Abstract
Rationale
Chronic exposure to drugs of abuse alters neural processes that normally promote learning and memory. A context that is repeatedly paired with reinforcing drugs will acquire secondary reinforcing properties (conditioned reward). However, the effects of conditioned reward on spatial learning are unknown.
Objective
Using the conditioned place preference procedure and Morris water maze task, we examined the role of conditioned reward or aversion in spatial learning.
Materials and methods
Groups of rats acquired morphine (10 mg/kg), cocaine (10 mg/kg), or oral sucrose (15%) conditioned place preference (CPP). Another group of morphine-dependent rats acquired conditioned place aversion (CPA) to a context paired with precipitated opiate withdrawal induced by naloxone injections (1 mg/kg). To examine the role of conditioned reward or aversion in spatial learning, rats were then exposed to the previously morphine-, cocaine-, sucrose- or naloxone-paired context for 10 min before training of spatial learning in the Morris water maze.
Results
Exposure to the morphine- or cocaine-paired but not the sucrose- or the naloxone-paired context decreased the latency to find the platform in the Morris water maze test.
Conclusions
Our results provide the first evidence that conditioned drug reward promotes spatial learning. We speculate that this enhancement of spatial learning by the drug-paired context may promote contextual-cue-induced relapse to drug taking by facilitating exploratory drug-seeking behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular, neuroanatomical, and neurophysiological studies have demonstrated mechanistic similarities between normal forms of learning and memory and central actions of reinforcing drugs; drugs of abuse and Pavlovian and instrumental learning processes act on similar neural pathways in the mesocorticolimbic brain reward system (Beninger and Gerdjikov 2004; Everitt and Robbins 2005; Hyman et al. 2006; Jentsch and Taylor 1999; Stewart 2004; White 1996). Repeated administration of reinforcing drugs, such as cocaine or morphine produces functionally significant alterations in neurobiological processes subserving reward-related learning and memory (Harris et al. 2004; Hyman et al. 2006; Olson et al. 2005; Schoenbaum et al. 2006; Taylor and Horger 1999; Taylor and Jentsch 2001; Valjent et al. 2006). These drug-induced neural alterations in reward-related learning and memory processes may result in enhanced control over drug-seeking behavior by reward-associated contextual stimuli (Berke 2003; Hyman 2005; Self 2004). Enhanced control over drug-seeking behavior by reward-related stimuli may in turn increase the vulnerability of drug users to relapse into drug use upon exposure to drug-associated contexts, such as places paired with their prior drug use (Ehrman et al. 1992; Gawin and Kleber 1986).
Repeated prior exposure to psychostimulants and psychomotor-stimulant induced sensitization enhance the acquisition of appetitive Pavlovian learning and the expression of Pavlovian-cue induced operant behavior (Pavlovian-to-instrumental transfer) when sensitized rats are tested in a drug-free state (Harmer and Phillips 1998; Taylor and Jentsch 2001; Wyvell and Berridge 2001). Moreover, accumbens amphetamine injections potentiates conditioned-reward-directed behaviors in rats tested during acute exposure to this drug (Phillips et al. 2003; Taylor and Robbins 1984; Wyvell and Berridge 2000). However, while previous studies have investigated the effects of acute or repeated psychostimulant drug exposure on the performance of conditioned-reward-directed behaviors, no studies have examined whether previous repeated exposure to conditioned drug rewards (for example the drug-paired context) enhances subsequent learning. An interesting question regarding drug-paired contexts is whether repeated prior pairings of a drug with a certain context can enhance subsequent spatial learning and memory processes.
After repeated pairings with drugs, the contextual environment acquires secondary reinforcing properties (conditioned reward) through Pavlovian learning (Schechter and Calcagnetti 1993). Through Pavlovian learning, rats will choose to spend more time in a location in which they have passively received an injection of psychostimulants or morphine than in another location paired with saline injection (conditioned place preference–CPP; Bardo and Bevins 2000; Tzschentke 1998). Previous exposure to drug-paired contexts through CPP enhances subsequent drug self-administration and reinstatement of drug-seeking behavior (Bardo and Bevins, 2000; Itzhak and Martin 2002). We hypothesized that CPP would facilitate subsequent spatial learning. To that effect, we investigated the effect of morphine- or cocaine-induced CPP on rat spatial learning using the Morris water maze test. Groups of rats acquired morphine (10 mg/kg), cocaine (10 mg/kg), oral sucrose (15%) CPP. Another group of morphine-dependent rats acquired conditioned place aversion (CPA) to a context paired with precipitated opiate withdrawal induced by naloxone injections (1 mg/kg). The sucrose CPP and the morphine CPA groups were included to assess the generality of the effect of our training procedure on subsequent performance and to examine roles of different cue stimuli (rewarding or aversive) in a spatial learning task. To examine the role of conditioned reward or aversion in spatial learning, rats were then exposed to the previous morphine-, cocaine-, sucrose-, or naloxone-paired context for 10 min before training of spatial learning in the Morris water maze.
Materials and methods
Subjects and drugs
One hundred seven male Sprague–Dawley rats, weighing 220–270 g, were housed individually (experiment 3) or in groups of four (experiment 1–2 and 4) under a constant temperature (23 ± 2°C) and maintained on a 12-h light/dark cycle with free access to food and water except in the case of rats in the sucrose training paradigm. All the treatments of the rats were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no.86–23, 1996). The procedures were approved by the local Committee of Animal Use and Protection. The drugs used were cocaine hydrochloride and morphine hydrochloride (Qinghai Pharmaceutical, China). The naloxone hydrochloride was purchased from Sigma (SI, USA). Cocaine, morphine, and naloxone were dissolved in saline.
Training for morphine and cocaine CPP
The apparatus for conditioned place preference training consisted of six identical three-chamber PVC boxes (Med Associates, USA) in light- and sound-controlled cubes. Two large chambers (27.9 cm long × 21.0 cm wide × 20.9 high cm) were separated by a smaller chamber (12.1 cm long × 21.0 cm wide × 20.9 cm high), with differences in wall color and floor texture. One large chamber was black with a floor of stainless-steel bars (4.8 mm placed on 1.6-cm centers). The other large chamber was white with a floor of a stainless-steel mesh (1.3 × 1.3 cm). The middle smaller chamber was gray with a smooth PVC floor. Three distinct chambers were separated by manual guillotine doors. According to previously study, rats had a significant natural preference for the black chamber over the white chamber, and bias could be neutralized by manipulating the ambient lighting within the CPP procedure (Roma and Riley 2005). The intensity of ambient illumination was adjusted to balance the side preferences for each large chamber. The conditioning sessions were performed in the light phase in the presence of white noise. To determine baseline preference (preconditioning), the rats were initially placed in the middle chamber with the doors removed for a period of 15 min. The time spent in the previously saline- or drug-paired chambers during the 15-min session was determined using MED-PC system (Med Associates, USA). Conditioning was performed using an unbiased, balanced protocol as in our previous studies (Lu et al. 2001; Wang et al. 2006). About 10% total subjects (13/120), which spent 150 s in one large chamber more than in another, were discarded.
Morphine and cocaine CPP training sessions consisted of 8 alternating days of drug and saline injections with the guillotine doors in place (Lu et al. 2000; Wang et al. 2006). The chamber in which morphine or cocaine was administered was assigned randomly. Thus, each rat was treated for 8 consecutive days with alternate injections by drugs (morphine 10 mg/kg, s.c.; cocaine 10 mg/kg, i.p.) and saline (1 ml/kg). The drug doses selected for CPP conditionings were based on our and other previous studies (Bardo et al. 1995; Lu et al. 2001; Wang et al. 2006). The most effective morphine and cocaine doses during acquisition of CPP were 10 mg/kg morphine, s.c. or 10 mg/kg cocaine, i.p. respectively (Bardo et al. 1995; Lu et al. 2001; Tzschentke 1998; Wang et al. 2006). After each injection, the rats were immediately confined to the conditioning chamber for 45 min before they returned to the home cage. On day 9, the rats were tested for the acquisition (postconditioning) of morphine or cocaine CPP under conditions identical to those described for the preconditioning test: The doors were removed and the rats were placed in the middle chamber and allowed to move freely for 15 min.
Training for sucrose CPP
The procedure for training sucrose CPP was modified from previous studies (Alderson et al. 2001; Delamater et al. 2000). Briefly, rats were individually housed with free access to drinking water but limited access to 10 g lab chow per day for 8 days before training. Under this rearing condition, rats maintained 80% body weight. The cover of the two large chambers of the CPP box was modified for liquid supply through a drinking tube. Before the preconditioning test and the training session, drinking water was removed from the home cage for 2 h. Preconditioning test was performed as described above except that plain water was available on both side. Sucrose CPP training consisted of 14 alternating days of sucrose and plain water available with the guillotine doors in place. During training for CPP, rats were allowed free access alternately to sucrose solution (15%) and water for 30 min through a tube. The chamber in which sucrose was delivered was assigned randomly. Our preliminary experiment demonstrated that this procedure produced the largest magnitude of sucrose CPP in our rats. On day 15, the rats were tested for 15 min for the acquisition of sucrose CPP under conditions identical to the preconditioning test.
Training for naloxone-conditioned place aversion
The procedure of naloxone-precipitated withdrawal-induced CPA in morphine-dependent rats was based on previous studies (Lu et al. 2000, 2005; Mucha 1987; Salem and Hope 1997; Watanabe et al. 2002, 2003). Rats received morphine s.c. injections twice a day for 7 days by an increasing dose regimen of 10, 15, 20, 25, 30, 35 or 40 mg/kg, respectively (Lu et al. 2005). From days 8 to 14, rats were given injections of morphine 40 mg/kg twice a day with a 12-h interval between injections (8:00 a.m. and 8:00 p.m.). On day 8, rats were tested for their preconditioning CPA at 2–4 h before the second morphine injection. Naloxone-conditioned place aversion training started on day 9. Conditioning was performed in six alternate sessions of naloxone and saline treatments. Thirty minutes after first injection of morphine 40 mg/kg (s.c.), rats were injected with naloxone 1 mg/kg (i.p.) or saline (1 ml/kg). After each injection of naloxone or saline, the rats were immediately confined to the conditioning chamber for 30 min before they returned to the home cage. On day 15, the rats were tested for the acquisition of naloxone CPA under conditions identical to those described for the CPP test.
Place navigation task
Rats were habituated to the swimming pool environment the day after the last CPP test. Two days after the last CPP test, a place navigation task was conducted using the Morris water maze (Morris et al. 1982, 1986). The apparatus consisted of a circular black-painted swimming pool (150 cm in diameter × 50 cm high) filled with water at 22–24°C and made opaque by the addition of black ink. The pool was located in a testing room containing numerous objects on the wall that the animals can use as cues for spatial orientation. The position of these environmental cues remained unchanged throughout the entire testing period. The pool was divided into four equal quadrants. The black platform was invisible by making the circular of Morris water maze black and the water opaque by addition of black ink. The invisible black platform (9 cm in diameter and submerged 1.5 cm below the water surface) was placed in the center of the quadrant I. On day 1, the rats were habituated to the swimming pool environment. They were placed individually on the platform for 20 s and in the pool for 60 s and allowed to swim. During the training period of the task, rats were given three trials per day to find the hidden platform for 5 consecutive days (maximum trial duration 60 s, 20-s reinforcement on the platform). Each rat was gently placed into the water, with the nose pointing toward the wall at one of the starting points designated on the rim center of quadrants II, III, and IV. Rats that failed to find the hidden platform were given a score of 60 s and then physically placed on the platform for 20 s. The time required for rats to climb onto the platform was recorded as escape latency. The mean of the three trials per training day was scored.
Experimental design
As described above, each experiment consisted of three phases: training for CPP (or CPA), test for expression of CPP (or CPA), and training for spatial learning in the Morris water maze. Experiments 1–3 examined the effects of drugs (experiments 1 and 2) or sucrose (experiment 3) conditioned reward on spatial learning and memory. After testing for acquisition of CPP for morphine, cocaine, or sucrose, rats were randomly assigned to three groups (conditioned reward, non-conditioned reward and control group, n = 8–10 per group) and subsequently tested for spatial learning in the Morris water maze task, as described above. During the testing session, separate groups of rats were given different treatments: (1) rats in the conditioned reward group were placed in the previously morphine-, cocaine- or sucrose-paired chamber for 10 min each day before placement in the water maze; (2) rats in the non-conditioned reward group were placed in the previously saline- or water-paired chamber for 10 min each day before placement in the water maze; (3) rats in the control group (prior reward-experience condition) were not exposed to any CPP chamber before placement in the water maze.
Experiment 4 examined the effects of conditioned aversion on spatial learning and memory. Rats were trained on acquisition of naloxone CPA as described above. Subsequently, rats were randomly assigned to three groups (conditioned aversion, non-conditioned aversion, and control group, n = 8 per group) and subsequently tested for spatial learning in the water maze task, as described above. During the test for spatial learning, separate groups of rats were given different treatments: (1) rats in the conditioned aversion group were placed in the previously naloxone-paired chamber for 10 min each day before placement in water maze; I2) rats in the non-conditioned aversion group were placed in the previously saline-paired chamber for 10 min each day before placement in the water maze; (3) rats in the control group (prior precipitated withdrawal condition) were not exposed to any chamber prior to placement in the water maze.
Statistical analysis
The amount of time spent in drug- or sucrose-paired sides, the scores of place preference or aversion, and the time of escape latency for finding the platform during the water maze test were expressed as means ± SEM. Data were analyzed using a mixed factorial design with treatment condition (conditioned reward, non-conditioned reward, and control) as the between-subjects factor and test day (preconditioning and postconditioning in CPP or CPA test and days 1–5 in test of water maze) as the within-subjects factor. A subsequent one-way analysis of variance (ANOVA) was used to reveal the effect of treatment condition at each test day. A post-hoc analysis using the Student–Newman–Keuls test was used to evaluate differences among treatment conditions in time spent in the drug- or sucrose-paired side or in escape latency for finding the platform. Statistical differences at P < 0.05 were considered significant.
Results
Effects of morphine conditioned reward on spatial learning
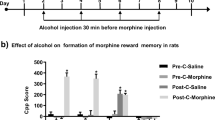
Figure 1a shows the time spent in the morphine-paired chamber during the preconditioning and postconditioning of CPP. Overall, as in our previous studies (Lu et al. 2000, 2001), rats in the different groups [conditioned reward, non-conditioned reward and control (prior reward-experience)] acquired morphine CPP after 8-day alternate injections of morphine and saline. As no significant differences were found on time spent in the morphine-paired chamber among the three groups during the preconditioning or postconditioning test session (P > 0.05), the data from three groups were combined. The one-way ANOVA analysis revealed a significant increase in the time spent in the morphine-paired chamber from the pre- to post-conditioning place preference tests [F (1, 29) = 49.1, P < 0.05].
Effects of morphine conditioned reward on spatial learning. a Acquisition of morphine CPP. Data are depicted as the time (mean ± SEM) spent in the morphine-paired chamber during the preconditioning and postconditioning of CPP. Different from preconditioning, *P < 0.05 (total n = 30, 3 groups). b Effects of exposure to previous morphine-paired chamber on spatial learning. Data are depicted as the time (mean ± sem) spent in finding the hidden platform. Different from the control group, *P < 0.05, n = 9–11 per group
Figure 1b shows the time spent in finding and climbing onto the hidden platform. Rats that were exposed to the previously morphine-paired side spent significantly less time to find the hidden platform, which indicated that exposure to a conditioned reward increased spatial learning and memory. Data analysis revealed significant effects of treatment condition [F (2, 27) = 16.6, P < 0.05), test day [F (4, 108) = 46.1, P < 0.05] and treatment condition × test day [F (8, 108) = 12.7, P < 0.05). However, no significant differences in swimming speed (14.5, 13.9, and 14.1 cm/s, for conditioning reward, non-conditioning reward, and control group, respectively) were observed among different groups (P > 0.05). Post-hoc group differences are indicated in Fig. 1b.
Effects of cocaine conditioned reward on spatial learning
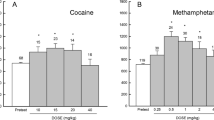
Figure 2a shows the time spent in the cocaine-paired chamber during the preconditioning and postconditioning of CPP. As in experiment 1, rats in the different groups (conditioned reward, non-conditioned reward, and control) acquired cocaine CPP after an 8-day alternate injections of cocaine and saline. As no significant differences were found on time spent in the cocaine-paired chamber among the three groups during the preconditioning or postconditioning test session (P > 0.05), the data from three groups were combined. One-way ANOVA analysis revealed a significant increase in the time spent in the cocaine-paired chamber from the pre- to post-conditioning place preference tests [F (1, 28) = 22.5, P < 0.05].
Effects of cocaine conditioned reward on spatial learning. a Acquisition of cocaine CPP. Data are depicted as the time (mean ± sem) spent in the cocaine-paired chamber during the pre-conditioning and post-conditioning of CPP. Different from pre-conditioning, *P < 0.05 (total n = 29, 3 groups). b Effects of exposure to previous cocaine-paired chamber on spatial learning. Data are depicted as the time (mean ± SEM) spent in finding the hidden platform. Different from the control group, *P < 0.05, n = 9–10 per group
Figure 2b shows the time spent in finding and climbing onto the hidden platform. Rats that were exposed to the previously cocaine-paired side spent significantly less time to find the hidden platform, which indicated that exposure to cocaine conditioned reward increased spatial learning and memory. Data analysis revealed significant effects of treatment condition [F (2, 26) = 38.8, P < 0.05), test day [F (4,104) = 65.8, P < 0.05) and treatment condition × test day [F (8, 104) = 15.4, P < 0.05). However, no significant differences in swimming speed (15.1, 14.7, and 15.3 cm/s, for conditioning reward, non-conditioning reward, and control group, respectively) were observed among different groups (P > 0.05). Post-hoc group differences are indicated in Fig. 2b.
Effects of sucrose conditioned reward on spatial learning
As in experiment 1, rats in the different groups (conditioned reward, non-conditioned reward, and control) acquired sucrose CPP after 14 days of alternate access to 15% sucrose solution and water. As no significant differences were found in time spent in the sucrose-paired chamber among the three groups during the preconditioning or postconditioning test session (P > 0.05), the data from the three groups were combined. The one-way ANOVA showed that there was a significant increase in the time spent in the sucrose-paired chamber from the pre- to post-conditioning place preference tests [F (1, 23) = 10.0, P < 0.05].
Rats that were exposed to the previously sucrose-paired side spent a similar time to find the hidden platform as the control and non-conditioned reward groups, indicating that previous exposure to sucrose conditioned reward does not affect spatial learning and memory. Data analysis revealed significant effects of test day [F (4, 84) = 15.0, P < 0.05) but not for treatment condition or treatment condition × test day (P > 0.05).
Effects of naloxone-conditioned aversion on spatial learning
Rats in the different groups (conditioned aversion, non-conditioned aversion, and control) acquired naloxone CPA after 6 days of alternate injections of naloxone and saline after chronic morphine administration. As no significant differences were found in time spent in the naloxone-paired chamber among the three groups during the preconditioning or postconditioning test session (P > 0.05), the data from the three groups were combined. The one-way ANOVA revealed a significant decrease in the time spent in the naloxone-paired chamber from the pre- to post-conditioning place preference tests [F (1, 23) = 28.9, P < 0.05].
No significant differences in time spent to find the hidden platform were found among groups, indicating that exposures to drug-conditioned aversion do not affect spatial learning and memory. Data analysis revealed significant effects for test day [F (4, 84) = 32.5, P < 0.05] but not for treatment condition or treatment condition × test day (P > 0.05).
Discussion
The present study examined the effects of cocaine- and morphine-conditioned reward on spatial learning and memory, as assessed with the Morris water maze task. The main finding in this report was that after the acquisition of CPP, rats exposed to a context previously paired with morphine or cocaine but not a context previously paired with sucrose or naloxone-precipitated withdrawal, spent significantly less time to find a hidden platform in the Morris water maze, relative to rats not previously exposed to a drug-paired context.
Contextual cues that are associated with morphine or psychostimulant availability can promote psychomotor sensitization (Badiani et al. 2000; Crombag et al. 2000). In our present study, no significant differences in swimming speed were observed among pre-exposed to the drug (cocaine or morphine) paired-, unpaired-context, and control groups. Thus, it is unlikely that rats pre-exposed to the cocaine or morphine-paired context have exhibited enhanced performance in the Morris water maze task due to the expression of psychomotor sensitization induced by the drug-paired context.
Previous studies have shown that prior exposure to drugs of abuse enhances the learning of appetitive Pavlovian-conditioned responses and spatial learning as assessed by the Morris water maze task in animals tested in a drug-free state. Repeated intermittent exposure to psychomotor stimulant drugs leads to enhanced acquisition of Pavlovian conditioning or of Pavlovian-cue-directed operant behaviors in animals tested in a drug-free state (Harmer and Phillips 1998, 1999; Taylor and Jentsch 2001; Wyvell and Berridge 2001). Previous exposure to morphine enhances spatial learning as assessed with the Morris water maze task (Yang et al. 2004). However, no studies have assessed the effects of exposure to a drug-paired context on spatial learning. The results of the present study provide the first demonstration that conditioned -drug reward promotes spatial learning and memory in rats.
Cocaine- or morphine-conditioned reward, but not sucrose conditioned reward, may have enhanced subsequent spatial learning and memory in the Morris water maze for several reasons. It has demonstrated that morphine or cocaine, but not sucrose, may have sensitized brain areas involved in spatial learning rendering them hypersensitive to spatial or contextual-stimuli predicting reinforcement. For example, repeated daily administration of cocaine has been shown to induce functional changes in voltage-dependent sodium channels in rat hippocampal CA1 pyramidal neurons, increasing the excitability of those neurons (Zhai et al. 1997). Cocaine administration increases the excitability of Shaffer collateral synapses of CA1 pyramidal neurons in the hippocampus, which can enhance processing of neuronal signals through hippocampal circuitry (Hammad and Wagner 2006) that is involved in spatial learning (Foster et al. 1989; McNaughton et al. 1983; O'Keefe 1983). Mu-opioid receptor agonists also increase the excitability of hippocampal neurons after electrical stimulation of synaptic afferents to the dentate gyrus and CA1 regions of the rat hippocampus; mu-opioid receptor induced suppression of gamma-aminobutyric acid (GABA) release onto GABA-A receptors facilitates excitatory activity of CA1 hippocampal neurons (McQuiston and Saggau 2003; Neumaier and Chavkin 1986). Interestingly, hippocampal circuitry that is necessary for spatial learning (Morris et al. 1990; Moser et al. 1993) is also necessary for both the acquisition and expression of established morphine and cocaine CPP (Meyers et al. 2006; Milekic et al. 2006). Thus, the hippocampal formation is important for directing approach behaviors elicited by cocaine or morphine contextual stimuli and also for using salient spatial stimuli to guide exploratory behavior necessary to attain reinforcement in navigation tasks. As cocaine- or morphine-conditioned stimuli induce a conditioned enhancement of brain reward function (Bespalov and Zvartau 1992; Kenny et al. 2003) and morphine and cocaine increase the excitability of hippocampal circuitry that is necessary for spatial learning (see above), previous cocaine or morphine context may facilitate spatial learning by sensitizing hippocampal circuitry necessary for spatial learning, increasing its sensitivity to spatial information predictive of reinforcement. In contrast, there is no evidence, to our knowledge, suggesting that sucrose reward alters the brain circuitry required for spatial learning in the same manner as drugs of abuse.
The other reason for different roles of drug-conditioned reward and sucrose conditioned reward in spatial learning may be due to different neuronal processes in mesolimbic system induced by natural reward-related cues vs cue associated with drugs of abuse. It has been reported that animals were trained to administer heroin associated with a compound audio-visual cue; re-exposure to the cue after 3 weeks of withdrawal reinstated heroin-seeking behavior, which resulted in immediate early gene (IEG) expression of ania-3 (a member of the Homer family), Mitogen-activated protein (MKP)-1, c-fos and nuclear receptor subfamily 4, group A, member 3 (Nr4a3) in the medial prefrontal cortex (mPFC), and of ania-3 in the orbital frontal cortex (OFC) and nucleus accumbens core (NAc). The activation patterns for heroin-seeking behaviors did not generalize to sucrose-seeking behaviors, indicating that the two behaviors involve different connectivity pathways of neuronal signaling (Koya et al. 2006). Thus, drugs of abuse, but not sucrose, may induce sensitization of hippocampal circuitry required for spatial learning accounting for the differences found in the present study wherein cocaine or morphine conditioned reward, but not sucrose conditioned reward, facilitated subsequent spatial learning and memory.
Another question raised by the present study is why conditioned reward, but not conditioned aversion, facilitates subsequent spatial learning and memory. Previous studies have shown that when drug reinforcement has been repeatedly paired with drug-associated cues, these conditioned cues can elicit Pavlovian approach responses and preference for the drug-paired environment (Bardo and Bevins 2000). In preclinical studies, stimuli that are associated with reinforcement and increased dopamine release in the mesolimbic system, such as intracranial self-stimulation (ICSS), have been shown to enhance the performance of a variety of learned responses including operant and spatial learning (Aldavert-Vera et al. 1996; Segura-Torres et al. 1988, 1991; Yoganarasimha et al. 1998). Stimulant drugs such as amphetamine and cocaine (as well as others), but not aversive stimuli such as those associated with opiate withdrawal, exaggerate the incentive salience of conditioned stimuli that are closely associated in space and time with self-administered drugs (Robinson and Berridge 1993). We therefore propose that exposure to the drug-paired environment acting as a conditioned reward may elicit incentive salience associated with reinforcement that could enhance the learning or retention of a spatial-navigation task. This hypothesis is supported by our current results. Rats that were pre-exposed to the aversive naloxone-paired and drug-unpaired side did not exhibit improved spatial learning and memory, unlike the rats pre-exposed to a morphine- or cocaine-paired environment that exhibited an enhancement of this behavior.
In summary, the present study investigated the effect of conditioned drug reward on spatial learning and memory using the CPP paradigm and the Morris water maze task. Our results suggest that acute exposure to the morphine or cocaine-paired context enhances subsequent spatial learning and memory.
References
Aldavert-Vera L, Segura-Torres P, Costa-Miserachs D, Morgado-Bernal I (1996) Shuttle-box memory facilitation by posttraining intracranial self-stimulation: differential effects in rats with high and low basic conditioning levels. Behav Neurosci 110:346–352
Alderson HL, Jenkins TA, Kozak R, Latimer MP, Winn P (2001) The effects of excitotoxic lesions of the pedunculopontine tegmental nucleus on conditioned place preference to 4%, 12% and 20% sucrose solutions. Brain Res Bull 56:599–605
Badiani A, Oates MM, Robinson TE (2000) Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacology (Berl) 151:273–82
Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward. Psychopharmacology 153:31–43
Bardo MT, Rowlett JK, Harris MJ (1995) Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51
Beninger RJ, Gerdjikov T (2004) The role of signaling molecules in reward-related incentive learning. Neurotox Res 6:91–104
Berke JD (2003) Learning and memory mechanisms involved in compulsive drug use and relapse. Methods Mol Med 79:75–101
Bespalov A, Zvartau EE (1992) Conditioned-reflex activation of electrical self-stimulation of the brain: a model of situational craving for narcotics. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni IP Pavlova 42:759–763
Crombag HS, Badiani A, Maren S, Robinson TE (2000) The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res 116:1–22
Delamater AR, Sclafani A, Bodnar RJ (2000) Pharmacology of sucrose-reinforced place-preference conditioning: effects of naltrexone. Pharmacol Biochem Behav 65:697–704
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107:523–529
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Foster TC, Castro CA, McNaughton BL (1989) Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science 244:1580–1582
Gawin FH, Kleber HD (1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43:107–113
Hammad H, Wagner JJ (2006) Dopamine-mediated disinhibition in the CA1 region of rat hippocampus via D3 receptor activation. J Pharmacol Exp Ther 316:113–120
Harmer CJ, Phillips GD (1998) Enhanced appetitive conditioning following repeated pretreatment with d- amphetamine. Behav Pharmacol 9:299–308
Harmer CJ, Phillips GD (1999) Enhanced conditioned inhibition following repeated pretreatment with d-amphetamine. Psychopharmacology (Berl) 142:120–131
Harris GC, Wimmer M, Byrne R, Aston-Jones G (2004) Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience 129:841–847
Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162:1414–22
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Itzhak Y, Martin JL (2002) Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology 26:130–134
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharamacology 146:373–390
Kenny PJ, Koob GF, Markou A (2003) Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci 117:1103–1107
Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB (2006) Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem 98:905–915
Lu L, Chen H, Su W, Ge X, Yue W, Su F, Ma L (2005) Role of withdrawal in reinstatement of morphine-conditioned place preference. Psychopharmacology (Berl) 181:90–100
Lu L, Liu D, Ceng X (2001) Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol 415:203–208
Lu L, Liu D, Ceng X, Ma L (2000) Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci 12:4398–4404
McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52:41–49
McQuiston AR, Saggau P (2003) Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol 90:1936–1948
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006) Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120:401–412
Milekic MH, Brown SD, Castellini C, Alberini CM (2006) Persistent disruption of an established morphine conditioned place preference. J Neurosci 26:3010–3020
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774–776
Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Morris RG, Schenk F, Tweedie F, Jarrard LE (1990) Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci 2:1016–1028
Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925
Mucha RF (1987) Is the motivational effect of opiate withdrawal reflected by common somatic indices of precipitated withdrawal? A place conditioning study in the rat. Brain Res 418:214–220
Neumaier JF, Chavkin C (1986) Opioid receptor activity in the dentate region of the rat hippocampus. NIDA Res Monogr 75:101–104
O’Keefe J (1983) Two spatial systems in the rat brain—implications for the neural basis of learning and memory. Prog Brain Res 58:453–464
Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ (2005) Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci 25:5553–5562
Phillips GD, Setzu E, Hitchcott PK (2003) Facilitation of appetitive Pavlovian conditioning by d-amphetamine in the shell, but not the core, of the nucleus accumbens. Behav Neurosci 117:675–684
Robinson TE, Berridge KC (1993) The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Roma PG, Riley AL (2005) Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav 82:163–169
Salem A, Hope W (1997) Effect of adenosine receptor agonists and antagonists on the expression of opiate withdrawal in rats. Pharmacol Biochem Behav 57:671–679
Schechter MD, Calcagnetti DJ (1993) Trends in place preference conditioning with a cross-indexed bibliography; 1957–1991. Neurosci Biobehav Rev 17:21–41
Schoenbaum G, Roesch MR, Stalnaker TA (2006) Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci 29:116–124
Segura-Torres P, Capdevila-Ortis L, Marti-Nicolovius M, Morgado-Bernal I (1988) Improvement of shuttle-box learning with pre- and post-trial intracranial self-stimulation in rats. Behav Brain Res 29:111–117
Segura-Torres P, Portell-Cortes I, Morgado-Bernal I (1991) Improvement of shuttle-box avoidance with post-training intracranial self-stimulation, in rats: a parametric study. Behav Brain Res 42:161–167
Self DW (2004) Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47(Suppl 1):242–255
Stewart J (2004) Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Nebr Symp Motiv 50:197–234
Taylor JR, Horger BA (1999) Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology 142:31–40
Taylor JR, Jentsch JD (2001) Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”). Biol Psychiatry 50:137–143
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA (2006) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A 103:2932–2937
Wang J, Fang Q, Liu Z, Lu L (2006) Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology 185:19–28
Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M (2002) Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol 88:399–406
Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M (2003) Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 170:80–88
White NM (1996) Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91:921–949 discussion 951–965)
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue- triggered “wanting” for sucrose reward. J Neurosci 21:7831–7840
Yang Y, Zheng X, Wang Y, Cao J, Dong Z, Cai J, Sui N, Xu L (2004) Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. J Neurosci 24:2412–2420
Yoganarasimha D, Shankaranarayana Rao BS, Raju TR, Meti BL (1998) Facilitation of acquisition and performance of operant and spatial learning tasks in self-stimulation experienced rats. Behav Neurosci 112:725–729
Zhai J, Wieland SJ, Sessler FM (1997) Chronic cocaine intoxication alters hippocampal sodium channel function. Neurosci Lett 229:121–124
Acknowledgments
This work was supported in part by the grants from Intramural Research Program of National Institute on Drug Abuse, NIH, USA, the 985 talent program of Peking University (nos. 985-2-046-121 and 985-2-027-39), the New Century Talent Scientist Grant of Ministry of Education, and the National Natural Science Foundation of China (nos. 30570576, 30670713, and 30611120528). We would like to thank Dr. Yavin Shaham for his help in preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, Hf., Zhang, ZY., Zhao, M. et al. Conditioned drug reward enhances subsequent spatial learning and memory in rats. Psychopharmacology 195, 193–201 (2007). https://doi.org/10.1007/s00213-007-0893-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0893-x