Abstract
Background
Cognitive deficits in schizophrenia are severe and do not respond well to available treatments. The development and validation of animal models of cognitive deficits characterizing schizophrenia are crucial for clarifying the underlying neuropathology and discovery of improved treatments for such deficits.
Materials and methods
We investigated whether single and repeated administrations of the psychotomimetic phencyclidine (PCP) disrupt performance in the five-choice serial reaction time task (5-CSRTT), a test of attention and impulsivity. We also examined whether PCP-induced disruptions in this task are attenuated by atypical antipsychotic medications.
Results
A single injection of PCP (1.5–3 mg/kg, s.c., 30-min pre-injection time) had nonspecific response-depressing effects. Repeated PCP administration (2 mg/kg for two consecutive days followed by five consecutive days, s.c., 30-min pre-injection time) resulted in decreased accuracy, increased premature and timeout responding, and increased response latencies. The atypical antipsychotic medications clozapine, risperidone, quetiapine, and olanzapine and the typical antipsychotic medication haloperidol did not disrupt 5-CSRTT performance under baseline conditions except at high doses. The response depression induced by a single PCP administration was exacerbated by acute clozapine or risperidone and was unaffected by chronic clozapine. Importantly, chronic clozapine partially attenuated the performance disruptions induced by repeated PCP administration, significantly reducing both the accuracy impairment and the increase in premature responding.
Conclusions
Disruptions in 5-CSRTT performance induced by repeated PCP administration are prevented by chronic clozapine treatment and may constitute a useful animal model of some cognitive symptoms of schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is associated with substantial cognitive impairments (Nuechterlein and Dawson 1984; Goldberg et al. 1988; Morice 1990; Nelson et al. 1990; Tamlyn et al. 1992; Paulus et al. 1996; Kuperberg and Heckers 2000) that constitute core aspects of the disorder (Elvevag and Goldberg 2000). These cognitive deficits are highly correlated with functional impairment and long-term disability (McGurk and Meltzer 2000; Sharma and Antonova 2003; Green et al. 2004). The newer atypical antipsychotic agents show some promise for ameliorating cognitive dysfunction (Meltzer and McGurk 1999; Bilder et al. 2002; Bender et al. 2005), but produce, at best, only partial recovery that falls well short of restoring normal functioning (Sharma and Antonova 2003; Keefe et al. 2004), while typical antipsychotic medications can worsen these deficits (Bilder et al. 1992; Mortimer 1997). Cognitive schizophrenia symptoms affect a wide range of cognitive domains and include attentional deficits (Nuechterlein and Dawson 1984; Laurent et al. 1999), cognitive impulsivity characterized by disinhibition of inappropriate responding (Kiehl et al. 2000; Weisbrod et al. 2000; Wykes et al. 2000; Badcock et al. 2002; Chan et al. 2006), cognitive inflexibility (Goldberg et al. 1988; Morice 1990), and decreased processing speed (Nelson et al. 1990). The development and validation of translational animal models of these deficits are crucial for the investigation of the neurobiological bases of cognitive schizophrenia symptoms and the search for improved treatments.
A promising approach to the development of such animal models is the use of phencyclidine (PCP), a dissociative anesthetic that noncompetitively blocks N-methyl-d-aspartate (NMDA) glutamate receptors. When administered to humans, acute PCP intoxication resembles a schizophrenia-like state (Luby et al. 1959; Bakker and Amini 1961; Allen and Young 1978; Castellani et al. 1982; Javitt 1987; Steinpreis 1996). Importantly, PCP, as well as its congeners MK-801 and ketamine, also disrupts cognitive function in both humans and animals (Handelmann et al. 1987; Hudzik and Wenger 1993; Krystal et al. 1994; Verma and Moghaddam 1996; Jentsch and Roth 1999; Stefani and Moghaddam 2005), producing deficits paralleling those present in schizophrenia (Pradhan 1984). Therefore, PCP may be a useful agent to induce schizophrenia-like cognitive deficits in animal models. A few studies have demonstrated that PCP-induced cognitive impairment may be attenuated by atypical antipsychotic medications (Jentsch et al. 1997a; Schroeder et al. 2000; Idris et al. 2005; Abdul-Monim et al. 2006). Intriguingly, in one of these studies, typical antipsychotic agents failed to ameliorate PCP-induced cognitive deficits (Abdul-Monim et al. 2006), mirroring the lack of responsiveness of cognitive schizophrenia symptoms to typical antipsychotic compounds. However, such studies are still few in number, and little is known about the potential of PCP to produce deficits that are sensitive to partial reversal with atypical antipsychotic agents (a situation that would mimic the clinical reality) in a number of important cognitive domains impaired in schizophrenia, particularly in animal models of attentional function.
The 5-CSRTT used in the present study is a well-established test of divided and sustained attention (Robbins 2002) that allows for the simultaneous examination of multiple aspects of cognition. Correct detection of a brief visual stimulus across five spatial locations provides a measure of attentional performance. Premature responses (i.e., responses before the onset of the light cue stimulus) gauge impulsivity or response disinhibition. Timeout responses (i.e., persistent responding after the onset of a penalizing timeout period) reflect inability to shift out of an initiated behavioral pattern, and therefore allow the evaluation of cognitive inflexibility. Finally, the animal’s latency to respond correctly presents a measure of its speed of processing; comparison with the animal’s latency to retrieve a food reward permits control for nonspecific effects on locomotion or motivation. Previous studies indicated that PCP administration disrupts 5-CSRTT performance (Le Pen et al. 2003; Greco et al. 2005). However, it is not known how atypical antipsychotic medications affect such PCP-induced cognitive deficits. Amelioration of PCP-induced 5-CSRTT disruption with atypical antipsychotic compounds would support the predictive validity of this model for cognitive impairments in schizophrenia (Geyer and Markou 1995). Moreover, in light of studies suggesting that different PCP regimens can significantly influence how PCP affects cognitive performance (Jentsch and Roth 1999), we endeavored to determine the regimen that best models schizophrenia-like cognitive deficits. We therefore compared the effects of a single PCP injection to the effects of repeated PCP administration on 5-CSRTT performance and then investigated whether these PCP effects were attenuated with atypical antipsychotic medications.
Materials and methods
Subjects
Male Wistar rats were purchased from Charles River Laboratories (Wilmington, MA) and housed two per cage on a 12-h:12-h reversed light–dark cycle (lights on at 6:00 a.m.). All testing was conducted during the animals’ dark cycle. Rats were allowed to reach a body weight of at least 275 g before being restricted to 15 g of food per day (in addition to the food pellets earned during testing) and before behavioral training was initiated. Rats received water ad libitum at all times except during testing. In total, 144 rats were used for the study. All experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the institute’s Animal Care and Use Committee.
Drugs
d-Phencyclidine sulfate (PCP) was obtained from the National Institute on Drug Abuse. Risperidone and haloperidol were purchased from Sigma-Aldrich (St. Louis, MO) and olanzapine from Anawa Trading SA (Wangen, Switzerland). Clozapine and quetiapine were provided as generous gifts by Novartis Pharma AG and AstraZeneca Pharmaceuticals, respectively. PCP was dissolved in 0.9% saline solution. Clozapine, olanzapine, risperidone, and quetiapine were dissolved in saline with one-sixteenth to one-eighth equivalents HCl 0.1 N; matched vehicle solutions were prepared from saline containing the same amounts of HCl 0.1 N. Haloperidol was diluted with saline from a stock solution of 5 mg/ml. PCP was administered by subcutaneous (s.c.) injection in a volume of 1 ml/kg (single administration) or 2 ml/kg (repeated administration). Antipsychotic compounds were administered by intraperitoneal (i.p.) injection in a volume of 2 ml/kg. In some experiments, clozapine was delivered via subcutaneous osmotic minipumps at a dose of 2, 4, or 6 mg kg−1 day−1; in those cases, clozapine was dissolved in HCl 0.1 N and diluted with saline (approximately half of the volume) according to the rats’ body weight.
Apparatus
Training and testing were conducted in operant conditioning boxes (Med Associates, St Albans, VT) containing a curved rear wall with nine contiguous apertures, with a photocell beam located at the entrance of each aperture to detect nosepoke response, and a 3-W stimulus light located at the rear of each aperture. Metal inserts blocked every alternate hole, leaving open holes 1, 3, 5, 7, and 9. In the opposite wall, a magazine connected to a food dispenser permitted the automatic delivery of food pellets (NOYES Precision Pellets 45 mg rodent food pellets; Research Diets, New Brunswick, NJ), with a photocell beam detecting head entries into the magazine. The apparatus was controlled by a PC running MedPC software (Med Associates). Operant conditioning boxes were located within light- and sound-attenuated chambers.
Five-choice serial reaction time task procedure
During two initial 20-min training sessions, five to ten pellets were placed in each open aperture and the magazine of the operant conditioning boxes to encourage rats to explore them. For the next three training sessions, all apertures were blocked with metal inserts, and a food pellet was delivered noncontingently into the magazine every 20 s to allow the rats to learn to retrieve food rewards from the magazine. The metal inserts were then removed from holes 1, 3, 5, 7, and 9 again, and rats were gradually trained on the 5-CSRTT task. The training procedure was based on the protocol established by Carli et al. 1983. Briefly, each trial was initiated by head entry into the food magazine. An initial pellet was delivered into the magazine at the start of each session to facilitate initiation of the first trial. After a 5-s intertrial interval (ITI), a light stimulus was presented in one of the response apertures. A nosepoke in this aperture within a limited hold period (correct response) resulted in the delivery of a food pellet into the magazine. Nosepokes in a wrong aperture (incorrect responses) were punished by a 5-s timeout, marked by extinction of the house light and no delivery of food reward. Failure to respond within the limited hold period (omission) likewise resulted in a timeout and no food reward, as did nosepokes in any aperture made before presentation of the target stimulus (premature responses). Nosepokes during the timeout period (timeout responses) reset the timeout. Each session lasted 30 min or until 100 trials had been completed, whichever occurred first. Duration of the light stimulus and limited hold period were initially set at 30 and 60 s, respectively, and gradually decreased over the course of training to their final length (stimulus duration, 1 s; limited hold, 5 s). Rats were trained until they had achieved criterion performance (>70% accuracy and <20 omissions) and stable baselines (<10% variation in accuracy over five consecutive days). On average, 40 sessions were required for rats to attain criterion performance. New cohorts of rats were trained for every experiment.
The following measures were recorded to assess task performance:
-
Accuracy: the number of correct responses divided by the sum of correct and incorrect responses [number of correct responses/(number correct responses + number of incorrect responses)×100]. Accuracy was only computed if correct + incorrect responses totaled ten or more.
-
Percent of correct responses: total number of correct responses divided by the total number of trials.
-
Percent of incorrect responses: total number of incorrect responses divided by the total number of trials.
-
Percent of omissions: total number of omissions divided by the total number of trials.
-
Total trials: total number of trials initiated (through a head entry into the food magazine after either a correct response or the end of the timeout following an incorrect response or omission) during the session.
-
Premature responses: total number of responses performed during the ITI, before presentation of the light stimulus.
-
Timeout responses: total number of responses performed during a timeout period.
-
Perseverative responses: nosepoke responses performed after a correct response but before collection of the reward. These responses were assessed in all experiments but are not reported here, as they did not show any systematic changes in any of the experiments (see “Discussion”).
-
Latency to correct response: time from the onset of the light stimulus to the performance of a correct nosepoke response.
-
Latency to incorrect response: time from the onset of the light stimulus to the performance of an incorrect nosepoke response. This latency measure was assessed in all experiments, but is not reported here, as it exhibited essentially the same drug effects as latency to correct response.
-
Latency to reward retrieval: time from the performance of a correct response to the retrieval of the food reward from the magazine.
Experimental design
Experiment 1: effects of a single PCP administration on 5-CSRTT performance
Rats were injected subcutaneously (s.c.) with 1.5, 2.25, or 3 mg/kg PCP or saline 30 min before 5-CSRTT testing (n = 5 or 6 per group). A between-subjects design was necessary due to our observation that PCP exposure significantly altered the effects of any subsequent PCP administrations (a phenomenon that was exploited in subsequent studies described below).
Experiment 2: effects of antipsychotic medications on 5-CSRTT performance under baseline conditions
Rats were injected intraperitoneally (i.p.) with 1, 2 or 3 mg/kg clozapine; 0.1, 0.2, or 0.3 mg/kg risperidone; 0.25, 0.5, or 1 mg/kg olanzapine; 2.5, 5 or 7.5 mg/kg quetiapine, 0.0125, 0.025, or 0.05 mg/kg haloperidol; or matched vehicle 1 h before 5-CSRTT testing. Drugs were administered at a concentration of 2 ml/kg to minimize possible aversive effects from the slightly acidic vehicle. Clozapine/haloperidol and risperidone/olanzapine were administered using a crossover design: A group of rats (n = 10) received clozapine and haloperidol, with half the rats receiving first all doses of clozapine according to a within-subjects Latin square design, followed by all doses of haloperidol in a second Latin square design; the other half received first haloperidol, then clozapine. Another group of rats (n = 9) received risperidone and olanzapine in a similar manner. This within-subjects crossover design was necessitated by the long training time required for rats to reach asymptotic performance in the 5-CSRTT. The low doses of atypical antipsychotic medications used here are unlikely to have produced significant carryover effects. Further, any such carryover effects that may have occurred were balanced across time and doses with the designs used and assessed statistically (see below). Quetiapine was administered to a separate group of rats (n = 13) according to within-subjects Latin square design. In all cases, a minimum 5-day washout period was allowed between drug administrations.
Experiment 3: effects of acute clozapine or acute risperidone on disruption of 5-CSRTT performance induced by a single PCP administration
Rats were injected i.p. with 1 or 2 mg/kg clozapine (n = 8 per dose), 0.1 mg/kg risperidone (n = 8), or vehicle (n = 9). Thirty minutes later, they received an s.c. injection of 2 mg/kg PCP. Thirty minutes after PCP administration, the rats were tested in the 5-CSRTT. Each rat received only one pretreatment/PCP combination; as in experiment 1, this between-subjects design was chosen due to the effects of PCP exposure on any subsequent PCP administrations.
Experiment 4: effects of chronic clozapine on disruption of 5-CSRTT performance induced by a single PCP administration
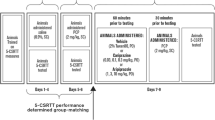
Rats were prepared with two consecutive 7-day osmotic minipumps delivering 2, 4, or 6 mg kg−1 day−1 clozapine or vehicle (n = 8 or 9 per group). Limited solubility of clozapine prevented the use of a single 14-day osmotic minipump. On the 12th day of clozapine/vehicle exposure, rats were injected with a single bolus dose of 2 mg/kg PCP s.c. 30 min before 5-CSRTT testing. Pumps were removed on the 14th day of clozapine/vehicle exposure, and rats were tested daily in the 5-CSRTT for ten additional days to assess the return of performance to baseline (see Fig. 1a for a diagram of the experimental design).
Experiment 5: effects of chronic clozapine on disruption of 5-CSRTT performance induced by repeated PCP administration
Rats received two initial s.c. injections of 2 mg/kg PCP separated by 24 h. The rats were then assigned into two groups that did not differ in accuracy, correct responses, incorrect responses, and premature responses both under baseline conditions and during the initial PCP exposure. One group (n = 11) was prepared with two consecutive 7-day osmotic minipumps delivering 4 mg kg−1 day−1 clozapine; the other group (n = 11) received pumps containing vehicle only. After three initial days of pump clozapine/vehicle exposure, all rats received five consecutive daily saline injections s.c. 30 min before 5-CSRTT testing, followed by five consecutive daily injections with 2 mg/kg PCP s.c. 30 min before 5-CSRTT testing. Overall, this design allowed for the comparison of the effects of clozapine vs vehicle (between-subjects factor) as well as of PCP vs saline (within-subjects factor), with all factorial combinations (vehicle/saline, vehicle/PCP, clozapine/saline, clozapine/PCP) explored. The mixed within-/between-subjects design was necessitated by the long training times called for by the task, and the large numbers of animals required in each group to reduce the considerable variation in PCP effects. However, other studies in our lab, in which saline was administered in place of PCP throughout pump exposure, demonstrated that the repeated injections alone do not induce any changes in any of the task parameters. Pumps were removed on the 14th day of clozapine exposure, and rats continued to be tested daily in the 5-CSRTT for 10 days (see Fig. 1b for a diagram of the experimental design). The examination of clozapine’s potential to prevent PCP effects resembles the prevention of recurring psychotic episodes in schizophrenia. PCP was administered at a concentration of 2 ml/kg because irritation of the injection site had been observed with repeated PCP injections at 1 ml/kg, even when varying the injection site.
Data analyses
Data from experiments 1 and 3 were analyzed using between-subjects one-way analyses of variance (ANOVA) with drug dose as the between-subjects factor. Data from experiment 2 were analyzed with one-way repeated-measures ANOVA, with drug dose as the within-subjects factor. A two-way mixed-design ANOVA was used to analyze data from experiments 4 and 5, with pump content (clozapine/vehicle) as the between-subjects factor and drug challenge (PCP/saline) as the within-subjects factor. Average values from the 5 days of saline injection during vehicle/clozapine treatment were compared to the values obtained on the day of the single PCP administration during vehicle/clozapine treatment (experiment 4) or averages of values obtained during the 5 days of PCP administration during vehicle/clozapine treatment (experiment 5). In addition, the vehicle-treated animals in experiment 5 were analyzed separately using one-way repeated-measures ANOVA, with injection day as the within-subjects factor, to explicitly assess the effects of repeated PCP administration over time in the absence of clozapine treatment. Where statistically significant effects were found in the ANOVA, post hoc comparisons among means were conducted using Newman–Keuls tests for one-way ANOVAs and Bonferroni tests for two-way ANOVAs. The level of significance was set at 0.05.
Results
Experiment 1: effects of a single PCP administration on 5-CSRTT performance
Casual observation of the subjects revealed no stereotyped movements after any of the PCP doses in this or later experiments. Some ataxia was observed after doses above 1.5 mg/kg.
A single administration of PCP decreased accuracy [F(3,16)=3.94, p < 0.05], an effect that reached significance at the 2.25-mg/kg dose (p < 0.05; Fig. 2a). Percent correct responses were suppressed by PCP [F(3,23)=10.99, p < 0.001] at the 2.25- and the 3-mg/kg PCP doses compared to the vehicle group (p < 0.001; Fig. 2b). Inspection of the data reveals a noticeable tendency of PCP to lower percent incorrect responses at the 3-mg/kg dose (Fig. 2c). PCP also increased percent of omissions [F(3,23)=8.94, p < 0.001] at the 2.25- and the 3-mg/kg doses (p < 0.01 and p < 0.001, respectively; data not shown) and decreased total trials [F(3,23)=3.41, p < 0.05; Fig. 2d]. PCP tended to reduce premature responses, although statistical significance was not reached (Fig. 2e). There was no effect of PCP on timeout responses (Fig. 2f). PCP administration increased the latency to correct response [F(3,21)=3.65, p < 0.05] at the 3-mg/kg dose (p < 0.05; Fig. 2g). Latency to reward retrieval was not affected (Fig. 2h).
Effects of single PCP administration in the 5-CSRTT. Accuracy (a), correct responses (b), incorrect responses (c), total trials (d), premature responses (e), timeout responses (f), latency to correct response (g), latency to reward retrieval (g) are shown as mean±SEM. Asterisks indicate statistically significant differences (*p < 0.05; ***p < 0.001) compared to the vehicle group
Experiment 2: effects of antipsychotic medications on 5-CSRTT performance under baseline conditions
Separate statistical analyses of data from rats that had received each antipsychotic compound either initially or after previous administration of another antipsychotic agent revealed no order effects; therefore, these groups were combined for further analyses.
None of the antipsychotic medications significantly altered accuracy in the 5-CSRTT (Table 1). Clozapine [F(3,39)=3.62, p < 0.05], risperidone [F(3,35)=6.33, p < 0.05], quetiapine [F(3,51)=3.42, p < 0.05], and olanzapine [F(3,35)=6.57, p < 0.01] decreased percent of correct responses; however, this effect reached significance only at the highest dose of each antipsychotic compound (3 mg/kg for clozapine, 0.3 mg/kg for risperidone, 7.5 mg/kg for quetiapine, 1 mg/kg for olanzapine; Table 1). None of the haloperidol doses affected percent of correct responses. Percent of incorrect responses was not altered by any antipsychotic compound. At the highest dose, clozapine [F(3,39)=3.42, p < 0.05], risperidone [F(3,35)=7.00, p < 0.01], and olanzapine [F(3,35)=7.37, p < 0.01] also increased percent of omissions (data not shown), and olanzapine [F(3,35)=7.66, p < 0.001] decreased total trials (p < 0.01; Table 1). In general, none of the antipsychotic medications affected premature responses or timeout responses. The only exception was a decrease in premature responses with risperidone [F(3,35)=5.03, p < 0.01)] at the 0.2- and 0.3-mg/kg doses (p < 0.05 and p < 0.01, respectively; Table 1). Clozapine [F(3,39)=5.54, p < 0.01] and risperidone [F(3,35)=6.67, p < 0.01] significantly increased latency to correct response at the highest doses tested (3 mg/kg for clozapine, 0.3 mg/kg for risperidone; p < 0.01; Table 1). None of the antipsychotic medications affected latency to reward retrieval (Table 1).
Experiment 3: effects of acute clozapine or risperidone on disruption of 5-CSRTT performance induced by a single PCP administration
No significant effects of PCP or antipsychotic medications on accuracy were found (Fig. 3a). PCP administration suppressed percent of correct responses [F(4,41)=10.08, p < 0.0001]. This effect was not prevented by clozapine or risperidone pretreatment (Fig. 3b). No significant effects of PCP or antipsychotic medications on percent of incorrect responses were observed (data not shown). PCP also increased percent of omissions [F(4,41)=5.812, p < 0.001; data not shown] and decreased total trials [F(4,41)=6.87, p < 0.001]. Clozapine or risperidone pretreatment did not reverse this effect; in fact, the reduction in total trials reached significance only after clozapine or risperidone, but not vehicle, pretreatment (p < 0.01 compared to baseline; Fig. 3c). No significant effects of PCP or antipsychotic agents on premature or timeout responding, latency to correct response, or latency to reward retrieval were found (data not shown).
Effects of acute atypical antipsychotic medication (clozapine or risperidone) treatment on 5-CSRTT performance disruption induced by a single PCP administration. Accuracy (a), correct responses (b), and total trials (c) are shown as mean±SEM. Asterisks indicate statistically significant differences (**p < 0.01; ***p < 0.001) compared to baseline. CLZ 1.0 = 1 mg/kg clozapine; CLZ 2.0 = 2 mg/kg clozapine; RIS 0.1 = 0.1 mg/kg risperidone
Experiment 4: Effects of chronic clozapine on disruption of 5-CSRTT performance induced by a single PCP administration
There was a significant decrease in accuracy after PCP injection [main effect of PCP: F(1,29)=46.27, p < 0.0001]. No main effect of clozapine and no interaction between clozapine and PCP were found (Fig. 4a). PCP treatment also significantly decreased percent of correct responses [main effect: F(1,29)=93.78, p < 0.0001], increased percent of omissions [main effect: F(1,29)=58.40, p < 0.0001], and decreased total trials [main effect: F(1,29)=41.98, p < 0.0001], but again, there was no clozapine main effect or clozapine × PCP interaction (Fig. 4b and data not shown). There were no PCP, clozapine, or interaction effects on percent of incorrect responses (data not shown). PCP increased premature [F(1,29)=11.91, p < 0.01] and timeout [F(1,29)=11.78, p < 0.01] responses. However, neither premature nor timeout responses were affected by clozapine, and there was no interaction (data not shown). PCP increased the latency to correct response [F(1,29)=39.30, p < 0.0001], but there was no main effect of clozapine and no interaction. There were no main or interaction effects on the latency to reward retrieval (data not shown).
Experiment 5: Effects of chronic clozapine on disruption of 5-CSRTT performance induced by repeated PCP administration
No stereotyped movements were observed after repeated PCP administration. Casual observation detected mild ataxia after the first injection, but not after later PCP injections.
Effects of repeated PCP in vehicle-treated animals
There was a significant main effect of repeated PCP on accuracy [F(7,87)=4.52, p < 0.001], with a significant decrease in accuracy on all days of PCP administration compared to baseline performance (p < 0.05; Fig. 5a). Repeated PCP also decreased percent of correct responses [F(7,87)=13.22, p < 0.0001] on all days (p < 0.001; Fig. 5b). Further, there was a significant main effect of repeated PCP on percent of incorrect responses [F(7,87)=3.34, p < 0.01], although none of the post hoc tests indicated significant changes on any specific days of PCP treatment (Fig. 5c). Inspection of the data suggests that the effect stems from the difference between percent of incorrect responses after the first PCP injection (which was slightly if nonsignificantly below baseline levels) and percent of incorrect response after later PCP administrations (when percent of incorrect responses tended to be increased). Percent of omissions was increased [F(7,87)=10.05, p < 0.0001] after the first, third, and sixth PCP administration (p < 0.001 and p < 0.05, respectively; data not shown). A main effect of PCP on total trials [F(7,87)=6.41, p < 0.0001] was due to a significant decrease in trials after the first PCP injection (p < 0.001); total trials were not significantly decreased after any of the later PCP administrations (Fig. 5d). Repeated PCP significantly affected premature responses [F(7,87)=7.23, p < 0.0001]. Premature responses tended to be decreased after the first initial PCP injection, although this effect did not reach significance. In contrast, later PCP injections led to increased premature responding compared to baseline levels (p < 0.05; Fig. 5e). There was also a significant effect of repeated PCP on timeout responses [F(8.87)=3.73, p < 0.01]. As with premature responding, the data show a tendency towards decreased timeout responding after the first initial PCP injection, followed by increased timeout responding after later PCP injections (Fig. 5f). Repeated PCP administration significantly increased latency to correct response [F(7,87)=3.90, p < 0.01] on all days of PCP exposure (p < 0.05; Fig. 5g). Latency to reward retrieval was not significantly altered by repeated PCP (Fig. 5h).
Effects of repeated PCP in the 5-CSRTT. Accuracy (a), correct responses (b), incorrect responses (c), total trials (d), premature responses (e), timeout responses (f), latency to correct response (g), latency to reward retrieval (h) are shown as mean±SEM. Asterisks indicate statistically significant differences (*p < 0.05; **p < 0.01; p*** < 0.001) compared to baseline
Effects of chronic clozapine on the effects of repeated PCP administration
ANOVA analysis indicated a significant main effect of repeated PCP on accuracy [F(1,20)=27.38, p < 0.0001; Fig. 6a]. Chronic clozapine attenuated this decrease in accuracy. While no main effect of clozapine and no interaction effects could be detected with the ANOVA, based on our a priori hypothesis outlined in “Introduction”, a t test comparing vehicle-treated and clozapine-treated animals after the third PCP injection (first day of repeated daily PCP exposure) revealed a significant difference (p < 0.05; Fig. 6a), indicating that rats in the clozapine group performed with higher accuracy than vehicle-treated rats. Inspection of the data confirmed a continuation of this tendency on all days of PCP administration. Moreover, on any day of PCP administration, fewer animals in the clozapine group performed at more than two standard deviations below their baseline accuracy (Table 2). While the ANOVA indicated a significant main effect of repeated PCP on percent of correct responses [F(1,20)=70.36, p < 0.0001], no effect of clozapine treatment and no clozapine × PCP interaction were observed (data not shown). However, a mixed-design ANOVA comparing levels of percent of incorrect responding on each day of repeated PCP exposure revealed lower levels of incorrect responding in clozapine-treated animals after PCP administration compared to vehicle-treated animals injected with PCP [F(1,80)=5.36, p < 0.05; Fig. 6b], although an ANOVA comparing averages across PCP and saline exposure days detected only a main effect of PCP to increase percent of incorrect responses [F(1,20)=6.10, p < 0.05]. Repeated PCP administration increased percent of omissions [F(1,20)=56.83, p < 0.0001] and decreased total trials [F(1,20)=41.01, p < 0.0001], but there was no effect of clozapine and no interaction effect (data not shown). ANOVA analysis of premature responses revealed significant main effects of repeated PCP [F(1,20)=79.73, p < 0.0001] and clozapine [F(1,20)=5.967, p < 0.05] treatment, as well as a clozapine × PCP interaction [F(1,20)=9.481, p < 0.01]. Post hoc tests indicated that clozapine did not affect premature responses in the absence of PCP, but significantly attenuated the PCP-induced increase in premature responses (Fig. 6c). Repeated PCP administration increased timeout responses [F(1,20)=80.11, p < 0.0001], but no effect of clozapine and no interaction effects were found (data not shown). Latency to correct response was significantly increased by repeated PCP [F(1,20)=28.90, p < 0.0001], but there was no effect of clozapine and no interaction effect. There was a small increase in latency to reward in clozapine-treated rats independent of PCP exposure [main effect of clozapine: F(1,20)=4.69, p < 0.05; data not shown].
Effects of chronic clozapine treatment on 5-CSRTT performance disruption induced by repeated PCP. Accuracy (a), incorrect responses (b), and premature responses (c) are shown as mean±SEM. Asterisk (*p < 0.05) indicates a statistically significant difference compared to the vehicle group and dagger sign (†p < 0.05) denotes a statistically significant difference compared to performance after saline injections; arrow pointing upwards or downwards indicates a PCP injection. CLZ Clozapine
Post-drug performance
In both experiments 4 and 5, performance of both the clozapine- and the vehicle-treated groups returned to baseline levels for all measures after cessation of PCP and clozapine/vehicle administration (data not shown).
Discussion
A single PCP injection tended to have nonspecific response-suppressive effects on 5-CSRTT performance that were not reversed by antipsychotic treatment in rats. In contrast, repeated PCP induced selective disruption of cognitive performance in the 5-CSRTT analogous to the deficit profile seen in schizophrenia. Chronic clozapine attenuated some of these deficits.
Effects of PCP
Attentional performance
Repeated PCP administration produced a robust decrease in attentional accuracy in the 5-CSRTT (Fig. 5a). Correct responding was selectively decreased, while incorrect responding was not altered or even tended to be slightly increased (Fig. 5b and c). In contrast, a single administration of PCP suppressed both correct and incorrect responses (Fig. 2b and c). However, reduced accuracy could be observed after a single PCP injection also (Fig. 2a), indicating that correct responses were disrupted somewhat more than incorrect responses. Thus, the single PCP injection may have produced a degree of specific attentional disruption. Accuracy in the 5-CSRTT is a conservative measure; it is based only on the ratio of correct and incorrect responses completed by the animal and is independent of the total number of trials and the number of omitted responses. Therefore, accuracy is unlikely to be confounded by nonspecific locomotor or motivational effects. The significant decrease in accuracy seen with repeated PCP administration is thus likely to reflect an actual attentional deficit instead of an artifact caused by sedation, locomotor disruption, lack of appetite, or other confounds. These results correspond well to previous findings indicating that PCP and other NMDA receptor antagonists induced decreases in accuracy in the 5-CSRTT (Grottick and Higgins 2000; Higgins et al. 2003a, 2005; Le Pen et al. 2003; Greco et al. 2005) or similar attentional tasks (Jin et al. 1997; Presburger and Robinson 1999; Mishima et al. 2002; Nelson et al. 2002; Rezvani and Levin 2003). Single, but not repeated, PCP administration also reduced the total number of trials initiated by the animals (Figs. 2d and 5d); however, rats still completed enough trials to allow for meaningful calculation and evaluation of the measures reflecting performance in this task.
Schizophrenia patients suffer from significant attentional deficits characterized by both decreased correct responses (errors of omission) and proportionally increased incorrect responses (errors of commission) in tests of attention (Nuechterlein and Dawson 1984; Laurent et al. 1999). The disruption of attentional performance observed here after repeated PCP administration may, therefore, model aspects of cognitive dysfunction present in schizophrenia.
Impulsivity
A single PCP injection tended to decrease premature responding in the 5-CSRTT (Fig. 2e), likely due to overall response depression (see above) rather than actions on impulsivity per se. Repeated PCP administration, however, strikingly increased premature responses starting with the second sequential injection (fourth injection overall; Fig. 5e), indicating decreased inhibition of inappropriate responding. Similar increases in impulsivity were seen after administration of NMDA receptor antagonists in previous studies in the 5-CSRTT or closely related tasks (Jin et al. 1997; Higgins et al. 2003a, b, 2005; Le Pen et al. 2003; Murphy et al. 2005), as well as in different cognitive tasks (Sanger and Jackson 1989; Sanger 1992; Jentsch et al. 2000; Compton et al. 2001; Jentsch and Taylor 2001; Higgins et al. 2003a, b; Jentsch and Anzivino 2004). Such impaired response inhibition mirrors the impulsivity exhibited by schizophrenia patients in go/no go tasks (Kiehl et al. 2000; Weisbrod et al. 2000; Wykes et al. 2000; Badcock et al. 2002; Chan et al. 2006).
Cognitive inflexibility
No effects on timeout responses were observed after a single PCP administration (Fig. 2f), but repeated PCP increased them noticeably (Fig. 5f). Increases in timeout responding suggest decreased ability to shift out of an initiated behavioral response, possibly reflecting cognitive, or at least behavioral, inflexibility and matching the cognitive inflexibility displayed by schizophrenia patients (Goldberg et al. 1988; Morice 1990). Performance in several tasks assessing cognitive flexibility is impaired after PCP administration (Jentsch et al. 2000; Jentsch and Taylor 2001; Laurent and Podhorna 2004; Shannon and Love 2004; Egerton et al. 2005; Rodefer et al. 2005). Previous studies did not report PCP effects on timeout responding in the 5-CSRTT; however, they note increases in perseverative responding, indicating a similar decrease in cognitive flexibility (Le Pen et al. 2003; Greco et al. 2005). Surprisingly, we did not observe any effects of PCP on perseverative responses beyond suppression after the first injection. A possible explanation is that perseverative responding is a more “restricted” measure than timeout responses. The animal can only perform one perseverative response per trial because a timeout is initiated after the first perseverative response. Thus, if a rat performs several repeated nosepokes after an initial correct response, only the first is recorded as a perseverative response, while the remainder registers as timeout responses. Thus, the PCP-induced cognitive inflexibility in our study may not have been pronounced enough to significantly affect perseverative responding, while the more sensitive measure of timeout responding recorded the additional perseveration.
Speed of processing
Single and repeated PCP administration increased the latency to correct responding (Figs. 2g and 5g), consistent with previous studies showing decreased performance speed after administration of NMDA antagonists in various cognitive tasks (Danysz et al. 1988; Jin et al. 1997; Grottick and Higgins 2000; Rezvani and Levin 2003) including the 5-CSRTT (Le Pen et al. 2003). Notably, latency to reward retrieval was unaffected (Figs. 2h and 5h), indicating that the change in latency to correct responding was not due to locomotor impairment or motivational deficits, but instead reflected a decrease in speed of processing. Decreased speed of processing is another typical cognitive dysfunction in schizophrenia (Nelson et al. 1990; Green et al. 2004) that is apparently modeled by PCP disruption of the 5-CSRTT.
Single versus repeated PCP administration
Repeated PCP appears to induce cognitive deficits with relevance to schizophrenia better than a single administration of PCP that led to general nonspecific suppression of responding in addition to any possible attentional disruption. A single PCP injection tended to suppress incorrect along with correct responses, at least at the highest dose, and also decreased total trials. Interestingly, however, repeated PCP selectively decreased correct responses, producing a significant and robust accuracy deficit suggestive of specific attentional disruption. Likewise, increases in premature and timeout responding, indicative of increased impulsivity and impaired cognitive flexibility, were only observed after repeated PCP administration.
Numerous studies have shown important differences in the effects of single versus repeated PCP administration. The former is more likely to cause nonspecific behavioral suppression, while specific cognitive deficits are more reliably observed after the latter (for review, see Jentsch and Roth 1999). A single PCP administration disrupts easy tasks dependent on simple sensory processes and associative learning (which are relatively spared in schizophrenia patients; Kesner et al. 1983; Tang and Franklin 1983; Tang and Ho 1988; DeNoble et al. 1990; Jones et al. 1990) and produces motivational deficits that may confound cognitive test results (Frederick et al. 1995). A number of studies have detected cognitive deficits after a single PCP injection (Danysz et al. 1988; Sanger and Jackson 1989; Sanger 1992; Compton et al. 2001; Le Pen et al. 2003; Jentsch and Anzivino 2004; Laurent and Podhorna 2004; Shannon and Love 2004; Egerton et al. 2005; Rodefer et al. 2005). The tasks used in some of these studies may have been less susceptible to nonspecific locomotor or motivational confounds. Moreover, differences in rat strain, salt formulation of PCP, PCP doses, routes of administration, and interval between PCP administration and behavioral testing may all have contributed to the different observations. Overall, the literature suggests that while a single PCP injection may evoke specific cognitive disruptions, such deficits are observed more reliably and freer from confounding nonspecific effects after repeated administration. This conclusion is supported by the results of this study.
Studies exploring the effects of subchronic PCP found specific disruption of various aspects of cognition (Jentsch et al. 1997a, b, 2000; Schroeder et al. 2000; Jentsch and Taylor 2001; Stefani and Moghaddam 2002; Laurent and Podhorna 2004; Rodefer et al. 2005; Abdul-Monim et al. 2006; Deschenes et al. 2006), with motor and motivational deficits largely absent (Jentsch et al. 1997a). However, many of these studies explored cognitive function after a significant washout period, when animals were no longer under the direct influence of PCP. This procedure is based on the hypothesis that subchronic PCP exposure leads to neurochemical changes that resemble the neuropathological state seen in schizophrenia. However, it should be noted that the schizophrenia-like state evoked by PCP in humans is present during PCP intoxication, not during PCP withdrawal or during prolonged post-PCP abstinence (Pradhan 1984). Notably, disruption of prepulse inhibition, a deficit of sensorimotor gating and pre-attentional processing present in both schizophrenia patients (Geyer et al. 1990; Braff et al. 1992) and PCP-treated animals (Mansbach and Geyer 1989; Geyer et al. 1990), occurs during chronic exposure to PCP while the drug is on board, but cannot be detected after treatment washout (Martinez et al. 1999; Schwabe et al. 2005). Similar negative results after subchronic PCP administration and subsequent drug washout have been obtained with some tests of working memory (Stefani and Moghaddam 2002) and cognitive flexibility (Deschenes et al. 2006). In our study, cognitive disruptions were prominent during repeated PCP exposure, but animals returned to stable baseline performance after drug washout. Therefore, the effects of repeated PCP exposure under the regimen used here appear to selectively affect behavior and cognition during further PCP exposure, but not during subsequent drug-free periods. This phenomenon may be due to the development of selective tolerance to nonspecific sedative, locomotor disruptive, or amotivational effects of PCP, unmasking the specific cognitive disrupting effects. Reduction in PCP-induced ataxia with repeated injections has been reported, with no significant ataxia detectable after the fifth injection (Melnick et al. 2002). These findings correspond well to the ataxia detected by casual observation in rats after the first, but not after later, PCP injections in our study. Podhorna and Didriksen (2005) report initial profound sensorimotor “side effects” after PCP administration to rats and mice that confound specific cognitive deficits in a Morris water maze task; these “side effects” were greatly reduced after three to ten daily PCP pre-injections, allowing clear observation of specific cognitive impairment. However, in the Podhorna and Didriksen study, the presence of PCP in the animals on the test day was still required for cognitive impairments to be detected.
Other aspects of PCP intoxication may conversely exhibit augmentation after repeated administrations (Xu and Domino 1994; Wolf 1998), a phenomenon that may be relevant to schizophrenia pathophysiology (Lieberman et al. 1997). In our study, increased premature and timeout responses developed only after repeated PCP injections, suggesting that sensitization may play a role in PCP-induced impulsivity and cognitive inflexibility. A combination of tolerance to the nonspecific effects of a single PCP administration and development of selective augmentation of specific PCP-induced cognitive impairments may therefore make repeated PCP a better inducing condition for schizophrenia-like cognitive deficits than a single PCP injection.
Effects of antipsychotic medications
Antipsychotic medications produced no significant effects on 5-CSRTT performance except at the highest doses tested at which all antipsychotic compounds, except haloperidol, depressed correct responding; clozapine, risperidone, and olanzapine increased omissions; and olanzapine decreased total trials. Risperidone decreased premature responding at a dose that did not significantly affect correct responses or omissions, possibly indicating reduced impulsivity. Correct latency was unaffected except for increases at the highest dose of clozapine and risperidone (Table 1). This pattern of results suggests that atypical antipsychotic medications do not affect 5-CSRTT performance except for a nonspecific response depression at higher doses and implies that any attenuation of PCP-induced 5-CSRTT disruptions seen in later experiments after antipsychotic agent treatment was not due to a main effect of antipsychotic agent treatment itself. Our findings that antipsychotic medications either did not affect or impaired baseline execution of the 5-CSRTT, but partially rescued performance disrupted by PCP (see below), are consistent with previous studies showing that antipsychotic medications impaired 5-CSRTT performance in normal rats (Passetti et al. 2003; Rezvani and Levin 2004; Rezvani et al. 2006), but improved 5-CSRTT execution in rats whose performance had been degraded by serotonin receptor agonists (Koskinen and Sirvio 2001) or prefrontal cortex lesions (Passetti et al. 2003).
Attenuation of PCP-induced disruption with atypical antipsychotic medications
Effects of acute antipsychotic medications on PCP-induced deficits
Acute administration of clozapine or risperidone did not attenuate the response suppression produced by a single PCP administration and, in fact, appeared to further depress responding in the 5-CSRTT (Fig. 3). This phenomenon may be due to an aversive state induced by acute clozapine or risperidone. Previous studies have shown aversive effects of acute clozapine, such as elevation of intracranial self-stimulation thresholds (an indicator of decreased reward function; Semenova and Markou 2003), as well as disruptions of attentional performance with acute clozapine or risperidone (Rezvani and Levin 2004; Rezvani et al. 2006). It is notable that the doses of clozapine and risperidone used here did not suppress 5-CSRTT responding by themselves. A previous study exploring PCP-induced cognitive impulsivity similarly failed to see attenuation of the deficit after acute clozapine administration (Compton et al. 2001). Other studies, however, have found improvements of PCP-induced cognitive impairments after acute treatment with atypical antipsychotic medications (Abdul-Monim et al. 2003, 2006; Idris et al. 2005). Possibly, the cognitive tasks used in these studies are less sensitive to the initial aversive effects of atypical antipsychotic agents, allowing for their beneficial effects to emerge after acute administration. However, in some of these studies, the ameliorating effects of the atypical antipsychotic compounds never reached statistical significance (Idris et al. 2005). Even in studies reporting beneficial effects of acute antipsychotic medications, impairment of performance beyond the PCP-induced disruption was seen with some doses of atypical antipsychotic compounds, including clozapine (Abdul-Monim et al. 2006).
Effects of chronic antipsychotic medications on PCP-induced deficits
Chronic administration of antipsychotic medications is usually required for the full clinical benefits in schizophrenia (Johnstone et al. 1978; Beckmann et al. 1979; Gelder et al. 2000). Further, in some animal studies, the beneficial effects of clozapine were seen only after chronic administration (Semenova and Markou 2003). Thus, we gave clozapine chronically through osmotic minipumps. Chronic clozapine exhibited no effects on the nonspecific response suppression caused by a single PCP administration (Fig. 4). In contrast, chronic clozapine attenuated the selective disruption of cognitive performance induced by repeated PCP (Fig. 5), again suggesting that repeated PCP induces cognitive deficits with relevance to schizophrenia better than a single PCP injection. Few studies have explored the effectiveness of chronically administered atypical antipsychotic agents in attenuating PCP-induced cognitive disruption. One study found that a deficit in the novel object recognition test induced by subchronic PCP administration was blocked by subchronic, but not acute, clozapine administration in rats (Hashimoto et al. 2005). In addition, repeated clozapine injections in monkeys reduced subchronic PCP-induced disruption of an object retrieval/detour task (Jentsch et al. 1997a).
In our study, clozapine attenuated the decrease in accuracy resulting from repeated PCP administration (Fig. 6a). This attenuation was partial and reached significance on only 1 day of repeated PCP administration (while this day was the first in the sequence of five daily PCP exposures, the profile of PCP-induced deficits is already reflective of repeated PCP effects due to the previous exposure to two initial PCP injections before pump implantation). However, inspection of the data suggests a lasting effect of clozapine pretreatment on performance on all 5 days of PCP administration. Clozapine-treated rats were less likely to respond to PCP with severe disruptions of attentional performance than vehicle-treated rats. On all days of PCP administration, fewer rats in the clozapine group performed at more than two standard deviations below their baseline performance compared to rats in the vehicle group (Table 2). The ability of clozapine to attenuate disruptions in accuracy depended largely on a reduction in incorrect responding (Fig. 6b) rather than effects on the number of correct responses or omissions. Clozapine, therefore, reversed PCP-induced deficits by facilitating the inhibition of incorrect responses, not by rescuing correct responding suppressed by repeated PCP. Clozapine also robustly attenuated the strong increase in premature responding induced by repeated PCP (Fig. 6c). Neither effect was due to a main effect of clozapine. The clozapine-treated group did not show differences in accuracy or premature responding in the absence of PCP and differed from the vehicle-treated group only during PCP administration. Clozapine did not have any effect on the increases in timeout responses and latency to correct response induced by repeated PCP.
The difficulty of achieving significant effects for the attenuation of accuracy deficits seen in this study reflects the great between-subjects variability in response to PCP, which led to large variances in each group despite the relatively large number of animals (n = 11) used here. Moreover, only limited effects of clozapine on cognitive, including attentional, deficits have been observed in schizophrenia patients (Meltzer et al. 1996; Sharma and Mockler 1998; Meltzer and McGurk 1999; Sharma and Antonova 2003). The fact that clozapine only exhibited partial effectiveness in this model is therefore not surprising and consistent with the human clinical situation. Thus, this model has predictive validity (Geyer and Markou 1995).
Conclusions
While a single PCP injection induced nonspecific response suppression in a cognitive test, repeated PCP led to performance disruptions resembling cognitive deficits in schizophrenia, such as disrupted attention, increased impulsivity, and decreased cognitive flexibility and speed of processing. Chronic clozapine, an atypical antipsychotic medication with some effectiveness in ameliorating cognitive deficits in schizophrenia, partially attenuated the attentional deficit and increased impulsivity. Thus, we conclude that repeated PCP-induced disruption of attention and response inhibition, as measured in the 5-CSRTT, may constitute a useful inducing condition in procedures assessing cognitive functions with relevance to cognitive deficits in schizophrenia.
References
Abdul-Monim Z, Reynolds GP, Neill JC (2003) The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J Psychopharmacol 17(1):57–65
Abdul-Monim Z, Reynolds GP, Neill JC (2006) The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res 169(2):263–273
Allen RM, Young SJ (1978) Phencyclidine-induced psychosis. Am J Psychiatry 135:1081–1084
Badcock JC, Michie PT, Johnson L, Combrinck J (2002) Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med 32(2):287–297
Bakker CB, Amini FB (1961) Observations on the psychotomimetic effects of sernyl. Compr Psychiatry 2:269–280
Beckmann B, Hippius H, Ruther E (1979) Treatment of schizophrenia. Prog Neuropsychopharmacol 3:47–52
Bender S, Dittmann-Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, Vorbach EU, Kuhn KU, Lambert M, Dittmann RW, Naber D (2005) Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs. clozapine. Int J Neuropsychopharmacol 9(2):135–145
Bilder RM, Lieberman JA, Kim Y, Alvir JM, Reiter G (1992) Methylphenidate and neuroleptic effects on oral word production in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 5:262–271
Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA (2002) Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 159(6):1018–1028
Braff DL, Grillon C, Geyer MA (1992) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49(3):206–215
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Castellani S, Giannini AJ, Boeringa JA, Adams PM (1982) Phencyclidine intoxication: assessment of possible antidotes. J Toxicol Clin Toxicol 19(3):313–319
Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK (2006) The components of executive functioning in a cohort of patients with chronic schizophrenia: a multiple single-case study design. Schizophr Res 81(2–3):173–189
Compton AD, Slemmer JE, Drew MR, Hyman JM, Golden KM, Balster RL, Wiley JL (2001) Combinations of clozapine and phencyclidine: effects on drug discrimination and behavioral inhibition in rats. Neuropharmacology 40(2):289–297
Danysz W, Wroblewski JT, Costa E (1988) Learning impairment in rats by N-methyl-d-aspartate receptor antagonists. Neuropharmacology 27(6):653–656
DeNoble VJ, Jones KW, Schaeffer CL, Bauerle LM (1990) 3-((±)-2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) and phencyclidine produce a deficit of passive avoidance retention in rats. Eur J Pharmacol 175(2):197–202
Deschenes A, Goulet S, Dore FY (2006) Rule shift under long-term PCP challenge in rats. Behav Brain Res 167(1):134–140
Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA (2005) Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl) 179(1):77–84
Elvevag B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14(1):1–21
Frederick DL, Gillam MP, Allen RR, Paule MG (1995) Acute behavioral effects of phencyclidine on rhesus monkey performance in an operant test battery. Pharmacol Biochem Behav 52(4):789–797
Gelder MG, López-Ibor JJ, Andreasen N (2000) New oxford textbook of psychiatry. Oxford University Press, New York, NY
Geyer MA, Markou A (1995) Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven, New York, pp 787–798
Geyer MA, Swerdlow NR, Mansbach RS, Braff DL (1990) Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull 25:485–498
Goldberg TE, Kelsoe JR, Weinberger DR, Pliskin NH, Kirwin PD, Berman KF (1988) Performance of schizophrenic patients on putative neuropsychological tests of frontal lobe function. Int J Neurosci 42(1–2):51–58
Greco B, Invernizzi RW, Carli M (2005) Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU(2/3) receptor agonist LY379268. Psychopharmacology (Berl) 179(1):68–76
Green MF, Kern RS, Heaton RK (2004) Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72(1):41–51
Grottick AJ, Higgins GA (2000) Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res 117(1–2):197–208
Handelmann GE, Contreras PC, O’Donohue TL (1987) Selective memory impairment by phencyclidine in rats. Eur J Pharmacol 140:69–73
Hashimoto K, Fujita Y, Shimizu E, Iyo M (2005) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol 519(1–2):114–117
Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R (2003a) Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology 44(3):324–341
Higgins GA, Enderlin M, Haman M, Fletcher PJ (2003b) The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 170(3):309–319
Higgins GA, Ballard TM, Enderlin M, Haman M, Kemp JA (2005) Evidence for improved performance in cognitive tasks following selective NR2B NMDA receptor antagonist pre-treatment in the rat. Psychopharmacology (Berl) 179(1):85–98
Hudzik TJ, Wenger GR (1993) Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey. J Pharmacol Exp Ther 265:120–127
Idris NF, Repeto P, Neill JC, Large CH (2005) Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and d-amphetamine in the rat. Psychopharmacology (Berl) 179(2):336–348
Javitt DC (1987) Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatr 9(1):12–35
Jentsch JD, Anzivino LA (2004) A low dose of the alpha2 agonist clonidine ameliorates the visual attention and spatial working memory deficits produced by phencyclidine administration to rats. Psychopharmacology (Berl) 175(1):76–83
Jentsch JD, Roth RH (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20(3):201–225
Jentsch JD, Taylor JR (2001) Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology 24(1):66–74
Jentsch JD, Redmond DE Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997a)Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277:953–955
Jentsch JD, Tran A, Le D, Youngren KD, Roth RH (1997b) Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17:92–99
Jentsch JD, Roth RH, Taylor JR (2000)Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biol Psychiatry 48(5):415–424
Jin J, Yamamoto T, Watanabe S (1997) The involvement of sigma receptors in the choice reaction performance deficits induced by phencyclidine. Eur J Pharmacol 319(2–3):147–152
Johnstone EC, Crow TJ, Frith CD, Carney MW, Price JS (1978) Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet 1(8069):848–851
Jones KW, Bauerle LM, DeNoble VJ (1990) Differential effects of sigma and phencyclidine receptor ligands on learning. Eur J Pharmacol 179(1–2):97–102
Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA (2004)Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry 161:985–995
Kesner RP, Hardy JD, Novak JM (1983) Phencyclidine and behavior: II. Active avoidance learning and radial arm maze performance. Pharmacol Biochem Behav 18(3):351–356
Kiehl KA, Smith AM, Hare RD, Liddle PF (2000) An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry 48(3):210–221
Koskinen T, Sirvio J (2001) Studies on the involvement of the dopaminergic system in the 5-HT2 agonist (DOI)-induced premature responding in a five-choice serial reaction time task. Brain Res Bull 1;54(1):65–75
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Kuperberg G, Heckers S (2000) Schizophrenia and cognitive function. Curr Opin Neurobiol 10(2):205–210
Laurent V, Podhorna J (2004) Subchronic phencyclidine treatment impairs performance of C57BL/6 mice in the attentional set-shifting task. Behav Pharmacol 15(2):141–148
Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T (1999) Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res 89(3):147–159
Le Pen G, Grottick AJ, Higgins GA, Moreau JL (2003) Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology 28(10):1799–1809
Lieberman JA, Sheitman BB, Kinon BJ (1997) Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 17(4):205–229
Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R (1959) Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psych 81(3):363–369
Mansbach RS, Geyer MA (1989) Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2:299–308
Martinez ZA, Ellison GD, Geyer MA, Swerdlow NR (1999) Effects of sustained phencyclidine exposure on sensorimotor gating of startle in rats. Neuropsychopharmacology 21(1):28–39
McGurk SR, Meltzer HY (2000) The role of cognition in vocational functioning in schizophrenia. Schizophr Res 45:175–184
Melnick SM, Rodriguez JS, Bernardi RE, Ettenberg A (2002) A simple procedure for assessing ataxia in rats: effects of phencyclidine. Pharmacol Biochem Behav 72(1–2):125–130
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25(2):233–255
Meltzer HY, Thompson PA, Lee MA, Ranjan R (1996)Neuropsychologic deficits in schizophrenia: relation to social function and effect of antipsychotic drug treatment. Neuropsychopharmacology 14(3 Suppl):27S–33S
Mishima K, Fujii M, Aoo N, Yoshikawa T, Fukue Y, Honda Y, Egashira N, Iwasaki K, Shoyama Y, Fujiwara M (2002) The pharmacological characterization of attentional processes using a two-lever choice reaction time task in rats. Biol Pharm Bull 25(12):1570–1576
Morice R (1990) Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br J Psychiatry 157:50–54
Mortimer AM (1997) Cognitive function in schizophrenia—do neuroleptics make a difference? Pharmacol Biochem Behav 56(4):789–795
Murphy ER, Dalley JW, Robbins TW (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 179(1):99–107
Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE (1990) Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med 20:357–365
Nelson CL, Burk JA, Bruno JP, Sarter M (2002) Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 161(2):168–179
Nuechterlein KH, Dawson ME (1984) Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 10:160–203
Passetti F, Levita L, Robbins TW (2003) Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav Brain Res 138(1):59–69
Paulus MP, Geyer MA, Braff DL (1996) Use of methods from chaos theory to quantify a fundamental dysfunction in the behavioral organization of schizophrenic patients. Am J Psychiatry 153:714–717
Podhorna J, Didriksen M (2005) Performance of male C57BL/6J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. Behav Pharmacol 16(1):25–34
Pradhan SN (1984) Phencyclidine (PCP): some human studies. Neurosci Biobehav Rev 8(4):493–501
Presburger G, Robinson JK (1999) Spatial signal detection in rats is differentially disrupted by delta-9-tetrahydrocannabinol, scopolamine, and MK-801. Behav Brain Res 99(1):27–34
Rezvani AH, Levin ED (2003) Nicotinic-glutamatergic interactions and attentional performance on an operant visual signal detection task in female rats. Eur J Pharmacol 465(1–2):83–90
Rezvani AH, Levin ED (2004) Nicotine-antipsychotic drug interactions and attentional performance in female rats. Eur J Pharmacol 486(2):175–182
Rezvani AH, Caldwell DP, Levin ED (2006) Chronic nicotine interactions with clozapine and risperidone and attentional function in rats. Prog Neuropsychopharmacol Biol Psychiatry 30(2):190–197
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163(3–4):362–380
Rodefer JS, Murphy ER, Baxter MG (2005) PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur J Neurosci 21(4):1070–1076
Sanger DJ (1992) NMDA antagonists disrupt timing behaviour in rats. Behav Pharmacol 3(6):593–600
Sanger DJ, Jackson A (1989) Effects of phencyclidine and other N-methyl-d-aspartate antagonists on the schedule-controlled behavior of rats. J Pharmacol Exp Ther 248(3):1215–1221
Schroeder U, Schroeder H, Schwegler H, Sabel BA (2000) Neuroleptics ameliorate phencyclidine-induced impairments of short-term memory. Br J Pharmacol 130(1):33–40
Schwabe K, Brosda J, Wegener N, Koch M (2005)Clozapine enhances disruption of prepulse inhibition after sub-chronic dizocilpine- or phencyclidine-treatment in Wistar rats. Pharmacol Biochem Behav 80(2):213–219
Semenova S, Markou A (2003) Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry 54(11):1249–1264
Shannon HE, Love PL (2004) Within-session repeated acquisition behavior in rats as a potential model of executive function. Eur J Pharmacol 498(1–3):125–134
Sharma T, Antonova L (2003) Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am 26(1):25–40
Sharma T, Mockler D (1998) The cognitive efficacy of atypical antipsychotics in schizophrenia. J Clin Psychopharmacol 18(2 Suppl 1):12S–19S
Stefani MR, Moghaddam B (2002) Effects of repeated treatment with amphetamine or phencyclidine on working memory in the rat. Behav Brain Res 134(1–2):267–274
Stefani MR, Moghaddam B (2005) Systemic Xand prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci 119(2):420–428
Steinpreis RE (1996) The behavioral and neurochemical effects of phenyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res 74(1–2):45–55
Tamlyn D, McKenna PJ, Mortimer AM, Lund CE,Hammond S, Baddeley AD (1992) Memory impairment in schizophrenia: its extent, affiliations and neuropsychological character. Psychol Med 22:101–115
Tang AH, Franklin SR (1983) Disruption of brightness discrimination in a shock avoidance task by phencyclidine and its antagonism in rats. J Pharmacol Exp Ther 225(3):503–508
Tang AH, Ho PM (1988) Both competitive and non-competitive antagonists of N-methyl-d-aspartic acid disrupt brightness discrimination in rats. Eur J Pharmacol 151(1):143–146
Verma A, Moghaddam B (1996) NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16:373–379
Weisbrod M, Kiefer M, Marzinzik F, Spitzer M (2000) Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol Psychiatry 47(1):51–60
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54(6):679–720
Wykes T, Reeder C, Corner J (2000) The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr Res 46(2–3):241–253
Xu X, Domino EF (1994) Phencyclidine-induced behavioral sensitization. Pharmacol Biochem Behav 47(3):603–608
Acknowledgements
This work was supported by NIMH grant MH062527 to AM. NA was supported by individual Pre-Doctoral Fellowship 15DT-0048 from the Tobacco-Related Disease Research Program (TRDRP) of the State of California. The authors would like to thank Dr. Amanda Harrison and Professor Ian Stolerman for consulting us on establishing the five-choice serial reaction time task in our laboratory, Ms. Jessica Benedict and Ms. Chelsea Onifer for technical assistance, Mr. Pete Sharp for excellent assistance with electronics and computer software, and Mr. Mike Arends for editorial assistance. Finally, the authors wish to thank Dr. Daniel Hoyer from Novartis Pharma AG for providing us with clozapine and Dr. Jeffrey Goldstein from AstraZeneca Pharmaceuticals for providing us with quetiapine. Part of this work was presented at the International Behavioral Neuroscience Society 15th Annual Meeting in 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amitai, N., Semenova, S. & Markou, A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology 193, 521–537 (2007). https://doi.org/10.1007/s00213-007-0808-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0808-x