Abstract
Rationale
Compounds selective for the GABAA receptors containing an α5 subunit have been reported to enhance performance in the hippocampally mediated delayed-matching-to-position version of the Morris water maze, in which reduction in the time required to find a hidden platform relative to an initial trial is used as an index of learning and memory.
Objective
In the present study, we have used one such compound, α5IA-II, to examine whether these effects occur during the encoding, consolidation or recall phases of this paradigm.
Methods
α5IA-II was administered in the absence or presence of the benzodiazepine site antagonist flumazenil, so as to limit its action to periods associated with encoding, consolidation and recall. Drug doses and timings of administrations were defined using occupancy data derived from an in vivo [3H]flumazenil binding assay. Similar experiments were carried out to study the memory-disruptive properties of chlordiazepoxide (CDP).
Results
The trial 1 to trial 2 difference was increased when α5IA-II was given before either trial 1 or trial 2, indicating an effect on the encoding and recall phases, respectively, of learning and memory. Conversely, α5IA-II had no effect on performance when given immediately after trial 1, suggesting that it had no effect on the consolidation phase. In contrast to the facilitation of performance produced by the α5-selective inverse agonist α5IA-II given during the encoding and recall but not the consolidation phase, the non-selective agonist CDP impaired performance when given during the encoding and recall phases, whilst having no effect on the consolidation phase.
Conclusions
These data further highlight the cognition-enhancing properties of GABAA α5-selective inverse agonists and define the functional specificity of these effects in terms of encoding and recall processes in the Morris water maze.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzodiazepines have long been known to disrupt memory in human (Ghoneim and Mewaldt 1975; Duka et al. 1996a) and animal studies (McNaughton and Morris 1987) as a result of their action at GABAA receptors, where they facilitate the effects of the neurotransmitter GABA. In contrast, drugs that act at the same binding site, but which decrease GABA’s effects (inverse agonists), have been reported to exert promnestic effects in both animal models (Venault et al. 1986; Jensen et al. 1987; Sarter and Stephens 1988) and human experiments (Duka et al. 1996b).
GABAA receptors are ligand (GABA)-gated ion channels that are pentameric assemblies of subunits derived from the 16 members of this family (α1-6, β1-3, γ1-3, δ, ɛ, θ and π; Simon et al. 2004) the majority of which comprise α, β and γ subunits arranged with a 2:2:1 stoichiometry (Minier and Sigel 2004). The benzodiazepine agonists, such as diazepam and chlordiazepoxide (CDP), exert their effects via a specific recognition site on particular subtypes of the GABAA receptor. More specifically, they enhance the inhibitory actions of GABA at GABAA receptors containing β, γ2 and either an α1, α2, α3 or α5 subunit (Sieghart 1995). On the other hand, non-selective benzodiazepine site inverse agonists, such as FG 7142, also affect these same GABAA subtypes but they decrease the inhibitory effects of GABA.
The opposing effects of benzodiazepine site agonists and inverse agonists at the receptor level are reflected in vivo. For example, in addition to their contrasting effects on memory, benzodiazepine site agonists are anxiolytic and anti-convulsant (Stephens et al. 1987; Argyropoulos and Nutt 1999) whereas non-selective inverse agonists are anxiogenic and pro-convulsant or convulsant (Petersen 1983; Dorow et al. 1983). These properties restrict the potential use of inverse agonists as palliative treatments for disorders where memory impairment is indicated.
Recent advances using transgenic mice and subtype-selective pharmacological tools have begun to dissect out which of the particular pharmacological properties of the non-selective agonists are associated with certain GABAA receptor subtypes (Rudolph and Möhler 2004). For example, α1-containing GABAA receptors are associated with the sedating effects of diazepam (Rudolph et al. 1999; McKernan et al. 2000) whereas the α2 and/or α3 subtypes are associated with the anxiolytic effects (Löw et al. 2000; Atack et al. 2005, 2006; Dias et al. 2005). With respect to the α5 subtype, preferential expression of these receptors within the hippocampus implies a role in hippocampal functions, such as learning and memory (Wisden et al. 1992; Fritschy and Möhler 1995; Sur et al. 1999; Sieghart and Sperk 2002). In accordance, reduced expression of α5 subunit-containing GABAA receptors has been reported to be associated with facilitation of aspects of cognition (Collinson et al. 2002; Crestani et al. 2002) and has resulted in these receptors to become a putative target for subtype-selective inverse agonists as cognition enhancers (Maubach 2003).
Subtype selectivity for the benzodiazepine site of GABAA receptors may be achieved in either of the two ways: either via selective affinity or selective efficacy (Atack 2005). Subtype-selective affinity is defined in terms of a compound having much higher affinity for a particular subtype, whereas with subtype-selective efficacy, a compound binds with equivalent affinity for the benzodiazepine site of the different GABAA receptors but has efficacy only at certain subtypes. Compounds possessing α5-selective inverse agonist efficacy have been described (Chambers et al. 2004; Sternfeld et al. 2004; Street et al. 2004). Of these, the best characterised is α5IA, a triazolophthalazine which has recently been shown to enhance performance in a hippocampal-dependent [delayed-matching-to-position (DMTP)] version of the Morris water maze (Figure 1; Dawson et al. 2006). Moreover, and unlike non-selective inverse agonists, this compound was not anxiogenic nor did it have pro-convulsant and kindling liabilities.
General scheme of the design of the delayed-matching-to-position version of the Morris water maze. The difference between the time taken to find the platform during trials 1 and 2 (the ‘savings’ time) is used as an index of the rats’ ability to ‘remember’ the location of the platform. When the rat locates the platform on trial 1, it is allowed to spend 30 s on the platform, so that it might use the visual cues around the maze to encode the position of the platform. This information is held in short-term memory and then, provided the interval between trials 1 and 2 is sufficient, becomes consolidated into a longer-term memory store from which it can be retrieved during the recall phase
The process of learning and memory can be roughly divided into three stages: encoding, consolidation and retrieval (recall) (Abel and Lattal 2001). The original information must enter the sensory channels (e.g., via visual, olfactory, auditory or tactile stimuli) and then be rapidly encoded into a form that passes into the short-term memory. Some of this information may then be consolidated into long-term storage. The final stage of processing is ‘recall’, involving the retrieval and use of information that was stored earlier. Whilst molecular genetic (gene deletion or gene mutation) and lesioning studies can identify genes and brain structures important for studying cognitive processes, they are not amenable to differential manipulation at each separate stage of memory processing, so that it is difficult to distinguish their effects on encoding, consolidation or retrieval. In contrast, pharmacological approaches offer the highest temporal specificity because they can be applied and removed from the system within a relatively short-time window. For example, a pharmacological approach to inactivating the hippocampus at appropriate times during or after training in a water maze (using an AMPA receptor antagonist) demonstrated that inactivation during encoding, consolidation and retrieval disrupted hippocampally mediated encoding, consolidation and recall of spatial memory (Riedel et al. 1999).
Because GABAA α5-selective inverse agonists have previously been reported to enhance performance in the DMTP water maze task (Chambers et al. 2004; Dawson et al. 2006), the purpose of the present study was to examine the effects of such a compound more systematically on the separate phases of encoding, consolidation and recall. This was achieved by administering an α5-selective inverse agonist, α5IA-II—a compound structurally related to α5IA (Sternfeld et al. 2004; Street et al. 2004; Stephens et al. 2005; Dawson et al. 2006), before trial 1 (to modulate the encoding phase), after trial 1 (to modulate the consolidation phase) or, after a delay, before trial 2, which occurs 4 h after trial 1 (to modulate the recall phase). To limit the action of α5IA-II to the desired phase of memory processing, the prototypic benzodiazepine site antagonist flumazenil was used to block the action of α5IA-II. Finally, given that clinical treatment with non-selective benzodiazepine site agonists gives rise to impairments on encoding (anterograde amnesia), but not to impairments on recall (retrograde amnesia; Ghoneim and Mewaldt 1975), the effects of CDP on water maze DMTP encoding, consolidation and recall were evaluated. This would provide important data for comparison to the effects of α5IA-II.
Materials and methods
Materials
α5IA-II(3-(5-methylisoxazol-3-yl)-6-(2-pyridyl)-1,2,4-triazolo[3,4-a]phthalazine) was synthesised by the Medicinal Chemistry Department of Merck, Sharp & Dohme (Sternfeld et al. 2004; Street et al. 2004) as were flumazenil and bretazenil. CDP hydrochloride was purchased from Sigma-Aldrich (Poole, Dorset, UK). [3H]flumazenil ([3H]Ro 15–1788; 70–87 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA, USA).
α5IA-II (1 mg/kg in 70% polyethyleneglycol 300), flumazenil (10 mg/kg in 0.5% methylcellulose) and CDP (5 mg/kg in saline) were all dosed via the i.p. route with a dosing volume of 1 ml/kg. The dose of 1.0 mg/kg α5IA-II was chosen on the basis of preliminary (unpublished) Morris water maze studies in which this dose produced a robust, reproducible cognitive-enhancing effect. The dose of 5.0 mg/kg CDP was selected on the basis that it has previously been shown to impair spatial learning in the Morris water maze (McNamara and Skelton 1993). The dose of 10.0 mg/kg flumazenil was chosen on the basis that this dose was shown to block the cognitive-enhancing effects of α5IA—a compound structurally related to α5IA-II (Dawson et al. 2006).
In vitro efficacy
Efficacy was measured using whole-cell patch-clamp electrophysiology of L(tk−) cells stably expressing human recombinant GABAA receptors. Cells expressing human β3 and γ2 plus either α1, α2, α3 or α5 subunit were grown on glass coverslips, and pipettes with a resistance of 5–10 MΩ were used to patch-clamp whole cells using an Axopatch-200B patch-clamp amplifier. The peak amplitude of the currents produced by a 5-s application of an EC20-equivalent concentration of GABA was compared to the corresponding GABA-evoked currents observed after a 30-s pre-treatment with α5IA-II. The modulation of the GABA EC20 current by α5IA-II was calculated as:
For each individual patch-clamped cell, a concentration–effect curve was constructed with curve-fitting being performed using a non-linear least square method using GraphPad Prism (GraphPad Software, San Diego, CA, USA). From these data, maximum efficacy and EC50 were determined for each cell (Brown et al. 2002).
In vivo experiments
All aspects of animal care and use were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986, and its associated guidelines.
Subjects
Male, hooded Lister rats were housed in groups of four in solid-bottomed cages with sawdust bedding. They were given free access to water and were given 15 g expanded diet daily after the experimental session. Outside the experimental session, they were kept in a humidity- and temperature-controlled room (21±1°C, 55±5% relative humidity). Rats were maintained on a 12-h photoperiod with lights on at 0700 h. All receptor occupancy studies and water maze studies were conducted between 0800 and 1600 h.
Receptor occupancy of α5IA-II and flumazenil
Male hooded Lister rats (250–300 g; Harlan, UK) were administered i.p. (dose volume=1 ml/kg; n=6–8/group) with either vehicle, α5IA-II (1 mg/kg in 70% polyethyleneglycol 300) or flumazenil (10 mg/kg in 0.5% methylcellulose) for periods of 0.25, 0.5, 1, 2 or 4.5 h. Three minutes before these times, rats were given an i.v. injection of [3H]flumazenil (1 ml/kg of a 15-μCi/ml solution made up in isotonic saline) following which they were killed by stunning and decapitation.
Brains were removed, weighed, rapidly homogenised (using a Polytron PT2100 homogenizer) in ten volumes of ice-cold buffer (50 mM Tris–HCl, pH 7.5) and 300 μl aliquots of homogenate were filtered through pre-soaked Whatman GF/B filters using a filtration manifold (Hoefer Scientific Instruments). Filters were then washed with 10 ml of buffer and then placed in vials, scintillation fluid added, and radioactivity counted using a Beckman LS 6500 scintillation counter. Non-specific binding was established in a separate group of rats using 5 mg/kg of the non-selective benzodiazepine ligand, bretazenil, with a 0.5-h pre-treatment time.
Typically, total counts in vehicle-treated animals were in the region of 2,000 dpm and non-specific binding in the region of 150 dpm. For vehicle- and drug-treated animals, specific binding was calculated (actual counts minus counts in the non-specific binding, i.e. bretazenil, animals) and occupancy was defined as the extent by which the specific binding in drug-treated animals was reduced relative to specific binding in the vehicle group.
The Morris water maze delayed-matching-to-position (DMTP) task
The Morris water maze is a 2-m-diameter tank filled with an opaque mixture of water and white dye maintained at 26–28°C and in which was placed a 10-cm-diameter platform, submerged 2 cm below the surface. The tank was surrounded by a curtain on which were attached high-contrast, black-and-white patterned pictures (42×30 cm), visible from the water surface, that served as spatial ‘extra-maze’ cues. A video camera was mounted directly above the centre of the pool and was connected (via a VCR) to an image analyser (VP 200, HVS Image, UK) which digitised the image. The dark heads of hooded Lister rats provided a high-contrast image against the white-coloured water that could be tracked and quantified using HVS Water 2020 software (HVS Image, UK) and which provided measures including latency to reach the platform, length of path taken, swimming speed and time spent in pre-defined areas of the pool.
Male hooded Lister rats (250–350 g; Harlan, UK) received four trials per day for each of 8–10 days during which the submerged platform was placed in a different location each day but remained constant throughout the day. The maximum trial length was 60 s and if the rat had not located the platform, the trial ended automatically and the rat was placed on the platform. The rat remained on the platform for a 30-s inter-trial interval (ITI). At the end of the ITI, the rat was placed into the pool again but at a different location; and upon release, the next trial began. This procedure was repeated until four trials had been completed. On trial 1, the latency to find the platform is relatively long, but it is shorter on trials 2, 3 and 4, demonstrating that the animal’s memory for the platform location improves each time it escapes to the hidden platform. The improvement in the animal’s memory was quantified by subtracting the trial 2 latency from the trial 1 latency to give the savings score. The savings score from each day was used to calculate the ‘mean savings’ value across either the training phase or the compound-testing phase.
Prior to compound testing, animals were assigned to treatment groups in such a way as to ensure that the level of performance (using mean savings) during the training phase was not significantly different (i.e. groups were balanced according to baseline performance). The compound treatment phase lasted for 5 days and was identical to the methodology described above with the exception that a 4-h delay period was inserted between trial 1 and trial 2. On drug-testing days, animals received trials 3 and 4 at 30 s intervals after trial 2 to reinforce the rule that, within any day, the platform position is fixed, but that it changes position between days. However, data from trials 3 and 4 were not evaluated for the purposes of the present report.
Effect of α5IA-II on encoding
Rats received injections of either vehicle or α5IA-II (n=20/group) 0.5 h before trial 1, and then either vehicle or flumazenil was administered immediately, 1.5 and 3 h after trial 1. This dosing regime of flumazenil was based upon its rapid clearance and relatively transient receptor occupancy (see Fig. 3). There were four groups (n=10/group) which were vehicle/vehicle (control); α5IA-II/vehicle, to demonstrate the effects of α5IA-II on the acquisition (encoding) of the platform location during trial 1; α5IA-II/flumazenil, in which the effects of α5IA-II are blocked by flumazenil administration after trial 1 and during the consolidation period; and vehicle/flumazenil, which controls for any potential effects of flumazenil in the α5IA-II/flumazenil group.
Effect of α5IA-II on consolidation
Rats received injections of either vehicle or α5IA-II (n=20/group) immediately after trial 1, and then either vehicle or flumazenil was administered 0.25 h before trial 2. Thus, there were four groups (n=10/group) which were vehicle/vehicle (control); α5IA-II/vehicle, to show the potential effects of α5IA-II on the consolidation of the memory trace acquired during the acquisition stage; α5IA-II/flumazenil, in which the potential effects of any α5IA-II that remain 4.5 h after administration on recall are blocked by flumazenil; and vehicle/flumazenil, which controls for any potential effects of flumazenil in the α5IA-II/flumazenil group.
Effect of α5IA-II on recall
Rats received injections of either vehicle or α5IA-II (n=20/group) 0.5 h before trial 2, and then either vehicle or flumazenil was administered 0.25 h before trial 2. Thus, there were four groups (n=10/group) which were vehicle/vehicle (control); α5IA-II/vehicle, to show the potential effects of α5IA-II on the retrieval of the memory of the platform location; α5IA-II/flumazenil, in which the potential effects of α5IA-II on recall are blocked by flumazenil before the retrieval of the memory during trial 2; and vehicle/flumazenil, which controls for any potential effects of flumazenil in the α5IA-II/flumazenil group.
Effect of CDP on encoding, consolidation and recall
Vehicle was administered 0.5 h before trial 1 with CDP being given either 0.5 h before trial 1, immediately after trial 1 or 0.5 h preceding trial 2 to examine its effects on encoding, consolidation and recall, respectively.
Statistical analyses
Mean savings and mean swim speed during the compound testing phase were subjected to a one-way analysis of variance (ANOVA) with one between factor of treatment (four levels). Post hoc analysis was conducted using linear contrasts or Newman–Keuls multiple range analysis at the 95% confidence level.
Results
Intrinsic efficacy of α5IA-II
Figure 2 shows the efficacy profile of α5IA-II at different subtypes of human recombinant GABAA receptors. There was a marked reduction in the GABA EC20-evoked current at the α5 subtype with the maximum efficacy being −45% (i.e. the GABA EC20 current is reduced by almost half in the presence of α5IA-II; Table 1). In comparison, the non-selective full inverse agonist DMCM has an efficacy of between −53 and −71% at the different subtypes, with α5 efficacy being −57% (Dawson et al. 2006). Hence, α5IA-II has efficacy at the α5 subtype which approaches full inverse agonism. In contrast, the efficacy at the α1, α2 and α3 subtypes is much less, α5IA-II being essentially an antagonist at the α2 subtype (maximum efficacy=−7%) and a weak partial inverse agonist at the α1 and α3 subtypes (efficacy=−14 and −17%, respectively). The functional affinity of α5IA-II (i.e. the EC50) ranges between 2.5 and 5.6 nM (Table 1) and is consistent with the binding affinity of this compound (Ki values ranging from 0.8 to 2.7 nM; Table 1). Because the efficacy profile of α5IA-II is that of a predominantly α5-selective inverse agonist with weak partial inverse agonism at the α1, α2 and α3 subtypes, the in vivo effects are likely to be primarily related to inverse agonism at the α5 subtype.
Efficacy of α5IA-II at recombinant human GABAA stably expressed in mouse fibroblast L(tk−) cells. The effects of α5IA-II on the current produced by a submaximal concentration of GABA (equivalent to an EC20) were measured by whole-cell patch clamp electrophysiology, and α5IA-II attenuated the GABA-evoked current to a greater extent at α5- compared to α1-, α2- or α3-containing GABAA receptors. Values shown are mean±SEM (n=3–4/data point). Inset shows the structure of α5IA-II
Occupancy of α5IA-II and flumazenil
The occupancy at the benzodiazepine site of rat brain GABAA receptors, achieved by the doses of α5IA-II and flumazenil used in these experiments, was measured as the ability of both compounds to inhibit the binding of [3H]flumazenil in vivo. A dose of 1 mg/kg α5IA-II produced sustained, and high levels of occupancy with maximum occupancy (80±2%) being achieved within 15 min of dosing with 21±3% remaining occupied 4.5 h after dosing (Fig. 3). In contrast, the occupancy of flumazenil was much more transient; maximum occupancy (98±1%) was achieved within the first 15 min, but by 1 h post-administration, occupancy had dropped to 30±4%, and by 2 h, there was essentially no occupancy remaining (occupancy=2±2%).
Time course of occupancy of the benzodiazepine site of rat brain GABAA receptors by a α5IA-II or b flumazenil. Rats received either vehicle, α5IA-II (1 mg/kg i.p. in 70% PEG 300 vehicle) or flumazenil (10 mg/kg i.p. in 0.5% methylcellulose vehicle) and were killed at various times later with occupancy being measured using a [3H]flumazenil in vivo binding assay. Values shown are mean±SEM (n=6–8/group)
These data were used to establish the requirements for repeated administration of flumazenil during the 4-h ITI when blocking the effects of α5IA-II during consolidation and to give flumazenil 15 min before trial 2 to block the effects of α5IA-II on recall.
Effect of α5IA-II on encoding
Treatment groups balanced on the basis of mean savings were such that there were no significant differences between groups before the compound treatment phase [F(3,36)=0.00, P=0.999]. Levene’s test for equality of variances between groups reveals these to be non-significantly different [F(3,36)=0.29, P=0.83] showing an equal range of performance across the treatment groups (data not shown).
There was a main effect of treatment on the savings between trials 1 and 2 [F(3,36)=3.03, P<0.05] with post hoc linear contrasts showing (Fig. 4) that the mean savings of the α5IA-II/vehicle group and the α5IA-II/flumazenil group were significantly higher than those of the group treated with vehicle/vehicle (F(1)=5.29, P<0.05 and F(1)=4.65, P<0.05, respectively). There was no effect of treatment on mean swim speed during the compound testing phase [F(3,36)=0.05, P=0.982] (data not shown).
Effect of α5IA-II on the encoding phase of the delayed-matching-to-position version of the Morris water maze. The effects of α5IA-II on the mean savings (i.e. the increased ability to find the platform in trial 2 in the α5IA-II/veh. group) was not blocked by administration of flumazenil immediately after trial 1 and during the period before trial 2 (the α5IA-II/flumazenil group). Data shown are the mean±SEM (n=10/group). *Significantly different to performance in the veh./veh. group, P<0.05
In keeping with its behaviourally silent profile, post hoc analysis revealed that treatment with flumazenil in combination with prior vehicle treatment had no effects in any of the above measures.
Effect of α5IA-II on consolidation
Treatment groups balanced on the basis of mean savings were such that there were no significant differences between groups before the compound treatment phase [F(3,36)=0.00, P=0.999]. Levene’s test for equality of variances between groups reveals these to be non-significantly different [F(3,36)=0.29, P=0.83] showing an equal range of performance across the treatment groups (data not shown).
There was no effect of treatment on the savings between trials 1 and 2 (F(3,36)=0.06, P=0.979). Hence, when administered before the ‘consolidation’ phase (after trial 1), α5IA-II did not significantly increase mean savings compared to vehicle-treated animals (Fig. 5).
Effect of α5IA-II on the consolidation phase of the delayed-matching-to-position version of the Morris water maze. There was no effect of α5IA-II on the mean savings (i.e. the increased ability to find the platform in trial 2) when administered immediately after Trial 1. Data shown are the mean±SEM (n=10/group)
Effect of α5IA-II on recall
Treatment groups balanced on the basis of mean savings were such that there were no significant differences between groups before the compound treatment phase [F(3,36)=0.01, P=0.999]. Levene’s test for equality of variances between groups reveals these to be non-significantly different [F(3,36)=0.31, P=0.816] showing an equal range of performance across the treatment groups (data not shown).
Figure 6 shows that there was a main effect of treatment (F(3,36)=2.92, P<0.05) with post hoc linear contrasts revealing that mean savings of the α5IA-II/vehicle group were significantly higher than those of the vehicle/vehicle group (F(1)=5.87, P<0.05), suggesting that α5IA-II can improve performance due to an effect on recall and that this effect could be blocked by flumazenil since the α5IA-II/flumazenil group showed no such enhanced performance.
Effect of α5IA-II on the recall phase of the delayed-matching-to-position version of the Morris water maze. When given before trial 2, α5IA-II enhanced the ability to find the platform in trial 2 (α5IA-II/veh. group) and this effect could be blocked by administration of flumazenil subsequent to α5IA-II administration but before trial 2. Data shown are the mean±SEM (n=10/group). *Significantly different to performance in the veh./veh. group, P<0.05
Effect of CDP on encoding, consolidation and recall
Treatment groups balanced on the basis of mean savings were such that there were no significant differences between groups before the compound treatment phase [F(3,36)=0.0, P=1.0]. Levene’s test for equality of variances between groups reveals these to be non-significantly different [F(3,36)=0.44, P=0.727] showing an equal range of performance across the treatment groups (data not shown).
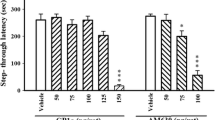
Figure 7 shows that pretreatment with CDP had marked effects on the performance of animals in the water maze DMTP task. During the compound testing phase, there was a main effect of treatment [F(3,36)=3.36, P<0.05]. Linear contrasts revealed that CDP had significant detrimental effects on savings when it was administered before trial 1 (encoding) [F(1)=4.15, P<0.05] and when given before trial 2 (recall) [F(1)=6.33, P<0.05]. There was no effect of CDP on savings when administered immediately after trial 1 (consolidation). There was no effect of CDP on mean swim speed during the compound testing phase [F(3,36)=0.4, P=0.752] (data not shown).
Effect of chlordiazepoxide (CDP) on the encoding, consolidation and recall phases of the delayed-matching-to-position version of the Morris water maze. When given before either trial 1 (Encoding) or trial 2 (Recall), CDP impaired the ability to find the platform in trial 2, indicating an effect on encoding and recall. However, when given after trial 1, CDP had no effect, suggesting that it plays no role during consolidation (Consolid.). Data shown are the mean±SEM (n=10/group). *Significantly different to performance in the veh. group, P<0.05
Discussion
α5 subunit-containing GABAA receptors are limited in their distribution in the brain and are most prominently located within the hippocampus (Wisden et al. 1992; Fritschy and Möhler 1995; Sur et al. 1999; Sieghart and Sperk 2002). Because the hippocampus has been suggested to play a key role in learning and memory, especially in relation to spatial information (Morris et al. 1982; Milner et al. 1998), it has been hypothesised that the α5 subtype of GABAA receptors plays a role in normal, hippocampally mediated cognitive functions (Maubach 2003), as well as in the amnestic effects of benzodiazepines (Dawson et al. 2006). Consistent with that notion, reduced expression of α5-containing receptors facilitated trace fear conditioning (Crestani et al. 2002), whereas deletion of the α5 subunit resulted in an improved performance in a spatial learning task that requires intact hippocampal function but did not affect learning that occurs independently of the hippocampus (Collinson et al. 2002).
The fact that loss of α5-containing GABAA receptors actually enhanced performance in the DMTP version of the water maze suggested that a compound which reduced the function of this receptor subtype (i.e. an inverse agonist) should enhance performance in this assay (Collinson et al. 2002). This idea found support in that two structurally diverse compounds (a triazolophthalazine and a pyrazolotriazine) with selective inverse agonist properties at α5-containing receptors both enhanced the performance of normal rats in this assay (Chambers et al. 2004; Dawson et al. 2006). In the present study, we employed a compound, α5IA-II, that is very similar in structure to the triazolophthalazine α5IA (Sternfeld et al. 2004; Street et al. 2004), to examine at which stage in the learning and memory process such compounds exert their effects. α5IA-II has an efficacy profile very similar to that of α5IA (Fig. 2) and an affinity (0.8–2.7 nM, depending on subtype) only slightly lower than that of its structural analogue (0.6–0.9 nM; Dawson et al. 2006). It occupies the benzodiazepine site of rat brain GABAA receptors well and with a potency (occupancy 0.5 h after 1 mg/kg dose of 76%; Fig. 3) that is comparable to that seen in mice (90%; Stephens et al. 2005).
Moreover, this occupancy is relatively sustained such that 4.5 h after dosing, the compound occupies 21% of GABAA receptors. Hence, when α5IA-II is dosed 0.5 h before trial 1, it has the potential to have effects throughout the 4-h consolidation period before recall and, indeed, during the recall phase itself. Therefore, to prevent α5IA-II having continued effects at different stages of the learning and memory process, the benzodiazepine site antagonist, flumazenil, was employed to block the effects of α5IA-II during these latter stages. However, because flumazenil is rapidly cleared, its occupancy is only relatively short lived (Fig. 3) and three separate doses were required to block the effects of α5IA-II during the 4-h period between trials 1 and 2 (Fig. 4).
The key results from the present series of experiments were that α5IA-II affects encoding and recall but does not affect the consolidation phases of performance in the DMTP version of the Morris water maze. With respect to encoding, it is striking that the effects of α5IA-II were not affected by the administration of flumazenil immediately after completion of trial 1 (Fig. 4). This suggests that the memory trace is produced quickly, either during the swim trial (up to 60 s) plus 30 s ITI on the hidden platform, or during the short time required for flumazenil to displace α5IA-II from its binding sites within the brain. That the memory trace may be rapidly acquired is further indirectly supported by the observation that administration of α5IA-II immediately after trial 1 (Fig. 5) has no effect on performance.
Consistent with the α5-selective inverse agonist improving subsequent performance when it was given during the initial encoding, the non-selective benzodiazepine site agonist CDP given just before initial acquisition impaired the rats in their subsequent performance during recall. Although we cannot be sure whether the action of CDP in this experiment is attributable to the effects on acquisition, rather than on consolidation or recall, the fact that when CDP was given immediately after the acquisition phase with no effects on subsequent recall suggests strongly that it did, indeed, block acquisition. These effects are unlikely to be due to motor impairment because CDP was without effects on mean swimming speed throughout the testing period (data not shown). The literature from human studies of the mnemonic effects of benzodiazepines indicates that when given after acquisition, benzodiazepines may facilitate memories of the events immediately before drug treatment (Ghoneim et al. 1984), presumably because they block the formation of memory for events experienced under the drug, so that these events no longer interfere with memory for events experienced immediately before drug administration (Hinrichs et al. 1984). That we saw no evidence in the present experiments that administration of CDP after acquisition exerted these kinds of retrograde facilitatory effects may be attributable to the familiarity of the home environment experienced after acquisition, which would be expected to make minimal demands on memory processing.
The data suggesting that an α5-selective inverse agonist enhances and a non-selective benzodiazepine site agonist (CDP) impairs performance when given during the recall phase are somewhat surprising because clinically, benzodiazepine agonists have been reported not to impair the retrieval (recall) of previously acquired information (Ghoneim and Mewaldt 1990). This raises the possibility that either the process of learning and memory is different in rats and man or, alternatively, that the so-called encoding, consolidation and recall stages of the water maze paradigm differ from the analogous phases in tests of human memory. In this latter regard, it may therefore be necessary to reconsider what the terms ‘consolidation’ and ‘recall’ represent in the present paradigm.
If the standard consolidation theory is applied to the present observations, one could question the validity of the term ‘consolidation’ in the water maze assay. Thus, it could be argued that the 4-h period between acquisition and recall in the water maze DMTP task is not long enough for full consolidation to take place and that information learned during the encoding phase has not been transferred to the neo-cortex, and hence become independent of the hippocampus (i.e. has not become “consolidated” in the classical sense). Indeed, the existence of temporally graded retrograde amnesia suggests that consolidation is a slow, gradual process with memory becoming ‘fixed’ as time passes and it becoming independent of the hippocampus (Squire and Alvarez 1995), presumably as a consequence of protein synthesis (Goelet et al. 1986; Davis and Squire 1984; Schafe and LeDoux 2000), although the extent to which these memory traces are ‘fixed’ is the subject of much debate (Nadel and Moscovitch 1997, 1998; Nader et al. 2000; Nadel and Land 2000).
It might be argued that the findings reported in this study are specific to the Morris water maze paradigm. However, α5IA-II has been shown to facilitate the performance of rats in the hippocampus-dependent contextual conditioning paradigm (Cobain 1999, unpublished data). Furthermore, α5IA—a compound structurally related to α5IA-II, has been reported to improve performance in contextual conditioning (Dawson et al. 2006). Although neither of these studies investigated the encoding, consolidation and recall processes individually, these findings suggest that the effects of α5IA-II from the present study are not specific to the Morris water maze. Further investigation of the effects of α5IA-II on encoding, consolidation and recall processes specific to contextual conditioning would clearly be of interest in light of the present findings.
Based on the preferential localization of the α5 subtype in the hippocampus, the effect of α5IA-II on recall is assumed to be hippocampally mediated. However, it should be recognised that α5-containing GABAA receptors are also found in the neo-cortex, albeit as much lower levels of expression than in the hippocampus (Sur et al. 1999). As a result, it is possible that the effects on recall of α5IA-II may be an effect on retrieval of consolidated information from the neo-cortex. Nevertheless, the most parsimonious explanation for the effects of α5IA-II on recall is that they are related to the hippocampus. With this in mind, it could be predicted that compounds such as α5IA-II may be less useful in facilitating recall of well learned, fully consolidated (i.e. neo-cortically located) material, but would be effective in facilitating recall of weak or labile, hippocampally mediated memories.
References
Abel T, Lattal KM (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11:180–187
Argyropoulos SV, Nutt DJ (1999) The use of benzodiazepines in anxiety and other disorders. Eur Neuropsychopharmacol 9(Suppl 6):S391–S392
Atack JR (2005) The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs 14:601–618
Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford K, McKernan RM, Dawson GR (2005) Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Br J Pharmacol 144:357–366
Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal S, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro J-L, Whiting P, Dawson GR, McKernan RM (2006) TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a non-sedating anxiolytic in rodents and primates. J Pharmacol Exp Ther 316:410–422
Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136:965–974
Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O’Connor D, Marshall G, Rycroft W, Macleod AM (2004) An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA α5 receptors with cognition enhancing properties. J Med Chem 47:5829–5832
Cobain MR (1999) The localization and function of the alpha5 subunit containing GABAA receptors in the rat. PhD thesis, Downing College, Cambridge
Collinson N, Kuenzi F, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PW, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci 22:5572–5580
Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A 99:8980–8985
Davis HP, Squire LR (1984) Protein synthesis and memory: a review. Psychol Bull 96:518–559
Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR (2006) An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther 316:1335–1345
Dias R, Sheppard WFA, Fradley RL, Garrett EM, Stanley JL, Marshall GR, Goodacre S, Lincoln RJ, Tye SJ, Cook S, Conley R, Hallett D, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan R, Dawson GR, Reynolds DS (2005) Anxiolytic properties of benzodiazepines are mediated through the GABAA α3 receptor subtype. J Neurosci 25:10682–10688
Dorow R, Horowsli R, Paschelke G, Amin M, Braestrup C (1983) Severe anxiety induced by FG 7142, a β-carboline ligand for benzodiazepine receptors. Lancet 2:98–99
Duka T, Curran HV, Rusted JM, Weingartner HJ (1996a) Perspectives on cognitive psychopharmacology research. Behav Pharmacol 7:401–410
Duka T, Ott H, Rohloff A, Voet B (1996b) The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills. Psychopharmacology (Berl) 123:361–373
Fritschy JM, Möhler H (1995) GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194
Ghoneim MM, Mewaldt SP (1975) Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacologia 44:257–262
Ghoneim MM, Mewaldt SP (1990) Benzodiazepines and human memory: a review. Anesthesiology 72:926–938
Ghoneim MM, Hinrichs JV, Mewaldt SP (1984) Dose response analysis of the behavioral effects of diazepam: I. Learning and memory. Psychopharmacology (Berl) 82:291–295
Goelet P, Castellucci VF, Schacher S, Kandel ER (1986) The long and short of long-term memory—a molecular framework. Nature 322:419–422
Hinrichs JV, Ghoneim MM, Mewaldt SP (1984) Diazepam and memory: retrograde facilitation produced by interference reduction. Psychopharmacology (Berl) 84:158–162
Jensen LH, Stephens DN, Sarter M, Petersen EN (1987) Bidirectional effects of β-carbolines and benzodiazepines on cognitive processes. Brain Res Bull 19:359–364
Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U (2000) Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290:131–134
Maubach K (2003) GABAA receptor subtype selective cognition enhancers. Curr Drug Target CNS Neurol Disord 2:233–239
McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garret L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci 3:587–592
McNamara RK, Skelton RW (1993) Effects of intracranial infusions of chlordiazepoxide on spatial learning in the Morris water maze. I. Neuroanatomical specificity. Behav Brain Res 59:175–191
McNaughton N, Morris RGM (1987) Chlordiazepoxide, an anxiolytic benzodiazepine impairs place navigation in rats. Behav Brain Res 24:39–46
Milner B, Squire LR, Kandel ER (1998) Cognitive neuroscience and the study of memory. Neuron 20:445–468
Minier F, Sigel E (2004) Techniques: use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci 25:499–503
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Nadel L, Land C (2000) Memory traces revisited. Nat Rev Neurosci 1:209–212
Nadel L, Moscovitch M (1997) Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7:217–227
Nadel L, Moscovitch M (1998) Hippocampal contributions to cortical plasticity. Neuropharmacology 37:431–439
Nader K, Schafe GE, LeDoux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726
Petersen EN (1983) DMCM: a potent convulsive benzodiazepine receptor ligand. Eur J Pharmacol 94:117–124
Riedel G, Micheau J, Lam AG, Roloff E, Martin SJ, Bridge H, Hoz L, Poeschel B, McCulloch J, Morris RG (1999) Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2:898–905
Rudolph U, Möhler H (2004) Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 44:475–498
Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H (1999) Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401:796–800
Sarter M, Stephens DN (1988) β-carbolines as tools in memory research: animal data and speculations. Psychopharmacol Ser 6:230–245
Schafe GE, LeDoux JE (2000) Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20:RC96, 1–5
Sieghart W (1995) Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47:181–234
Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2:795–816
Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA (2004) Analysis of the set of GABAA receptor genes in the human genome. J Biol Chem 279:41422–41435
Squire LR, Alvarez P (1995) Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5:169–177
Stephens DN, Schneider HH, Kehr W, Jensen LH, Petersen E, Honore T (1987) Modulation of anxiety by beta-carbolines and other benzodiazepine receptor ligands: relationship of pharmacological to biochemical measures of efficacy. Brain Res Bull 19:309–318
Stephens DN, Pistovkacova J, Worthing L, Atack JR, Dawson GR (2005) Role of GABAA α5-containing receptors in ethanol reward in the mouse: the effects of targeted gene deletion and a selective inverse agonist. Eur J Pharmacol 526:240–250
Sternfeld F, Carling RW, Jelley RA, Ladduwahetty T, Merchant KJ, Moore KW, Reeve AJ, Street LJ, O’Connor D, Sohal B, Atack JR, Cook S, Seabrook GR, Wafford KA, Tattersall FD, Collinson N, Dawson GR, Castro JL, MacLeod AM (2004) Selective, orally active γ-aminobutyric acidA α5 receptor inverse agonists as cognition enhancers. J Med Chem 47:2176–2179
Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, McKernan RM, Sohal B, Cook S, Pike A, Dawson GR, Bromidge FA, Wafford KA, Seabrook GR, Thompson SA, Marshall G, Pillai GV, Castro JL, Atack JR, MacLeod AM (2004) Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazines and analogues as subtype-selective inverse agonists for the GABAAα5 benzodiazepine binding site. J Med Chem 47:3642–3657
Sur C, Fresu L, Howell O, McKernan RM, Atack JR (1999) Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res 822:265–270
Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, Rossier J (1986) Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature 321:864–866
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collinson, N., Atack, J.R., Laughton, P. et al. An inverse agonist selective for α5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology 188, 619–628 (2006). https://doi.org/10.1007/s00213-006-0361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0361-z