 2003). On the basis that Htr2a rather than Htr2c receptors mediate oxytocin responses in normal mice (Zhang et al. 2002) and the fact that hypothalamic Htr2a receptor binding sites are increased in SERT −/− mice (Li et al. 2003), these data suggest that Htr2a receptor function is upregulated in the hypothalamus of SERT −/− mice.
However, DOI also induces head twitches in mice via Htr2a receptor activation (González-Maeso et al. 2003; Willins and Meltzer 1997), and this effect was markedly reduced in SERT −/− mice compared to SERT +/+ mice (Qu et al. 2005). Qu et al. (2005) also showed that SERT −/− mice displayed diminished Htr2a-mediated activation of the PLA2 arachidonic acid signaling pathway. Other studies have found modest, brain region-selective and ligand-dependent ([3H]-MDL 100,907 vs [125I]-DOI) changes in Htr2a receptor binding in SERT −/− mice (Li et al. 2003; Rioux et al. 1999) and normal Htr2a mRNA levels throughout the brain of these mice (Li et al. 2003). Increased Htr2a binding in the hypothalamus and decreased Htr2a binding in the striatum in SERT −/− mice (Li et al. 2003) might underlie the increased oxytocin response and decreased head twitch response described in SERT −/− mice.
Htr3 receptors are the only ionotropic, ligand-gated serotonin receptor subtype (Hoyer et al. 2002; Mossner et al. 2004). In SERT −/− mice, Htr3 receptor binding was increased by 46% in the frontal cortex, 42% in the parietal cortex, and 18% in the stratum orions of the CA3 region of the hippocampus, relative to SERT +/+ mice (Mossner et al. 2004). Htr3 receptor density was also increased by 47% in the frontal cortex of SERT +/− mice (Mossner et al. 2004). Htr3a subunit mRNA levels in the CA1 region of the hippocampus were also increased by 65 and 57% in SERT +/− and −/− mice, respectively, compared to SERT +/+ mice (Mossner et al. 2004). Thus, while to date there have been limited investigations of the pharmacologic function of Htr2a, Htr2c, and Htr3 receptors in SERT −/− mice, due in part to a relative paucity of selective agonists at these receptors, those studies that have been conducted demonstrate altered expression and function of these receptors in SERT −/− mice.
Drugs acting on the GABA system
The functional properties of GABAB receptors in SERT −/− mice have been investigated. Application of the GABAB agonist baclofen to brainstem slices resulted in a concentration-dependent inhibition of cell firing of DRN neurons in SERT +/+ and −/− mice, but the range of concentrations required for this inhibitory effect was much higher in SERT −/− mice, indicative of reduced sensitivity (Mannoury la Cour et al. 2004). Baclofen also induced a concentration-dependent membrane hyperpolarization and a decrease in R in of DRN neurons in SERT +/+ and −/− mice, again with maximal effects occurring at markedly higher concentrations in SERT −/− mice. The inhibitory effects of baclofen were blocked by GABAB antagonists (CGP52432 or saclofen), confirming selective action at GABAB receptors. By contrast, parallel experiments with the GABAA agonist muscimol resulted in a similar inhibition of discharge of DRN neurons in SERT +/+ and −/− mice, specifying that the differences described above were specific to GABAB receptors. Moreover, these effects were specific to the DRN, as genotype differences in sensitivity to baclofen were not seen in CA1 hippocampal preparations. Baclofen induced an (CGP52432-reversible) increase in GTP-γ-S binding in DRN and hippocampal slices, which was also markedly lower in SERT −/− mice compared to SERT +/+ mice in the former region only (Mannoury la Cour et al. 2004).
These changes in GABAB resemble the aforementioned desensitization and downregulation of Htr1a autoreceptors in the DRN and the corresponding absence of changes in hippocampal Htr1a postsynaptic receptors that are seen in SERT −/− mice (Fabre et al. 2000; Mannoury la Cour et al. 2001). In fact, the Htr1a agonist 5-CT caused similar, but non-additive, effects to baclofen-evoked GTP-γ-S binding in DRN slices, suggesting the possibility of an alteration in a common transduction pathway in SERT −/− mice (Mannoury la Cour et al. 2004).
There are several lines of evidence for a role of serotonin in seizure disorders. Low levels of serotonin augment seizure activity (Jobe et al. 1999), while extracellular serotonin is increased after a seizure (Shouse et al. 2001) and may represent a compensatory response. In addition, anticonvulsants acting via GABA increase serotonin (Dailey et al. 1997a, b), and SRIs and Htr1a agonists demonstrate anticonvulsant activity dependent upon the availability of serotonin (Lu and Gean 1998; Pasini et al. 1996). Male SERT −/− mice were less vulnerable to pentylenetetrazole (PTZ)-induced seizures than male SERT +/+ mice, as demonstrated by the finding that the ED50 for clonic seizure was 29% higher in male SERT −/− mice than male SERT +/+ mice (55 and 39 mg/kg, respectively; Fig. 7a; Engel and Murphy, unpublished data). These differences were specific for male mice and were not observed in female mice (Fig. 7a,b).
PTZ-induced seizures. Male, but not female, SERT −/− mice were less sensitive to PTZ-induced clonic seizures, as male SERT −/− mice showed decreased vulnerability to PTZ-induced clonic seizures (a); the ED50 for male SERT +/+ mice was 39 mg/kg vs 55 mg/kg in male SERT −/− mice. Female SERT −/− mice showed similar levels of PTZ-induced seizures compared to female SERT +/+ mice (b); the ED50 for female SERT +/+ mice was 42 mg/kg vs 44 mg/kg in female SERT −/− mice (Engel and Murphy, unpublished data). * Significant difference from SERT +/+ mice
Male SERT −/− mice also displayed a 24% decrease in the number of GABAA binding sites in forebrain membranes as assessed by muscimol binding (Engel and Murphy, unpublished data). No genotype differences in binding at benzodiazepine (flunitrazepam) or GABAB (baclofen) sites were evident. Male SERT −/− mice also showed a significant (19%) decrease in GABA-stimulated Cl− uptake compared to their male SERT +/+ counterparts. Again, these alterations were found only in male, but not female, SERT −/− mice (Engel and Murphy, unpublished data). Modulation of GABA-stimulated Cl− uptake by pentobarbital or the neurosteroid pregnanolone was not different with respect to either gender or genotype.
Consistent with the absence of differences in flunitrazepam binding, the anxiolytic-like effects of the benzodiazepine diazepam (0.1 mg/kg) were intact in SERT −/− mice as measured using the elevated zero maze (Fig. 6b) and the light–dark exploration test (Fig. 6c; Wichems et al. 2000). In fact, SERT −/− mice had elevated baseline anxiety-like behaviors, which were essentially “normalized” by diazepam.
Drugs acting on the dopamine system
Several recent studies have examined a possible compensatory role for DAT or organic cation transporters in mediating serotonin uptake in SERT mutant mice (Mossner et al. 2006; Schmitt et al. 2003; Shen et al. 2004; Zhou et al. 2002). Shen et al. (2004) investigated the effects of systemic administration of the selective DAT blocker GBR12909 on serotonin levels in SERT mutant mice. While GBR12909 did not alter serotonin levels in SERT +/+ mice, there was a significant increase in extracellular serotonin in the caudate putamen of SERT −/− mice, suggesting that DAT can act as a transporter of serotonin under conditions where SERT function is absent. Ectopic serotonin immunostaining in dopamine neurons in substantia nigra and ventral tegmental area was also found in SERT −/− mice, and to a lesser extent in SERT +/− mice, whereas this did not occur in SERT +/+ mice (Zhou et al. 2002). This was prevented by the DAT inhibitor GBR12935, further implicating a DAT-mediated mechanism (Zhou et al. 2002).
Pan et al. (2001) also examined serotonin uptake in midbrain–hindbrain cultures after treatment with fluoxetine or the DAT blocker nomifensine. In SERT +/+ mice, fluoxetine dose-dependently inhibited serotonin uptake, whereas nomifensine had comparatively weak effects. In SERT −/− mice, baseline serotonin uptake was decreased as expected, while the residual uptake capacity that was retained was inhibited by nomifensine. Thus, it appears that cultured dopaminergic neurons of SERT −/− mice might demonstrate a capacity for DAT to transport serotonin that is revealed when the higher affinity SERT is not available (Pan et al. 2001). Along similar lines, SRI treatment leads to accumulation of serotonin in striatal dopaminergic axons and terminals, and serotonin is co-released with dopamine in nonmutant rats (Zhou et al. 2005). Interestingly, double SERT/MAO-A knockout mice also showed aberrant serotonin uptake in dopaminergic terminals, whereas triple SERT/MAO-A/DAT knockout mice did not show this aberrant uptake, again implicating DAT in the abnormal serotonin uptake observed in these mice (Mossner et al. 2006).
Application of serotonin followed by staining 2 days later with antiserotonin antibodies revealed enhanced staining (i.e., stronger immunoreactivity in cell body, processes, and varicosities) in midbrain–hindbrain neuronal cultures in both SERT +/+ and −/− mice (Pan et al. 2001). However, there was an increase in the number of serotonin positive neurons in neuronal cultures from SERT −/− mice only. In SERT +/+ neuronal cultures, the increased intensity of serotonin staining was blocked by the SERT blockers fluoxetine and imipramine, but not the DAT blocker nomifensine. In contrast, both SERT and DAT blockade attenuated the serotonin-induced increase in the number of serotonin immunoreactive cells (Pan et al. 2001).
Possible changes in the behavioral effects of dopamine-acting agents have not yet been closely examined. In one evaluation, the selective D1 agonist SKF 38939 (20 mg/kg ip) increased locomotor activity equally in mice of all three genotypes (Fig. 2f; Wendland and Murphy, unpublished data).
Concluding comments
Complete ablation or an ∼50% reduction in SERT expression and function in mice leads to changes in responses to serotonin receptor agonists and SERT inhibitors, and altered responses to drugs from different classes. These studies, discussed in the present review and summarized in Tables 1, 2, and 3, illustrate the remarkable number of adaptive drug responses that are attributable to targeted disruption of SERT.
As described, SERT −/− mice display increased baseline levels of anxiety-like behavior (Ansorge et al. 2004; Holmes et al. 2003b, c; Kalueff et al. 2007; Lira et al. 2003). A similar high-anxiety phenotype has been observed in 5-HT1A knockout mice (Pattij et al. 2002a, b), and in contrast to the findings in SERT −/− mice, mice overexpressing SERT display a low-anxiety (paroxetine-reversible) phenotype (Jennings et al. 2006). SERT −/− mice also show increased baseline stress responses (Ansorge et al. 2004; Li et al. 1999, 2000; Tjurmina et al. 2002) and heightened levels of “depression-related” immobility in the tail suspension test (Holmes et al. 2002b; Zhao et al. 2006), as well as alterations in REM sleep (Alexandre et al. 2006; Wisor et al. 2003), suggestive of a depressive-like phenotype.
Pharmacological assessments have been helpful in improving our understanding of the effects of constitutive alterations in SERT expression, and thus the phenotype of SERT mutant mice. For example, early postnatal treatment with the serotonin synthesis inhibitor PCPA or the Htr1a antagonist WAY 100635 rescued many aspects of the altered phenotype observed in SERT −/− mice, establishing a role for elevated extracellular serotonin and activity at Htr1a receptors during early development for a number of the aspects of the phenotype of SERT −/− mice (Alexandre et al. 2006). Further, the anxiety-like phenotype and heightened stress response were somewhat mimicked in SERT +/+ and +/− mice via early postnatal administration of fluoxetine (Ansorge et al. 2004).
SERT mutant mice offer a tool to provide insight into altered SERT expression in humans. Human SERT spans 37.8 kb on chromosome 17q11.2 and is composed of 14 exons encoding a protein of 630 amino acids (Lesch et al. 1994; Ramamoorthy et al. 1993). In addition to several regulatory domains controlling selective expression in serotonergic neurons, transcriptional activity of human SERT is modulated by a repetitive element of varying length in the 5′ flanking region located ~1.8 kb upstream of the transcription start site, termed the SERT gene-linked polymorphic region (5HTTLPR). This has been the most studied human SERT variant, as there is an approximately twofold difference in mRNA, transporter sites, and serotonin uptake in cells from individuals with SS vs LALA genotypes (Hu et al. 2006; Lesch et al. 1996). The 5HTTLPR and a similar insertion/deletion variant are only present in humans and higher nonhuman primates and not other species with SERT (Lesch et al. 1997; Wendland et al. 2006). Murine SERT, located at chromosome 11.42, possesses other transcriptional control elements highly similar to the human gene, including cAMP-dependent components plus some evidence for alternative exon 1 usage in different tissues (Bengel et al. 1997; Heils et al. 1998; Sakai et al. 2003).
SERT +/− mice have ∼50% less expression and transport function and can be viewed as a model for humans with the SS genotype. SERT +/− mice frequently demonstrate intermediate drug responses, between SERT +/+ and −/− mice (e.g., Figs. 2b and 5b), in keeping with the ∼50% intermediate reductions in SERT expression, as shown in studies of SERT binding sites and uptake (Fig. 1a; Bengel et al. 1998; Montañez et al. 2003; Perez et al. 2006; Sora et al. 2001). There are also examples where SERT +/− mice do not differ from SERT +/+ mice (e.g., Figs. 1d, e, 2c, and 3b,c), a hint that humans with the SS 5HTTLPR genotype might be predicted to have a variable or sometimes subtle phenotype in drug responses. Nonetheless, in other assays, responses in SERT +/− mice were as marked as in SERT −/− mice (e.g., Fig. 5d). An exception was an exaggerated response in SERT +/− mice in the neurochemical response to fluvoxamine (Fig. 1c). It is possible, therefore, that unanticipated changes in specific brain areas such as the CA3 subregion of the hippocampus (Montañez et al. 2003) might lead to paradoxical responses partly mediating side effects found with different SRIs.
Another potential modifier that also is relevant to humans is gender. Although drug responses are often similar in male and female SERT +/− and −/− mice, some exceptions include a relative insensitivity of male SERT −/− mice to the induction of clonic seizures by the benzodiazepine convulsant agent PTZ (Fig. 7a); in contrast, the responses to PTZ of female SERT −/− mice were not different from SERT +/+ female mice (Fig. 7b). A more subtle difference was observed in the temperature reductions produced by Htr1a agonist 8-OH-DPAT. While both male and female SERT −/− mice were insensitive to a low dose of 8-OH-DPAT, male SERT +/− mice were as sensitive to the drug as SERT +/+ mice, while female SERT +/− mice were as insensitive as SERT −/− mice (Fig. 5c,d; Li et al. 1999, 2000). Further, female SERT +/− and −/− mice had somewhat greater reductions in brainstem Htr1a binding sites (the suggested site of action for this drug’s hypothermic effects).
Predictably, SRIs such as fluoxetine and fluvoxamine are ineffective in SERT −/− mice on behavioral and other measures, whereas the effects of other antidepressants, for example, those inhibiting NET, continue to exert their typical antidepressant effects in mice with reduced SERT expression (Ansorge et al. 2004; Fabre et al. 2000; Holmes et al. 2002b; Montañez et al. 2003). These findings serve to clarify that SRIs exert neurochemical, antidepressant, and anti-anxiety effects initially via their action at SERT (Sanchez and Hyttel 1999) and not, for example, by direct effects on the Htr2c receptor, which had been invoked as a possible explanation for fluoxetine’s acute effects on some responses known to be Htr2c-mediated (Palvimaki et al. 1996) nor via dopamine- or sodium or potassium channel-mediated effects or nicotinic receptor effects, which are sometimes considered as alternate possibilities (Garcia-Colunga et al. 1997; Kobayashi et al. 2003; Lenkey et al. 2006; Molteni et al. 2006). These responses parallel and support reports that humans with the lesser-expressing SS genotype of the 5HTTLPR polymorphism are relatively insensitive to the therapeutic effects of SRIs (Serretti et al. 2007). It will be of interest to determine whether other drug responses found to be altered in SERT mutant mice are also influenced by coding and noncoding functional human SLC6A4 variants, of which at least ten have been identified to date.
Overall, some of the phenotypic alterations in SERT −/− mice appear to be due to changes occurring during critical periods of neurodevelopment, as the lack of SERT has been present since conception. Support for this idea comes from both animal and human research. For example, several of the phenotypic changes observed in SERT −/− mice can be blocked by early postnatal administration of PCPA (to block serotonin synthesis) or WAY 100635 (to block Htr1a receptors), or by Htr1b knockout, during a critical period of development (Alexandre et al. 2006; Persico et al. 2001). Also, some of the behavioral features of the phenotype observed in SERT −/− mice have been mimicked by early postnatal administration of SRIs (Ansorge et al. 2004). In humans, individuals with the S allele of the 5HTTLPR have also been shown to have similar elevations in anxiety and related emotional characteristics (Greenberg et al. 2000; Lesch et al. 1996) resembling some murine phenotypes, again suggestive of a role for neurodevelopment in these phenotypic alterations (for a review, see Holmes et al. 2003b). These findings are seemingly in contrast to the anti-anxiety and antidepressant effects of SRIs administered chronically in adult animals and humans. Future animal research with temporally and spatially regulated conditional disruption of SERT will help to untangle the disparity between findings on the effects of genetic or pharmacologic blockade of SERT in early development compared to pharmacologic blockade of SERT by SRIs later in life.
References
Adrien J (2002) Neurobiological bases for the relation between sleep and depression. Sleep Med Rev 6:341–351
Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J (2006) Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci 26:5554–5564
Andrews AM, Murphy DL (1993a) 2′-NH2-MPTP in Swiss Webster mice: evidence for long-term (6-month) depletions in cortical and hippocampal serotonin and norepinephrine, differential protection by selective uptake inhibitors or clorgyline and functional changes in central serotonin neurotransmission. J Pharmacol Exp Ther 267:1432–1439
Andrews AM, Murphy DL (1993b) Fluoxetine and desipramine selectively attenuate 2′-NH2-MPTP-induced depletions in serotonin and norepinephrine. Eur J Pharmacol 250:215–221
Andrews AM, Murphy DL (1993c) Sustained depletion of cortical and hippocampal serotonin and norepinephrine but not striatal dopamine by 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP): a comparative study with 2′-CH3-MPTP and MPTP. J Neurochem 60:1167–1170
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306:879–881
Bagdy G (1996) Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res 73:277–280
Bengel D, Johren O, Andrews AM, Heils A, Mossner R, Sanvitto GL, Saavedra JM, Lesch KP, Murphy DL (1997) Cellular localization and expression of the serotonin transporter in mouse brain. Brain Res 778:338–345
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53:649–655
Bill DJ, Knight M, Forster EA, Fletcher A (1991) Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol 103:1857–1864
Bonaventure P, Umans L, Bakker MH, Cras P, Langlois X, Luyten WH, Megens AA, Serneels L, Van Leuven F, Leysen JE (1999) Humanization of mouse 5-hydroxytryptamine1B receptor gene by homologous recombination: in vitro and in vivo characterization. Mol Pharmacol 56:54–67
Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J (2003) Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci 18:2203–2212
Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A (2006) Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res 30:1957–1965
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
D’Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH (1987) Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A 84:4322–4326
Dailey JW, Reith ME, Yan QS, Li MY, Jobe PC (1997a) Anticonvulsant doses of carbamazepine increase hippocampal extracellular serotonin in genetically epilepsy-prone rats: dose response relationships. Neurosci Lett 227:13–16
Dailey JW, Reith ME, Yan QS, Li MY, Jobe PC (1997b) Carbamazepine increases extracellular serotonin concentration: lack of antagonism by tetrodotoxin or zero Ca2+. Eur J Pharmacol 328:153–162
Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A (2006) Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci 26:6431–6438
Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A (2005) Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A 102:5582–5587
Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP (2000) Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci 12:2299–2310
Fox MA, Jensen CL, Murphy DL (2006) Mediation of exaggerated serotonin syndrome-like behaviors and temperature responses in serotonin transporter knockout mice by 5-HT1A and 5-HT7 serotonin receptors: a possible model and mechanism for differential human vulnerability to the serotonin syndrome. Neuropsychopharmacology 31:S221–S222
Fox MA, Murphy DL (2006) Exaggerated serotonin syndrome in serotonin transporter knockout mice. Int J Neuropsychopharmacol 9:S174–S175
Garcia-Colunga J, Awad JN, Miledi R (1997) Blockage of muscle and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac). Proc Natl Acad Sci U S A 94:2041–2044
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379:606–612
Gobbi G, Murphy DL, Lesch K, Blier P (2001) Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther 296:987–995
González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC (2003) Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 23:8836–8843
Goodwin GM, De Souza RJ, Green AR (1985) The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology 24:1187–1194
Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL (2000) Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet 96:202–216
Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR (2002) Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience 115:153–161
Hariri AR, Holmes A (2006) Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci 10:182–191
Heils A, Wichems C, Mossner R, Petri S, Glatz K, Bengel D, Murphy DL, Lesch KP (1998) Functional characterization of the murine serotonin transporter gene promoter in serotonergic raphe neurons. J Neurochem 70:932–939
Heydorn WE (1999) Paroxetine: a review of its pharmacology, pharmacokinetics and utility in the treatment of a variety of psychiatric disorders. Expert Opin Investig Drugs 8:417–441
Holmes A, Murphy DL, Crawley JN (2002a) Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 161:160–167
Holmes A, Yang RJ, Murphy DL, Crawley JN (2002b) Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27:914–923
Holmes A, Li Q, Murphy DL, Gold E, Crawley JN (2003a) Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2:365–380
Holmes A, Murphy DL, Crawley JN (2003b) Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry 54:953–959
Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003c) Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28:2077–2088
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D (2006) Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 78:815–826
Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, Olverman HJ, Hastie ND, Harmar AJ, Shen S, Sharp T (2006) Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci 26:8955–8964
Jobe PC, Dailey JW, Wernicke JF (1999) A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol 13:317–356
Jones BJ, Blackburn TP (2002) The medical benefit of 5-HT research. Pharmacol Biochem Behav 71:555–568
Kalueff AV, Fox MA, Gallagher PS, Murphy DL (2007) Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav 6:389–400
Këlai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L (2003) Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol 38:386–389
Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL (2005) Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49:798–810
Kobayashi T, Washiyama K, Ikeda K (2003) Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol 138:1119–1128
Lenkey N, Karoly R, Kiss JP, Szasz BK, Vizi ES, Mike A (2006) The mechanism of activity-dependent sodium channel inhibition by the antidepressants fluoxetine and desipramine. Mol Pharmacol 70:2052–2063
Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P (1994) Organization of the human serotonin transporter gene. J Neural Transm Gen Sect 95:157–162
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A (1997) The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm 104:1259–1266
Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL (1999) Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther 291:999–1007
Li Q, Wichems C, Heils A, Lesch KP, Murphy DL (2000) Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci 20:7888–7895
Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL (2003) Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem 84:1256–1265
Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL (2004a) Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci 24:10868–10877
Li Q, Ma L, Innis RB, Seneca N, Ichise M, Huang H, Laruelle M, Murphy DL (2004b) Pharmacological and genetic characterization of two selective serotonin transporter ligands: 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine (AFM) and 3-amino-4-[2-(dimethylaminomethyl-phenylthio)]benzonitrile (DASB). J Pharmacol Exp Ther 308:481–486
Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA (2003) Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry 54:960–971
Lu KT, Gean PW (1998) Endogenous serotonin inhibits epileptiform activity in rat hippocampal CA1 neurons via 5-hydroxytryptamine1A receptor activation. Neuroscience 86:729–737
Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L (2001) Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci 21:2178–2185
Mannoury la Cour C, Hanoun N, Melfort M, Hen R, Lesch KP, Hamon M, Lanfumey L (2004) GABA(B) receptors in 5-HT transporter- and 5-HT1A receptor-knock-out mice: further evidence of a transduction pathway shared with 5-HT1A receptors. J Neurochem 89:886–896
Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ (1992) Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol 107:15–21
Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM (2004) Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods 140:169–181
Molteni R, Calabrese F, Bedogni F, Tongiorgi E, Fumagalli F, Racagni G, Riva MA (2006) Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int J Neuropsychopharmacol 9:307–317
Montañez S, Owens WA, Gould GG, Murphy DL, Daws LC (2003) Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem 86:210–219
Mossner R, Schmitt A, Hennig T, Benninghoff J, Gerlach M, Riederer P, Deckert J, Lesch KP (2004) Quantitation of 5HT3 receptors in forebrain of serotonin transporter deficient mice. J Neural Transm 111:27–35
Mossner R, Simantov R, Marx A, Lesch KP, Seif I (2006) Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci Lett 401:49–54
Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B (1998) Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry 59(Suppl 15):4–12
Numis AL, Unger EL, Sheridan DL, Chisnell AC, Andrews AM (2004) The role of membrane and vesicular monoamine transporters in the neurotoxic and hypothermic effects of 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH(2)-MPTP). Mol Pharmacol 66:718–727
Palvimaki EP, Roth BL, Majasuo H, Laakso A, Kuoppamaki M, Syvalahti E, Hia J et al (1996) Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor. Psychopharmacology (Berl) 126:234–240
Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R (2001) Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Brain Res Dev Brain Res 126:125–129
Pasini A, Tortorella A, Gale K (1996) The anticonvulsant action of fluoxetine in substantia nigra is dependent upon endogenous serotonin. Brain Res 724:84–88
Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RA, van der Gugten J, Olivier B (2002a) Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology 27:380–390
Pattij T, Groenink L, Oosting RS, van der Gugten J, Maes RA, Olivier B (2002b) GABA(A)-benzodiazepine receptor complex sensitivity in 5-HT(1A) receptor knockout mice on a 129/Sv background. Eur J Pharmacol 447:67–74
Peng W, Simantov R (2003) Altered gene expression in frontal cortex and midbrain of 3,4-methylenedioxymethamphetamine (MDMA) treated mice: differential regulation of GABA transporter subtypes. J Neurosci Res 72:250–258
Perez XA, Andrews AM (2005) Chronoamperometry to determine differential reductions in uptake in brain synaptosomes from serotonin transporter knockout mice. Anal Chem 77:818–826
Perez XA, Bianco LE, Andrews AM (2006) Filtration disrupts synaptosomes during radiochemical analysis of serotonin uptake: comparison with chronoamperometry in SERT knockout mice. J Neurosci Methods 154:245–255
Perrault G, Morel E, Zivkovic B, Sanger DJ (1992) Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav 42:45–47
Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F (2001) Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci 21:6862–6873
Porsolt RD (2000) Animal models of depression: utility for transgenic research. Rev Neurosci 11:53–58
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Qu Y, Villacreses N, Murphy DL, Rapoport SI (2005) 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 180:12–20
Raap DK, Evans S, Garcia F, Li Q, Muma NA, Wolf WA, Battaglia G, Van De Kar LD (1999) Daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J Pharmacol Exp Ther 288:98–106
Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A 90:2542–2546
Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, Hamon M, Martres MP (1999) Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett 262:113–116
Rudnick G, Wall SC (1993) Non-neurotoxic amphetamine derivatives release serotonin through serotonin transporters. Mol Pharmacol 43:271–276
Sakai K, Hasegawa C, Okura M, Morikawa O, Ueyama T, Shirai Y, Sakai N, Saito N (2003) Novel variants of murine serotonin transporter mRNA and the promoter activity of its upstream site. Neurosci Lett 342:175–178
Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I (2001) Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci 21:884–896
Sanchez C, Hyttel J (1999) Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 19:467–489
Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP (2003) Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res 71:701–709
Serretti A, Kato M, De Ronchi D, Kinoshita T (2007) Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 12:247–257
Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I (2004) Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29:1790–1799
Sheridan DL, Wichems CH, Murphy DL, Andrews AM (1999) The effects of PCPA on monoamine neurotransmitter levels in mice with a disruption of the serotonin transporter gene. 29th Annual Meeting of the Society for Neuroscience
Shouse MN, Staba RJ, Ko PY, Saquib SF, Farber PR (2001) Monoamines and seizures: microdialysis findings in locus ceruleus and amygdala before and during amygdala kindling. Brain Res 892:176–192
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR (1998) Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A 95:7699–7704
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR (2001) Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A 98:5300–5305
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370
Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D (2006) Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol 17:271–287
Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL (2002) Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 143:4520–4526
Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R (2007) 3,4-Methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry (in press)
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Unger EL, Mazzola-Pomietto P, Murphy DL, Andrews AM (2002) 2′-NH(2)-MPTP [1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine] depletes serotonin and norepinephrine in rats: a comparison with 2′-CH(3)-MPTP [1-methyl-4-(2′-methylphenyl)-1,2,3,6-tetrahydropyridine]. J Pharmacol Exp Ther 303:527–533
van der Kooy D (1987) Place conditioning: a simple and effective method for assessing the motivational properties of drugs. In: Bozarth MA (ed) Methods of assessing the reinforcing properties of abused drugs. Springer, Berlin, pp 229–240
Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ (2006) Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet 36:163–172
Wichems CH, Andrews AM, Heils A, Li Q, Lesch KP, Murphy DL (1998) Spontaneous behavior differences and altered responses to psychomotor stimulants in mice lacking the serotonin transporter. 28th Annual Meeting of the Society for Neuroscience
Wichems CH, Li Q, Holmes A, Crawley JN, Tjurmina O, Goldstein D, Andrews AM, Lesch KP, Murphy DL (2000) Mechanisms mediating the increased anxiety-like and excessive responses to stress in mice lacking the serotonin transporter. 30th Annual Meeting of the Society for Neuroscience
Willins DL, Meltzer HY (1997) Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther 282:699–706
Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM (2003) Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport 14:233–238
Xu Y, Sari Y, Zhou FC (2004) Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res 150:151–161
Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D’Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD (2002) Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci 22:9635–9642
Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, Murphy DL, Lanthorn TH, Holmes A (2006) Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience 140:321–334
Zhou FC, Lesch KP, Murphy DL (2002) Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res 942:109–119
Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA (2005) Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron 46:65–74
Acknowledgment
This research was supported by the NIMH Intramural Research program. The authors wish to thank our many collaborators and colleagues throughout the world whose studies provided the basis for this review, and especially Teresa Tolliver, Sharon Engel, and Christine Wichems for contributions to initial studies of these mice, and also Theresa Deguzman for very helpful assistance with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fox, M.A., Andrews, A.M., Wendland, J.R. et al. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology 195, 147–166 (2007). https://doi.org/10.1007/s00213-007-0910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0910-0