Abstract
Rationale
The nicotinic receptor agonist, isoarecolone, has ‘nicotine-like’ subjective properties as detected by rats in a discrimination paradigm. However, isoarecolone lacks the intra-accumbens dopamine-releasing effects, a feature akin to most abused substances. In the five-choice serial reaction time task, isoarecolone can enhance attention and thus may be developed as a cognitive enhancer.
Objective
The present experiments assess the dependence profile of isoarecolone in rodent models of nicotine dependence.
Method and results
Tests for cross-substitution in which isoarecolone is substituted for nicotine [0.3 mg/kg/infusion (inf)] self-administration suggest isoarecolone to have nominal reinforcing properties (0.3 or 1.0 mg/kg/inf); intake of isoarecolone declined over three test sessions in which responding was no different from saline extinction, and behaviour was reinstated by re-presenting nicotine. In a model of nicotine-seeking behaviour, rats having been extinguished by removal of nicotine (0.03 mg/kg/inf) and associated cues, the presentation of priming doses of nicotine (0.1–0.4 mg/kg s.c.) with the cues robustly reinstated responding of nicotine-seeking behaviour. Tests with priming doses of isoarecolone (1–20 mg/kg s.c.) shown previously to generalise to nicotine in discrimination tests produced significant levels of reinstatement but the responses were significantly less compared to nicotine-induced reinstatement.
Conclusion
Overall, these results suggest that isoarecolone with its unique profile of behavioural activity should be further examined for treating chronic diseases that are characterised by attentional dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is little doubt about the role of nicotine in tobacco smoking behaviour (Stolerman and Jarvis 1995). The reinforcing effects of nicotine are thought to be the primary reason why humans inhale tobacco smoke, which can be routinely demonstrated in laboratory animals using the intravenous self-administration procedure. As shown in various species, relatively high rates of responding can be supported by scheduled delivery of intravenous nicotine infusions (inf) (Goldberg et al. 1981; Risner and Goldberg 1983; Corrigall and Coen 1989; Shoaib et al. 1997; Stolerman et al. 1999).
In view of the diversity of behavioural effects produced by nicotine, an extensive series of studies from Stolerman’s laboratory have collated results with nicotine and nicotinic analogues with regard to their pharmacological selectivity and relative potency in a variety of behavioural tasks (Goldberg et al. 1989; Stolerman 1990). This approach highlighted a robust correlation between the in vitro binding and in vivo potency with some outliers that suggest the existence of multiple neuronal nicotinic receptor subtypes mediating the diverse behavioural effects of nicotine. Both isoarecolone and epibatidine were outliers indicating these compounds to have different profiles of action.
Isoarecolone displays a unique profile of neurochemical and behavioural effects, which deserves further investigation. Initial behavioural studies confirmed this nicotinic analogue to possess ‘nicotine-like’ properties as detected by rats in a nicotine discrimination task (Reavill et al. 1987). However, unlike nicotine, isoarecolone produces minimal activation of locomotor activity in nicotine-dependent rats (Reavill et al. 1987), an observation supported by the relatively weak dopamine-releasing properties in the nucleus accumbens of rats (Mirza et al. 1996). In a later and more extensive biochemical study, isoarecolone was found to evoke mecamylamine-sensitive dopamine release in a concentration-dependent manner from preloaded cortical or striatal synaptosomes; however, the analogue was 20 times less potent than nicotine and was less efficacious, producing a maximal response up to 50% of that observed for nicotine (Whiteaker et al. 1995). These findings suggesting partial agonist action were supported by behavioural observations in which isoarecolone produced nominal hyperactivity in both saline- or nicotine-treated rats compared to nicotine (Whiteaker et al. 1995).
Isoarecolone has been investigated for effects on cognitive performance. In a delayed matching to sample paradigm in monkeys, isoarecolone was shown to be effective in enhancing short-term memory (Buccafusco et al. 1995). More recently, isoarecolone has been evaluated in a rodent model of attention. Using the five-choice serial reaction time task, graded doses of isoarecolone enhanced performance (Hahn et al. 2003). However, unlike nicotine which enhanced accuracy, omission errors and latency measures in the attention task, isoarecolone selectively enhanced accuracy without affecting the other measures which are thought to be primarily dependent on dopaminergic systems (Hahn et al. 2003).
For any cognitive enhancer, its therapeutic potential will depend largely upon its dependence-producing profile. As previously observed in a nicotine discrimination paradigm, it is likely that isoarecolone may also possess some of these stimulus properties. Given the reduced dopamine-releasing properties of isoarecolone, it is predicted that this partial agonist may exhibit weak reinforcing properties. Thus, the aim of these experiments was to evaluate the relative reinforcing effects of isoarecolone. A cross-substitution experiment in which rats previously trained to self-administer intravenous injections of nicotine will be presented with various doses of isoarecolone. If isoarecolone has marginal reinforcing efficacy, then it is predicted that over three test sessions, behaviour will extinguish, similar to the profile observed for saline substitution.
To complement this investigation, isoarecolone was also examined for its ability to reinstate nicotine-seeking behaviour. An increasing number of reports demonstrate that the priming doses of nicotine can reinstate nicotine-seeking behaviour in subjects previously extinguished by saline substitution (Chiamulera et al. 1996; Shaham et al. 1997; Andreoli et al. 2003; Cohen et al. 2005).
Materials and methods
Animals
Male hooded Lister rats (Harlan UK, Bicester) initially weighing 200–250 g were housed individually in a room maintained at 20–22°C with a regular light–dark cycle (light from 8 a.m. to 8 p.m.). Once surgically prepared with an intravenous catheter, rats received their daily diet (20–24 g) approximately 1–2 h after the end of the self-administration session. All these studies complied with all local and national ethical requirements and were carried out according to the Animals (Experimental Procedures) Act, 1986 under licence from the UK Home Office.
Nicotine self-administration procedure
Surgery
For self-administration studies, under surgical anaesthesia (a mixture of medetomidine 0.3 mg/kg and ketamine 70 mg/kg, i.p.), rats were implanted with a chronic Silastic catheter into the external jugular vein, as described previously by Shoaib et al. (1997). The catheter was connected to an L-shaped connector (Plastics-One, Roanoke, VA, USA) that was mounted on nylon mesh embedded in the subcutaneous cavity on the back of the animal. Daily flushing with 0.9% physiological saline containing Baytril (enrofloxacin) (0.16 mg/kg/day) maintained the patency of the intravenous catheter. Once animals regained body weights above pre-operative weights, the self-administration sessions started.
Apparatus
Twelve standard operant chambers (MED-Associates, VT, USA) were used that consisted of a Plexiglas enclosure with one house light, two levers, one tether and fluid swivel. One lever was defined as active, and presses on it resulted in fluid infusions; presses on the other lever were recorded but had no programmed consequence. Catheters were connected to an infusion pump (Razal, MED-Associates, IN, USA). The operant chambers were controlled by a microcomputer using the MED-Associates (Lafayette, IN, USA) MED-PC software package.
Self-administration procedure
In 1-h limited access sessions, rats were given the opportunity to lever press for intravenous infusions of nicotine (0.03 mg/kg/inf), as described previously (Shoaib et al. 1997). A stimulus light was utilised to signal availability of nicotine, which was turned off for 20 s during the time-out period. No other visual stimuli were employed for training. Once the rats showed response accuracy with at least 80% of the responses on the active lever and with stable intake of nicotine (±2 infusions) over 2 days, the number of responses required to produce an infusion was increased progressively up to three (3-response fixed ratio FR-3).
Tests for cross-substitution
Tests with isoarecolone began in rats that exhibited stable levels of nicotine self-administration on an FR-3 schedule (less than 30% variability from the mean number of infusions self-administered over three sessions). Each unit dose of isoarecolone (0.3–3.0 mg/kg/inf) was tested in three three-successive sessions. At least 3 days of testing with nicotine was allowed to re-establish baseline responding between each dose. The order of the unit doses presented was randomly selected which included a saline-substitution test.
Tests for reinstatement of nicotine-seeking behaviour
In a separate group of rats trained to self-administer nicotine (0.03 mg/kg/inf) to stable criterion, extinction was performed not only by removing nicotine but also by eliminating the cues that predicted the onset of the nicotine injection [infusion pumps were turned off, and the stimulus light was left on for the whole session (time-out period not used)]. Under these conditions, the rates of extinction were much faster relative to conventional extinction when nicotine was just removed, and lever-press responses dropped to below 20% of nicotine intake baseline levels (data not shown). Behaviour was extinguished for at least six sessions until criteria were satisfied (individual session responses equal to or less than 20% of stable baseline responses, variability within ±20% for a 3-day period). The tests for reinstatement were conducted by administering graded doses of nicotine (0.1–0.4 mg/kg s.c.) and presenting the cues (stimulus light signalling time-out) and saline infusions (pump noise) contingently upon each lever-press response. Similarly, graded doses of isoarecolone (1.0–20.0 mg/kg s.c.) were also tested in a randomised sequence of tests. Extinction was re-established after each reinstatement test.
Drugs
Nicotine bitartrate (BDH, Poole, Dorset) was dissolved in isotonic saline. The pH of nicotine solution was adjusted to 7 with dilute NaOH. No changes in pH were made for intravenous solutions of isoarecolone. Isoarecolone oxalate (Pfizer, CT, USA) was dissolved in saline. Both nicotine and isoarecolone were administered subcutaneously in a volume of 1 ml/kg. Doses of drugs were expressed as those of the base.
Statistical analysis
Data from self-administration experiments in the form of total responses or infusions self-administered were analysed using repeated measures ANOVA (SPSS, version 12). Data from the reinstatement tests were analysed in the form of total responses over the test session with respect to within-subject repeated measures ANOVA (SPSS, version 12). Post hoc (Tukey’s t test) tests were conducted to identify significant differences from controls.
Results
Acquisition of nicotine self-administration
Reliable nicotine self-administration behaviour was observed in both groups of rats. Over the course of training, rats learned to press the active lever with minimal number of responses on the inactive lever. As the discrimination between the active and inactive levers improved, the fixed ratio was increased progressively to a final ratio of 3. Under these final conditions, rats self-administered on average approximately 12 infusions during each 60-min session (data not illustrated).
Cross-substitution tests
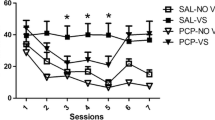
In rats lever pressing for intravenous nicotine injections (0.03 mg/kg/inj) under a fixed-ratio 3 schedule of reinforcement, the substitution of saline produced a gradual decline in the number of infusions over the three test sessions (Fig. 1). Similarly, the substitution of various doses of isoarecolone (0.3, 1.0 and 3.0 mg/kg/inf) failed to maintain responding in which extinction was observed for all doses over the three test-days; the number of infusions self-administered decreased over sessions [F(7,49)=2.2–12.2, P<0.05 to 0.001]. The largest unit dose of isoarecolone tested suppressed behaviour [F(7,42)=12.2, P<0.001], which produced residual effects as was evident during sessions 7 and 8 (P<0.05) when nicotine was reintroduced (Fig. 1).
Cross-substitution tests with isoarecolone in rats trained to self-administer nicotine (0.03 mg/kg/inf). The top panel shows the profile of extinction when saline is substituted for nicotine. The lower panels highlight the number of infusions of isoarecolone self-administered over three test sessions. Each point represents mean±SEM number of infusions in a 60-min session. Symbols show differences from session 3, the last nicotine self-administration session (Tukey’s t tests): *P<0.05. Sessions 7 and 8 show the recovery of responding when nicotine was reintroduced
Tests for reinstatement of nicotine-seeking behaviour
Priming doses of nicotine administered systemically with contingent presentation of cues produced robust reinstatement of nicotine-seeking behaviour in rats (Fig. 2). Significant increases were evident on the active lever [F(3,21)=14.2, P<0.0001] compared to inactive responses [F(3,21)=2.01, n.s.]. The same rats, within a randomised order, tested with isoarecolone with contingent presentation of cues also exhibited reinstatement of nicotine-seeking behaviour; however, isoarecolone was not as effective compared to nicotine. Increases on the active lever were apparent across isoarecolone dose [F(4,28)=8.0, P<0.0001] with little change on inactive lever presses [F(4,28)=0.46, n.s.]. The largest dose of isoarecolone (20 mg/kg s.c.) failed to reinstate nicotine-seeking behaviour, a dose previously reported to suppress food-maintained responding (Reavill et al. 1987).
Reinstatement of responding after extinction of nicotine self-administration; priming effects of nicotine and isoarecolone. The bars represent mean±SEM number of lever-press responses on the previously active (black bars) and inactive (open bars) levers during a 60-min reinstatement test. Symbols show differences from saline (Tukey’s t tests): *P<0.05
Discussions
Over a range of unit doses, the partial agonist isoarecolone did not show any signs of sustaining lever-press responding, and the profile over the tests appeared no different from saline extinction. Responding declined gradually over cross-substitution tests as observed with saline substitution in rats trained to self-administer nicotine. Extinction was incomplete partly because of the cues that were present during these cross-substitution tests. These initial findings suggest that isoarecolone has nominal reinforcing effects despite possessing nicotine-like discriminative stimulus effects. By contrast, isoarecolone reinstated nicotine-seeking behaviour in much the same way as observed with priming doses of nicotine, effects that may be linked to the pharmacological similarities in the discriminative stimulus properties of these two drugs. Because the experiment was not designed to establish the role of the contingently presented cues, without distinguishing the control condition, it is not clear if the modest reinstatement was due to isoarecolone, the cues or the combination of the two.
The present findings with isoarecolone support the notion that dopamine release in the nucleus accumbens is one of the key events that mediates the reinforcing effects of nicotine because isoarecolone has reduced dopamine-releasing properties (Whiteaker et al. 1995; Mirza et al. 1996). However, it is not clear which nicotinic receptor subtypes are involved in mediating the behavioural effects of isoarecolone. Receptor binding studies with isoarecolone have not identified any specific nicotinic receptor subtype; most receptor subtypes showed relatively weak affinity in relation to nicotine (Hahn et al. 2003).
The finding that isoarecolone can reinstate nicotine-seeking behaviour leads to a number of interpretations with regard to the reinstatement model and potential mechanisms of action. Firstly, the discriminative stimulus properties shared by nicotine and isoarecolone represent one common mechanism which may explain these findings. Similarities in the discriminative stimulus properties may be one factor behind reinstatement of drug-seeking behaviour. However, there are exceptions as highlighted by DeVries et al. (1999) who have demonstrated reinstatement by various dopaminergic agonists that generalise to cocaine’s discriminative stimulus properties but do not necessarily reinstate cocaine-seeking behaviour (DeVries et al. 1999). It would therefore be worthwhile to explore further these inter-relationships by examining other nicotinic analogues that generalise to the nicotine discriminative stimulus for their effects to reinstate nicotine-seeking behaviour. A second possibility is that the effects of isoarecolone may be non-specific, and thus general increases in activity may account for the increases in rates of responding. This explanation is not plausible because isoarecolone does not elevate dopamine levels nor does it increase locomotor activity. The reduced involvement of dopamine does raise an interesting argument with regard to dopamine and isoarecolone-induced reinstatement of nicotine-seeking behaviour. It would appear that dopamine may not be involved in reinstating nicotine-seeking behaviour, although evidence to support this is sparse. The D1 receptor antagonist has been shown to dose-dependently attenuate cue-induced reinstatement of nicotine-seeking behaviour (Cohen et al. 2005). Finally, the reinstatement effects produced in the present experiment featured both nicotine prime plus the contingent presentation of drug-associated cues. Experiments have shown drug-associated cues to interact with the priming doses of the drug to augment the reinstatement effect. Acute exposure to nicotine has been shown to enhance conditioned reinforcement (Olausson et al. 2004). Given the extensive literature suggesting a prominent role for conditioned cues within nicotine self-administration models (Caggiula et al. 2001; Chaudhri et al. 2005), it is conceivable that priming doses of isoarecolone may also serve to enhance conditioned reinforcement, although unlike nicotine, this may not be dependent upon dopaminergic neurotransmission.
Enhancement of performance by isoarecolone may not be unique to just the reinstatement or conditioned reinforcement paradigm. Similar effects may also help to explain the improvement on accuracy as measured using the five-choice serial reaction time task (Hahn et al. 2003). As predicted, isoarecolone had minimal effects on the dopamine-related measures in the 5-CSRTT; latency to respond and omission errors were unaffected. Similarly, in other models assessing cognitive performance, isoarecolone has been shown to enhance performance (Buccafusco et al. 1995). This suggests that isoarecolone or a structurally similar analogue may offer the potential for development as a cognitive enhancer. However, further experiments are warranted to characterise its pharmacological selectivity. Furthermore, before a final conclusion may be made regarding abuse liability, isoarecolone should be examined for reinforcing properties by training to self-administer in naïve groups of rats.
References
Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA (2003) Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 28:1272–1280
Buccafusco JJ, Jackson WJ, Gattu M, Terry AV (1995) Isoarecolone-induced enhancement of delayed matching to sample performance in monkeys: role of nicotinic receptors. Neuroreport 6:1223–1227
Caggiula AR, Donny AC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Chaudhri N, Caggiula AR, Donny AC, Palmatier MI, Liu X, Sved AF (2005) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–366
Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M (1996) Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 127:102–107
Cohen C, Perrault G, Griebel G, Soubrie P (2005) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155
Corrigall WA, Coen KM (1989) Nicotine maintains self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99:473–478
DeVries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren LJMJ (1999) Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 143:254–260
Goldberg SR, Spealman RD, Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214:573–575
Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS (1989) Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 97:295–302
Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP (2003) Attentional effects of nicotinic agonists in rats. Neuropharmacology 44:1054–1067
Mirza NR et al (1996) The nicotinic receptor agonists (−)-nicotine and isoarecolone differ in their effects on dopamine release in the nucleus accumbens. Eur J Pharmacol 295:207–210
Olausson P, Jentsch JD, Taylor JR (2004) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171:173–178
Reavill C, Spivak CE, Stolerman IP, Waters JA (1987) Isoarecolone can inhibit nicotine binding and produce nicotine-like discriminative stimulus effects in rats. Neuropharmacology 26:789–792
Risner ME, Goldberg SR (1983) A comparison of nicotine and cocaine self-administration in the dog: fixed ratio and progressive ratio schedules of intravenous drug infusion. J Pharmac Exp Ther 224:319–326
Shaham Y, Adamson LK, Grocki S, Corrigall WA (1997) Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 130:396–403
Shoaib M, Schindler CW, Goldberg SR (1997) Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129:35–43
Stolerman IP (1990) Behavioural pharmacology of nicotine: implications for multiple brain nicotinic receptors. In: Bock G, Marsh J (eds) CIBA Foundation Symposium 152: the biology of nicotine dependence. Wiley, Chichester, pp 3–16
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117:2–10
Stolerman IP, Naylor C, Elmer GI, Goldberg SR (1999) Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 141:297–306
Whiteaker P, Garcha HS, Wonnacott S, Stolerman IP (1995) Locomotor activation and dopamine release produced by nicotine and isoarecolone in rats. Br J Pharmacol 116:2097–2105
Acknowledgements
We wish to thank the Medical Research Council and the University of Newcastle for funding this research. Also, we thank Dr. Nimish Sidhpura for the assistance in training some of the rats and Professor Ian Stolerman for his encouragement and advice in facilitating this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoaib, M. Effects of isoarecolone, a nicotinic receptor agonist in rodent models of nicotine dependence. Psychopharmacology 188, 252–257 (2006). https://doi.org/10.1007/s00213-006-0498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0498-9