Abstract
Rationale
Common biological effects by all three conventional anti-bipolar drugs, the lithium ion (Li+), carbamazepine, and valproic acid, are important because identical effects may provide information about the pathophysiology of affective disorders. It has been reported that chronic treatment with either drug in vivo down-regulates the turnover of arachidonic acid in brain. This reaction is catalyzed by Ca2+-dependent phospholipase A2 (cPLA2), the expression of which was down-regulated by Li+ or carbamazepine but not by valproic acid; expression of two other PLA subtypes, iPLA2 and sPLA2 was unaffected. cPLA2 is amply expressed in astrocytes, and in the present study, effects of 1–4 weeks of treatment with clinically relevant concentrations of each of the three anti-bipolar drugs on cPLA2, iPLA2, and sPLA2 mRNA and protein expression were determined in primary cultures of mouse astrocytes by reverse transcription polymerase chain reaction (RT-PCR) and immunoblotting.
Results
Two or more weeks treatment with Li+ concentrations below 2 mM, carbamazepine or valproic acid up-regulated mRNA and protein expression of cPLA2, but had no effect on iPLA2 and sPLA2, showing enzyme specificity. The effect occurred more rapidly at higher than lower concentrations but also tended to end after 4 weeks at the higher concentrations. Two millimolar Li+ caused an initial increase of cPLA2 followed by a decrease after 3 and 4 weeks. Topiramate had no effect, indicating specificity for anti-bipolar drugs.
Conclusions
Both up- and down-regulation of cPLA2 gene expression are involved in the mechanisms of action of anti-bipolar drugs; astrocytes are a target for these drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium salts (Li+), valproic acid (VPA), and carbamazepine (CBZ) are the three classical anti-bipolar drugs, which all have to be administered for a couple of weeks before their therapeutic action becomes manifest. Li+ has been used as a mood stabilizer for more than 50 years (Schou 2001). The anticonvulsants valproate (VPA) and carbamazepine (CBZ) were originally developed as antiepileptic drugs and were later found to be effective in the treatment of bipolar disorder, but this does not apply to all anticonvulsants, as, for example, topiramate has no antimanic effect (Goodnick 2006). Li+, VPA, and CBZ have nothing in common in chemical structures, and only a few metabolic parameters in the brain are affected similarly by all of them. Shared effects by all three drugs are important because common mechanisms of action may provide information about the pathophysiology of bipolar disorder and facilitate drug development.
Most of the common effects by Li+, CBZ, and VPA have been observed in cultured astrocytes (Lubrich and van Calker 1999; Wolfson et al. 2000; Hertz et al. 2004), where they all affect uptake of myo-inositol, or in cultured neurons (Williams et al. 2002; Di Daniel et al. 2006), where they affect process dynamics of developing neurons. In intact brain, Rapoport and coworkers have established that after 6 weeks (Li+ and CBZ) or 30 days (VPA) of treatment of rats with doses leading to therapeutically relevant drug levels, each drug decreases the in vivo turnover of arachidonic acid by deacylation from glycerophospholipids followed by re-acylation (Chang et al. 1996, 2001; Bazinet et al. 2006); this effect is specific for arachidonic acid, as docosahexaenoic acid turnover was not affected in a similar manner (Rapoport and Bosetti 2002; Bazinet et al. 2005, 2006).
The enzyme that specifically hydrolyzes the acyl bond of arachidonic acid from the sn-2 position of glycerophospholipids in the cell membrane is Ca2+-dependent phospholipase A2 (cPLA2). Chronic treatment with Li+ for 6 weeks decreases the expression of cPLA2 in rat brain, whereas Li+ has no effect on two other types of PLA2, secretory PLA2 (sPLA2) and intracellular PLA2 (iPLA2) (Rintala et al. 1999; Weerasinghe et al. 2004; Basselin et al. 2005). The down-regulation was documented for the IVA subtype (85 kD) of cPLA2, also called cPLA2a, one of the six paralogs of cPLA2 (Ghosh et al. 2006), but other paralogs were not discussed. Similar results were found in rat chronically treated with CBZ (Ghelardoni et al. 2004), whereas chronic treatment with VPA did not influence cPLA2 in the brain in vivo (Chang et al. 2001).
Among the cells that express cPLA2 are astrocytes (Sun et al. 2005), a glial cell type that accounts for 20–30% of the total volume in brain cortex (Williams et al. 1980; Wolff and Chao 2004). Indeed, in the brain in vivo, the majority of cPLA2a in the gray matter is located in astrocytes (Stephenson et al. 2004; Lautens et al. 1998; Balboa et al. 2002), but no information is available about the cellular location of cPLA2b and cPLA2c in the brain. The activity of cPLA2a may be regulated by transmitters, as astrocytes display receptors for a large number of transmitters (Hansson and Rönnbäck 2004; Fiacco and McCarthy 2006), including serotonin and ATP, known to catalyze the formation of free arachidonic acid (AA) from glycerophospholipid in C6 glioma cells (Garcia and Kim 1997) and primary cultures of astrocytes (Xu et al. 2002). In the present study, we have investigated (1) the mRNA expression of cPLA2a, cPLA2b, and cPLA2c in astrocytes and neurons; (2) the effects of therapeutically relevant concentrations of Li+, VPA, and CBZ on gene and protein expression of cPLA2, sPLA2, and iPLA2 in primary cultures of mouse astrocytes (to investigate the potential role of astrocytes as a drug target and enzyme specificity of the drug effect), and (3) the effect of topiramate, an anticonvulsant which has no anti-bipolar effect, on gene and protein expression of cPLA2 (to study drug specificity).
Experimental methods
Chemicals for preparation of medium and most other chemicals, including VPA, CBZ and topiramate were purchased from Sigma (St. Louis, MO, USA). Lithium carbonate (Li2CO3) was obtained from Shanghai Hengxin Chemical Reagent (Shanghai, China). Santa Cruz Biotechnology (Santa Cruz, CA, USA) provided the first antibody raised against cPLA2 recognizing cPLA2a (85 kDa), cPLA2b (114 kDa), and cPLA2c (61 kDa), and Sigma (St. Louis, MO, USA) supplied the first antibody, raised against β-actin (42 kDa). The second antibody goat anti-mouse IgG HRP conjugate was purchased from Promega (Madison, WI, USA).
Primary cultures of astrocytes were prepared as previously described (Hertz et al. 1978, 1998) with minor modifications. The neopallia of the cerebral hemispheres were aseptically isolated, vortexed to dissociate the tissue, filtered through nylon meshes with pore sizes of 80 and subsequently 10 μm, diluted in culture medium, and planted in Falcon Primaria culture dishes. The culture medium was a Dulbecco’s medium with 7.5 mM glucose, initially containing 20% horse serum, and the cultures were incubated at 37°C in a humidified atmosphere of CO2/air (5:95%). The culturing medium was exchanged with fresh medium of similar composition on day 3, and subsequently, every 3–4 days. From day 3, the serum concentration was reduced to 10%, and after the age of 2 weeks, 0.25 mM dibutyryl cyclic AMP (dBcAMP) was included in the medium. Such cultures have been used in our laboratories for more than 25 years (Hertz et al. 1978), and they are highly enriched in astrocytes [>95% purity of glial fibrillary protein (GFAP)- and glutamine synthetase-expressing astrocytes (Hertz et al. 1985)]. Addition of dBcAMP leads to a morphological and functional differentiation as evidenced by the extension of cell processes, increases in several metabolic activities, and expression of voltage sensitive L-channels for calcium (Ca2+) (Hertz et al. 1989; Meier et al. 1991; Zhao et al. 1996). All four drugs were dissolved in phosphate-buffered saline (PBS). Lithium carbonate at final concentrations of 1.0, 0.5, or 0.25 mM (corresponding to Li+ concentrations of 2, 1, or 0.5 mM), VPA at final concentrations of 1 mM or 100 μM, CBZ at final concentrations of 50 or 25 μM, topiramate at concentration of 100 μM, or PBS (control) was added to the culture medium after 2 weeks of culturing (i.e., at the time the cultures had reached confluency) and was present during continued culturing for another 1, 2, 3, or 4 weeks.
Cerebellar granule neurons were cultured as previously described (Peng et al. 1991) with minor modifications. Briefly, 7-day-old mouse pups were rapidly decapitated and the brains taken out. The cerebella were aseptically separated from the remainder of the brain, and after removal of the meninges, the tissue was cut into cubes of ∼0.4 mm side dimensions, exposed to trypsin in a calcium–magnesium-free salt solution, reintroduced into tissue culture medium, passed through nylon sieves, and seeded into polylysine-coated standard 35-mm tissue culture dishes (Wuzhou Medical Plastic Factory, Zhejiang, China), using one cerebellum per culture dish. The cultures were grown in Dulbecco’s medium in which the glucose concentration was increased to 30 mM (to eliminate feeding during the culturing) and the K+ concentration to 24.5 mM, the glutamine concentration was decreased to 0.8 mM and 7% horse serum was added. After 2 days, cytosine arabinoside was added to the medium to a final concentration of 40 μM to curtail the number of astrocytes that develop in the cultures. The cells were used at the age of 7–8 days.
For determination of mRNA expression of different subtypes of PLA2, a cell suspension was prepared by discarding the culturing medium, adding Trizol to cultures on ice, and scraping the cells off the culture dish. The RNA pellet was precipitated with isopropyl alcohol, washed with 70% ethyl alcohol, and dissolved in 10 μl sterile, distilled water, and an aliquot was used for determination of the amount of RNA (Kong et al. 2002). One microgram of RNA extract was used for RT, which was initiated by a 5-min incubation at 65°C of RNA extract with Random Hexamer at a final concentration of 12.5 ng/μl and deoxyribonucleotide triphosphates (dNTPs) (TaKaRa Biotechnology, Dalian, China) at a final concentration of 0.5 mM. The mixture was rapidly chilled on ice and briefly spun, and 4 μl 5× First-Strand Buffer, 2 μl 0.1 M dithiotreitol and 1 μl RNaseOUT Recombinant RNase Inhibitor (40 U/μl) were added. After the mixture had been incubated at 42°C for 2 min, 1 μl (200 U) of Superscript II (Gibco) was added, and the incubation at 42°C continued for another 50 min. Subsequently, the reaction was inactivated by heating to 70°C for 15 min, and the mixture was chilled and briefly centrifuged.
Polymerase chain reaction (PCR) amplification was performed in a Robocycler thermocycler with 0.2 μM of sense or antisense and 0.375 U of Taq-polymerase (TaKaRa Biotechnology, Dalian, China) for cPLA2a, cPLA2b, cPLA2c, iPLA2, sPLA2, and TATA-binding protein (TBP), used as a housekeeping gene (Table 1). Brain and liver tissues were used as positive controls of three types of cPLA2. cDNA 2.5, 5, and 10 μg, and 25–45 cycles were tested for each pair of primers. The amount of cDNA used was 5 μg. Thirty cycles were used for iPLA2 and sPLA2, 35 cycles for cPLA2b and cPLA2c, and 40 cycles for cPLA2a and TBP. Initially, the template was denatured by heating to 94°C for 2 min, followed by 2-min amplification cycles, each consisting of two 45-s periods and one 60-s period, the first at 94°C, the second at 59°C for cPLA2a, 56°C for cPLA2b, cPLA2c, and iPLA2 or 55°C for sPLA2 and TBP, and the third at 72°C. The final step was extension at 72°C for 10 min. The PCR products were separated by 1% agarose gel electrophoresis, and captured by Fluorchem 5500 (Alpha Innotech, San Leandro, CA, USA).
For determination of protein expression of cPLA2, the cells were washed with ice-cold phosphate-buffered saline (PBS) containing 7.5 mM glucose, scraped off the dishes, and harvested in 0.5 ml of ice-cold buffer A [0.25 M sucrose, 10 mM HEPES, the phosphatase inhibitors alpha-mercaptoethanol (10 mM) and phenylmethyl sulfonyl fluoride (1 mM), and 1 mM sodium orthovanadate, pH 7.4] and homogenized to make a whole cell lysate. The protein content was determined in the homogenates by the Bradford method (Bradford 1976), using bovine serum albumin as the standard. Samples containing 50 μg protein were applied on slab gels of 10% polyacrylamide. After transfer to nitrocellulose membranes, the samples were blocked by 5% skimmed milk powder in TBS-T (30 mM Tris–HCl, 125 mM NaCl, 0.1% Tween 20) for 1 h. The nitrocellulose membranes were incubated with the first antibody, specific to either cPLA2 at 1 × 1,000 dilution or β-actin (used for housekeeping) at 1 × 4,000 dilution for 2 h at room temperature. After washing, specific binding was detected by goat-anti-mouse horseradish peroxidase-conjugated secondary antibody at 1 × 1,000 dilution. Staining was visualized by ECL detection reagents (Amersham Biosciences, Buckinghamshire, UK), followed by exposure to film (FuJi Photo Film, Tokyo, Japan). The results were collected by Fluorchem imaging system. Band density was measured with Window AlphaEaseTM FC 32-bit software.
Ratios between cPLA2 mRNA or protein and the respective housekeeping gene or protein (TBP and β-actin) were determined and averaged. All drug-treated cultures were compared to controls from the same batch at the same age in culture. The differences between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) multiple comparison test for unequal replications. The level of significance was set at p < 0.05.
Results
Expression of cPLA2
Figure 1 shows that the expression of mRNA for cPLA2a in astrocytes equals that in brain tissue, whereas less cPLA2a is present in neurons. Much smaller amounts of mRNA for cPLA2b was found in brain or the cultured cells, compared to that in liver, and cPLA2c was expressed in brain and in neurons, but not in astrocytes. A similar result was found in Western blot that predominantly cPLA2a, an 85-kDa band was recognized by the antibody against cPLA2 in astrocytes (results not presented). In the following, only drug effects on this paralog will be discussed, but this does not exclude that other cPLA2 paralogs could also be affected by treatment with anti-bipolar drugs.
Expression of cPLA2a, cPLA2b, and cPLA2c mRNA in primary cultures of astrocytes, with TATA-binding protein (TBP) as a housekeeping gene. The first lane, to the far left, represents a DNA ladder, the next 1 PCR product of cPLA2a from brain, 2 from astrocytes, 3 from cerebellar granule neurons, 4 PCR product of cPLA2b from brain, 5 from astrocytes, 6 from cerebellar granule neurons, 7 from liver, 8 PCR product of cPLA2c from brain, 9 from astrocytes, 10 from cerebellar granule neurons, 11 PCR product of TBP from brain, 12 from astrocytes, 13 from cerebellar granule neurons, and the last, 14, from liver. One μg of RNA extract was used in RT for all the samples. The size of PCR product of cPLA2a is 430 bp, of cPLA2b 273 bp, of cPLA2c 329 bp, and of TBP 236 bp
Effects of Li+
As shown in Fig. 2a, 2 weeks of treatment with 1 mM lithium carbonate (2 mM Li+) induced an up-regulation of mRNA expression of cPLA2a in astrocytes, whereas 1 week of treatment had no effect and treatment for 3 or 4 weeks led to a down-regulation. The up-regulation was reflected in the averaged densitometrically determined ratios between expression of cPLA2a and of TBP by a statistically significant increase from the control after 2 weeks follow by a decrease after 3 and 4 weeks (Fig. 2b). Protein expression of cPLA2a, determined by an 85-kDa band in Western blotting with β-actin as housekeeping protein (Fig. 3a), was also unaffected by 1 week of treatment with 1 mM lithium carbonate, significantly increased after 2 weeks of treatment, and significantly reduced after 3 and 4 weeks of treatment (Fig. 3b).
mRNA expression measured by RT-PCR of cPLA2a in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 1 mM lithium carbonate (2 mM lithium). a A representative experiment showing mRNAs for cPLA2a in the upper row and for TBP, as a house-keeping gene, in the lower row, in control cultures and the corresponding results in lithium-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and TBP (five samples from five different batches of cultures) in control cultures and cultures treated with 1 mM lithium carbonate. P < 0.05: * vs control cultures from the same batch and treatment period
Protein expression measured by immunoblotting of cPLA2a (85 kDa) in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 1 mM lithium carbonate (2 mM lithium). a A representative experiment showing proteins for cPLA2a in the upper row and for β-actin, as a house-keeping protein, in the lower row, in control cultures and the corresponding results in lithium-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and β-actin (four samples from four different batches of cultures) in control cultures, cultures treated with 1 mM lithium carbonate. P < 0.05: * vs control cultures from the same batch and treatment period
In contrast to the biphasic change of cPLA2a expression during exposure to 1 mM lithium carbonate, lithium carbonate at concentrations of 0.5 and 0.25 mM (i.e., 1.0 and 0.5 mM Li+) caused only an up-regulation of mRNA expression of cPLA2a (Fig. 4a). At both concentrations, the mRNA levels were increased after 2, 3, and 4 weeks of exposure to Li+, but after 4 weeks of treatment, the response was smaller with 0.5 mM lithium carbonate than with 0.25 mM (Fig. 4b). The cPLA2a protein was also increased but the response was slower than the mRNA increase, as it was not seen until after 3 and 4 weeks (Fig. 5).
mRNA expression measured by RT-PCR of cPLA2a in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 0.5 or 0.25 μM lithium carbonate (1 and 0.5 mM lithium). a A representative experiment showing mRNAs for cPLA2a in the upper row and for TBP, as a house-keeping gene, in the lower row, in control cultures and the corresponding results in lithium-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and TBP (four samples from four different batches of cultures) in control cultures, cultures treated with 0.5 mM lithium carbonate, and cultures treated with 0.25 mM lithium carbonate. P < 0.05: * vs control cultures from the same treatment period; ** vs control cultures and cultures treated with 0.5 mM lithium carbonate from the same batch and treatment period
Protein expression measured by immunoblotting of cPLA2a (85 kDa) in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 0.5 or 0.25 mM lithium carbonate (1 and 0.5 mM lithium). a A representative experiment showing proteins for cPLA2a in the upper row and for β-actin, as a house-keeping protein, in the lower row, in control cultures and the corresponding results in lithium-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and β-actin (four samples from four different batches of cultures) in control cultures, cultures treated with 0.5 mM lithium carbonate, and cultures treated with 0.25 mM lithium carbonate. P < 0.05: * vs. control cultures from the same batch and treatment period
Effects of CBZ
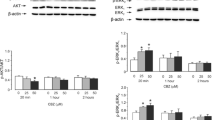
The effects of 25 or 50 μM CBZ on mRNA expression of cPLA2a were almost indistinguishable from those of 0.25 and 0.5 mM lithium carbonate. Two weeks of drug treatment induced a significant up-regulation of the expression, which persisted at 3 weeks (Fig. 6). At 50 μM CBZ, there was no significant difference between treated cultures and control cultures after 4 weeks, whereas the mRNA expression of cPLA2a remained increased during exposure to 25 μM CBZ. A similar difference was observed in the expression of the cPLA2a protein after 4 weeks, but after 2 weeks, an increase was only observed at 50 μM CBZ (Fig. 7).
mRNA expression measured by RT-PCR of cPLA2a in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 50 or 25 μM CBZ. a A representative experiment showing mRNAs for cPLA2a in the upper row and for TBP, as a house-keeping gene, in the lower row, in control cultures and the corresponding results in CBZ-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means±SEM of scanned ratios between cPLA2a and TBP (four samples from four different batches of cultures) in control cultures, cultures treated with 50 μM CBZ, and cultures treated with 25 μM CBZ. P < 0.05: * vs. control cultures from the same batch and treatment period
Protein expression measured by immunoblotting of cPLA2a (85 kDa) in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 50 μM or 25 μM CBZ. a A representative experiment showing proteins for cPLA2a in the upper row and for β-actin, as a house-keeping protein, in the lower row, in control cultures and the corresponding results in CBZ-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and β-actin (four samples from four different batches of cultures) in control cultures, cultures treated with 50 μM CBZ, and cultures treated with 25 μM CBZ. P < 0.05: * vs control cultures from the same batch and treatment period
Effects of VPA
The results with 1 mM and 100 μM VPA were similar to those with CBZ. Again, there was no effect after 1 week of treatment, but both concentrations caused an up-regulation of cPLA2a mRNA after 2 and 3 weeks treatment, whereas the up-regulation after 4 weeks persisted only at 100 μM (Fig. 8). One millimolar VPA also up-regulated cPLA2a protein after 2 and 3 weeks of treatment but not after treatment for 4 weeks, whereas 100 μM VPA caused a small, but significant up-regulation after 3 weeks and a larger up-regulation after 4 weeks of treatment (Fig. 9).
mRNA expression measured by RT-PCR of cPLA2a in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 1 mM or 100 μM VPA. a A representative experiment showing mRNAs for cPLA2a in the upper row and for TBP, as a house-keeping gene, in the lower row, in control cultures and the corresponding results in VPA-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and TBP (four samples from four different batches of cultures) in control cultures, cultures treated with 1 mM VPA, and cultures treated with 100 μM VPA. P < 0.05: * vs. control cultures from the same treatment period; ** vs control cultures and cultures treated with 100 μM VPA from the same batch and treatment period
Protein expression measured by immunoblotting of cPLA2a (85 kDa) in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 1 mM or 100 μM VPA. a A representative experiment showing proteins for cPLA2a in the upper row and for β-actin, as a house-keeping protein, in the lower row, in control cultures and the corresponding results in VPA-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and β-actin (four samples from four different batches of cultures) in control cultures, cultures treated with 1 mM VPA, and cultures treated with 100 μM VPA. P < 0.05: * vs control cultures from the same batch and treatment period; ** vs control cultures and cultures treated with 1 mM VPA from the same batch and treatment period
Lack of effects by topiramate
Treatment with 100 μM topiramate, a drug that is ineffective as an anti-bipolar medication had no effect on either cPLA2a mRNA or cPLA2a protein during up to 4 weeks of treatment (Figs. 10 and 11).
mRNA expression measured by RT-PCR of cPLA2a in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 100 μM topiramate. a A representative experiment showing mRNAs for cPLA2a in the upper row and for TBP, as a house-keeping gene, in the lower row, in control cultures and the corresponding results in topiramate-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and TBP (four samples from four different batches of cultures) in control cultures and cultures treated with 100 μM topiramate
Protein expression measured by immunoblotting of cPLA2a (85 kDa) in primary cultures of mouse astrocytes treated for 1 to 4 weeks with 100 μM topiramate. a A representative experiment showing proteins for cPLA2a in the upper row and for β-actin, as a house-keeping protein, in the lower row, in control cultures and the corresponding results in topiramate-treated cultures after 1, 2, 3, or 4 weeks of treatment. b All results are means ± SEM of scanned ratios between cPLA2a and β-actin (four samples from four different batches of cultures) in control cultures and cultures treated with 100 μM topiramate
Lack of effects by anti-bipolar drugs on iPLA2 and sPLA2
Li+, AVP, and CBZ at concentrations affecting cPLA2a mRNA had no effect on the expression of either iPLA2 (Table 2) or sPLA2 (Table 3). Nor did topiramate have any effect (Tables 2 and 3).
Discussion
Drug effects
In agreement with observations in the brain in vivo (Rintala et al. 1999; Weerasinghe et al. 2004), prolonged chronic treatment with 2 mM Li+ induced a down-regulation of cPLA2a in primary cultures of astrocytes. However, after 2 weeks of treatment, there was an up-regulation, a response not observed in the brain in vivo. A change in the direction of a functional response in astrocytes during prolonged treatment with a psychoactive drug has precedence, as we have previously reported that long-term treatment (2–3 weeks) of astrocyte cultures with fluoxetine, which has 5-HT2B receptor agonist activity, causes an up-regulation of the glycogenolytic response to renewed administration of fluoxetine, whereas short-term treatment (1 week) abolishes the fluoxetine-induced hydrolysis of glycogen (Kong et al. 2002).
Lower concentrations of lithium carbonate led within the period studied only to an up-regulation, but it is possible that longer exposure times might have resulted in a down-regulation, similar to that reported in the rat brain by Rintala et al. (1999), who used a treatment period of 6 weeks. Unfortunately, these authors did not report results after shorter exposure to Li+. The concept that the length of the treatment period may affect the response is supported by the findings with carbamazepine and valproic acid where the effect of higher concentrations (50 μM; 1 mM) peaked after 2–3 weeks of treatment and was abolished or greatly reduced after 4 weeks, whereas that to the lower concentration (25 μM; 100 μM) was pronounced after 4 weeks, but also developed more slowly. Thus, with all three drugs, high concentrations resulted in a rapid up-regulation, which within 4 weeks was abolished, greatly reduced, or even transformed to a down-regulation, whereas lower concentrations evoked a slower response and no reduction of the stimulation after 4 weeks of treatment. In contrast, topiramate, which has no anti-bipolar effect had no effect on cPLA2a expression, and mRNA expression of iPLA2 and sPLA2 was unaltered by Li+, VPA, and CBZ. Thus, the observed effects (up-regulation and down-regulation) show both enzyme specificity and drug specificity, and it was in a systematic fashion dependent upon drug concentration and the length of the treatment period. The enzyme specificity is identical to that reported in the brain in vivo after administration of either Li+ or carbamazepine (Rintala et al. 1999; Weerasinghe et al. 2004; Ghelardoni et al. 2004; Basselin et al. 2005), but no published data seem to be available about the effect of topiramate on cPLA2a expression in intact brain. Also, in contrast to the present observations, VPA was found not to affect cPLA2 expression in the brain in vivo (after 30 days of treatment), although it had the same effect on turnover of arachidonic acid as the other two anti-bipolar drugs (Chang et al. 2001).
The concentrations used in the present study are probably pharmacologically relevant. Thus, Soares et al. (2001) found that Li+ brain concentrations varied from 0.23 to 0.55 mEq/l at a plasma Li+ concentration of 0.7 mM, a concentration within the lower range of the therapeutically relevant level (Sproule 2002), and Moore et al. (2002) reported a brain/plasma ratio of 0.9. Accordingly, by studying the range 0.5–2.0 mM Li+ the pharmacologically relevant concentration range has probably been included (Sproule 2002).
Similar considerations apply to the concentrations used of VPA and CBZ. A therapeutically relevant plasma concentration of VPA is 0.6 mM, but Vajda et al. (1981) observed that the level of VPA in CSF was only 7.6–25.0% of its plasma concentration (50–100 μg/ml), i.e., 30–150 μM, and a brain–serum ratio of 15% was also reported by Wieser (1994). Again, the pharmacologically relevant concentration range is likely to have been covered by using a low concentration (100 μM) and a high concentration (1 mM), the results of which only varied in the time course. The concentration ratio between brain and plasma of CBZ has been determined as 1.4–1.6 in epileptic patients (Friis et al. 1978), but the protein binding of this drug makes estimates of the free concentration in the incubation medium (which contains 10% serum) and cells uncertain. The higher CBZ concentration used in the current study is, however, close to the mean plasma CBZ concentration of 53.6 ± 5.2 μmol/l in the study by Ghelardoni et al. (2004) and to the highest concentration in the therapeutic range reported in bipolar patients, 17–51 μmol/l (Petit et al. 1991; Bialer et al. 1998).
Relevance of astrocytes
In agreement with previous observations (Stephenson et al. 2004; Lautens et al. 1998; Balboa et al. 2002), cPLA2a was expressed to a large degree in astrocytes, whereas less expression was observed in neurons. In contrast, cPLA2c was mainly expressed in neurons. In the present context, it is of interest that cPLA2a is the only PLA2 that has specificity for phospholipid substrates containing arachidonic acid (Ghosh et al. 2006). Arachidonic acid interferes in a complex manner with the free cytosolic concentration of calcium ions ([Ca2+]i) in astrocytes (Sergeeva et al. 2003; Yang et al. 2005; Alloisio et al. 2006). This is important because astrocytic [Ca2+]i has signaling functions both within individual cells, during propagation of Ca2+ waves across the astrocytic syncytium, and in interactions between astrocytes and neurons (Cornell-Bell et al. 2004; Haas et al. 2006; Scemes and Giaume 2006; Fiacco and McCarthy 2006). Moreover, the released arachidonic acid can be further oxidized to eicosanoids, converted to endocannabinoids or re-acylated in the membrane, a process that in the past has been quantitatively greatly underestimated (Purdon et al. 2002). Among the eicosanoids, PGE2 may be of special interest, as it can be formed in astrocytes (Hewett 1999), and an enzyme catalyzing its terminal synthesis is decreased in the frontal and temporal cortex of bipolar patients, with a trend towards normalization by medication (Maida et al. 2006).
References
Alloisio S, Aiello R, Ferroni S, Nobile M (2006) Potentiation of native and recombinant P2X7-mediated calcium signaling by arachidonic acid in cultured cortical astrocytes and human embryonic kidney 293 cells. Mol Pharmacol 69:1975–1983
Balboa MA, Varela-Nieto I, Lucas KK, Dennis EA (2002) Expression and function of phospholipase A2 in brain. FEBS Lett 531:12–17
Basselin M, Chang L, Seemann R, Bell JM, Rapoport SI (2005) Chronic lithium administration to rats selectively modifies 5-HT2A/2C receptor-mediated brain signaling via arachidonic acid. Neuropsychopharmacol 30:461–472
Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ (2005) Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacol (Berl) 182:180–185
Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ (2006) Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry 59:401–407
Bialer M, Levy RH, Perucca E (1998) Does carbamazepine have a narrow therapeutic plasma concentration range? Ther Drug Monit 20:56–59
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI (1996) Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett 220:171–174
Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI (2001) Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem 77:796–803
Cornell-Bell AH, Jung P, Trinkaus-Randall V (2004) Decoding calcium wave signaling. In Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 661–687
Di Daniel E, Cheng L, Maycox PR, Mudge AW (2006) The common inositol-reversible effect of mood stabilizers on neurons does not involve GSK3 inhibition, myo-inositol-1-phosphate synthase or the sodium-dependent myo-inositol transporters. Mol Cell Neurosci 32:27–36
El Marjou M, Montalescot V, Buzyn A, Geny B (2000) Modifications in phospholipase D activity and isoform expression occur upon maturation and differentiation in vivo and in vitro in human myeloid cells. Leukemia 14:2118–2127
Fiacco TA, McCarthy KD (2006) Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia 54:676–690
Friis ML, Christiansen J, Hvidberg EF (1978) Brain concentrations of carbamazepine and carbamazepine-10,11-epoxide in epileptic patients. Eur J Clin Pharmacol 14:47–51
Garcia MC, Kim HY (1997) Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2Areceptor in rat C6 glioma cells. Brain Res 768:43–48
Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F (2004) Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry 56:248–254
Ghosh M, Tucker DE, Burchett SA, Leslie CC (2006) Properties of the Group IV phospholipase A2 family. Prog Lipid Res 45:487–510
Goodnick PJ (2006) Anticonvulsants in the treatment of bipolar mania. Expert Opin Pharmacother 7:401–410
Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H (2006) Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16:237–246
Hansson E, Rönnbäck L (2004) Astrocytic receptors and second messenger systems. In: Hertz L (ed) Non-Neuronal Cells of the Nervous System: Function and Dysfunction. Elsevier, Amsterdam, pp 475–501
Hertz L, Schousboe A, Boechler N, Mukerji S, Fedoroff S (1978) Kinetic characteristics of the glutamate uptake into normal astrocytes in cultures. Neurochem Res 3:1–14
Hertz L, Juurlink BHJ, Szuchet S (1985) Cell cultures. In: A Lajtha A (ed) Handbook of neurochemistry (2nd ed, Vol. 8) Plenum, New York, pp 603–661
Hertz L, Bender AS, Woodbury D, White SA (1989) Potassium induced calcium uptake in astrocytes and its potent inhibition by a calcium channel blocker. J Neurosci Res 22:209–215
Hertz L, Peng L, Lai JC (1998) Functional studies in cultured astrocytes. Methods 16:293–310
Hertz L, Chen Y, Bersudsky Y, Wolfson M (2004) Shared effects of all three conventional anti-bipolar drugs on the phosphoinositide system in astrocytes. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 1033–1048
Hewett SJ (1999) Interferon-gamma reduces cyclooxygenase-2-mediated prostaglandin E2 production from primary mouse astrocytes independent of nitric oxide formation. J Neuroimmunol 94:134–143
Ivanov A, Pero RS, Scheck A, Romanousky AA (2002) Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol 283:R1104–R1117
Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA (1995) A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem 22:22378–22385
Kong EKC, Peng L, Chen Y, Yu ACH, Hertz L (2002) Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem Int 27:113–120
Lautens LL, Chiou XG, Sharp JD, Young WS 3rd, Sprague DL, Ross LS, Felder CC (1998) Cytosolic phospholipase A2 (cPLA2) distribution in murine brain and functional studies indicate that cPLA2 does not participate in muscarinic receptor-mediated signaling in neurons. Brain Res 809:18–30
Lindbom J, Ljungman AG, Lindahl M, Tagesson C (2001) Expression of members of the phospholipase A2 family of enzymes in human nasal mucosa. Eur Respir J 18:130–138
Lubrich B, van Calker D (1999) Inhibition of the high-affinity myo-inositol uptake: a common mechanism of action of anti-bipolar drugs? Neuropsychopharmacol 21:519–529
Maida ME, Hurley SD, Daeschner JA, Moore AH, O’Banion M (2006) Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res 1103:164–172
Meier E, Hertz L, Schousboe A (1991) Neurotransmitters as developmental signals. Neurochem Int 19:1–15
Moore CM, Demopulos CM, Henry ME, Steingard RJ, Zamvil L, Katic A, Breeze JL, Moore JC, Cohen BM, Renshaw PF (2002) Brain-to-serum lithium ratio and age: an in vivo magnetic resonance spectroscopy study. Am J Psychiatry 159:1240–1242
Peng L, Juurlink BHJ, Hertz L (1991) Differences in transmitter release, morphology, and ischemia-induced cell injury between cerebellar granule cell cultures developing in the presence and in the absence of a depolarizing potassium concentration. Dev Brain Res 63:1–12
Petit P, Lonjon R, Cociglio M, Sluzewska A, Blayac JP, Hue B, Alric R, Pouget R (1991) Carbamazepine and its 10,11-epoxide metabolite in acute mania: clinical and pharmacokinetic correlates. Eur J Clin Pharmacol 41:541–546
Purdon AD, Rosenberger TA, Shetty HU, Rapoport SI (2002) Energy consumption by phospholipid metabolism in mammalian brain. Neurochem Res 27:1641–1647
Rapoport SI, Bosetti F (2002) Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry 59:592–596
Rintala J, Seemann R, Chandrasekaran K, Rosenberger TA, Chang L, Contreras MA, Rapoport SI, Chang MC (1999) 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport 10:3887–3890
Scemes E, Giaume C (2006) Astrocyte calcium waves: what they are and what they do. Glia 54:716–725
Schou M (2001) Lithium treatment at 52. J Affect Disord 67:21–32
Sergeeva M, Strokin M, Wang H, Ubl JJ, Reiser G (2003) Arachidonic acid in astrocytes blocks Ca(2+) oscillations by inhibiting store-operated Ca(2+) entry, and causes delayed Ca(2+) influx. Cell Calcium 33:283–292
Soares JC, Boada F, Spencer S, Mallinger AG, Dippold CS, Wells KF, Frank E, Keshavan MS, Gershon S, Kupfer DJ (2001) Brain lithium concentrations in bipolar disorder patients: preliminary (7)Li magnetic resonance studies at 3 T. Biol Psychiatry 49:437–443
Sproule B (2002) Lithium in bipolar disorder: can drug concentrations predict therapeutic effect? Clin Pharmacokinet 41:639–660
Stephenson DT, Manetta JV, White DL, Chiou XG, Cox L, Gitter B, May PC, Sharp JD, Kramer RM, Clemens JA (2004) Phospholipase A2 in the central nervous system: implication for neurodegeneration diseases. J Lipid Res 46:205–213
Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A, Sun, AY, Weisman GA (2005) Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol 31:27–41
Vajda FJ, Donnan GA, Phillips J, Bladin PF (1981) Human brain, plasma, and cerebrospinal fluid concentration of sodium valproate after 72 h of therapy. Neurology 31:486–487
Weerasinghe GR, Rapoport SI, Bosetti F (2004) The effect of chronic lithium on arachidonic acid release and metabolism in rat brain does not involve secretory phospholipase A2 or lipoxygenase/cytochrome P450 pathways. Brain Res Bull 63:485–489
Wieser HG (1994) Comparison of valproate level in human plasma, cerebrospinal fluid and brain tissue following administration of various preparations of valproate and valpromide. Schweiz Rundsch Med Prax 83:1111–1116
Williams V, Grossman RG, Edmunds SM (1980) Volume and surface area estimates of astrocytes in the sensorimotor cortex of the cat. Neurosci 5:115–1151
Williams RS, Cheng L, Mudge AW, Harwood AJ (2002) A common mechanism of action for three mood-stabilizing drugs. Nature 417:292–295
Wolff J, Chao TI (2004) Cytoarchitectonics of non-neuronal cells in the central nervous system. In Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 1–52
Wolfson M, Bersudsky Y, Zinger E, Simkin M, Belmaker RH, Hertz L (2000) Chronic treatment of human astrocytoma cells with lithium, carbamazepine or valproic acid decreases inositol uptake at high inositol concentrations but increases it at low inositol concentrations. Brain Res 855:158–161
Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, Sun GY (2002) Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachidonic acid release in primary murine astrocytes. J Neurochem 83:259–270
Yang KT, Chen WP, Chang WL, Su MJ, Tsai KL (2005) Arachidonic acid inhibits capacitative Ca2+ entry and activates non-capacitative Ca2+ entry in cultured astrocytes. Biochem Biophys Res Commun 331:603–613
Zhao Z, Hertz L, Code WE (1996) Effects of benzodiazepines on potassium-induced increase in free cytosolic calcium concentration in astrocytes: interactions with nifedipine and the peripheral-type benzodiazepine antagonist PK 11195. Can J Physiol Pharmacol 74:273–277
Acknowledgements
This study was supported by Grant No. 30572180 and No. 30370451 from the National Natural Science Foundation of China. We thank Mrs. Xiaolin Yang for her valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Gu, L., Zhang, H. et al. Up-regulation of cPLA2 gene expression in astrocytes by all three conventional anti-bipolar drugs is drug-specific and enzyme-specific. Psychopharmacology 194, 333–345 (2007). https://doi.org/10.1007/s00213-007-0853-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0853-5