Abstract
The chronic, excessive consumption of alcohol results in significant modification of selective neural systems of the brain structure, physiology, and function. Quantitative MR structural imaging, diffusion tensor imaging (DTI), and functional MRI (fMRI), together with neuropsychological challenges, have enabled rigorous in vivo characterization of the results of alcoholism on the brain in the human condition. Neuroimaging has also enabled longitudinal study for the examination of alcoholism’s dynamic course through periods of drinking and sobriety. Controlled studies have revealed compelling evidence for alcohol-related brain structural and functional modification—some longstanding, some transient, and some compensatory. Patterns of circuitry disruption identified through structural and functional MRI studies suggest a central role for degradation of frontocerebellar neuronal nodes and connecting circuitry affecting widespread brain regions and contributing to alcoholism’s salient, enduring, and debilitating cognitive and motor deficits—executive dysfunction, visuospatial impairment, and ataxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A salient characteristic of alcohol use disorders is the consumption of dangerous quantities of alcohol despite knowledge of the adverse effects of such behavior. The development of tolerance and dependence marks change in brain structure, function, and basic physiology. Our studies of the persistent brain structural and behavioral changes manifest by even well-detoxified alcoholic individuals attest to the importance of these alcoholism-induced brain insults to which the alcoholic must adapt. This neuroadaptation allows for some restoration of function with sobriety in a damaged brain, albeit at the expense of functional reserve and plasticity, and may contribute to the self-sustaining nature of alcoholism.
Here, we review evidence for alcoholism-related brain structural and functional modification—some longstanding, some transient, and some compensatory—from our quantitative in vivo MRI and neuropsychological studies of detoxified alcoholic men and women. Identified patterns of circuitry disruption from structural and functional studies suggest a central role for degradation of frontocerebellar neuronal nodes and connecting circuitry affecting widespread brain regions and contributing to the cognitive and motor sequelae of alcoholism. Descriptions of the MR imaging methods and quantification approaches considered herein are presented elsewhere in didactic reviews (Adalsteinsson et al. 2002; Pfefferbaum and Sullivan 2005b; Rosenbloom et al. 2003).
In vivo evidence for brain macrostructural compromise in chronic alcoholism
Cross-sectional studies
Depending on age, the brain of the detoxified alcoholic can appear as ravaged as that of a patient with Alzheimer’s disease, although the behavioral and neuropsychological consequences are typically far less severe and the brain dysmorphology is at least partially reversible. Both cortical gray matter (Fein et al. 2002; Jernigan et al. 1991) and white matter sustain widespread volume loss (Pfefferbaum et al. 1992), which is greatest in the prefrontal cortex and prefrontal and frontal white matter (Kubota et al. 2001) in older alcoholics (Pfefferbaum et al. 1997). In most brain regions and structures examined, we have observed an age–alcoholism interaction, where older alcoholics have greater volume shrinkage for their age than younger alcoholics. In one study, this interaction was shown to be independent of the possibility that older alcoholics had the opportunity to drink more alcohol for a longer time than younger alcoholics (Pfefferbaum et al. 1992).
In addition to the cortex, subcortical and brainstem structures are affected in uncomplicated alcoholism, that is, in alcoholics free of the severe syndromes, arising from alcohol-associated nutritional deficiencies or electrolytic imbalance. These clinically dramatic conditions include Marchiafava–Bignami disease, which primarily affects the corpus callosum and results in a disconnection syndrome; central pontine myelinolysis, which affects myelin in central pons and can cause paraplegia; and alcoholic cerebellar degeneration, which results in severe ataxia of gait and posture. In contrast to these clinically-defined conditions with characteristic radiological signs, quantitative analysis of MRI has been required to identify brain structural and functional deficits in uncomplicated alcoholics. Nonetheless, the brain structures affected in uncomplicated alcoholics are the same as those affected in these clinical syndromes (albeit to a lesser degree) and include the corpus callosum (Estruch et al. 1997; Hommer et al. 1996; Pfefferbaum et al. 1996), pons (Sullivan et al. in press; Sullivan and Pfefferbaum 2001), cerebellar hemispheres, and vermis (Sullivan et al. 2000a). Perhaps the most studied alcohol-related nutritional deficiency condition is Korsakoff’s syndrome (Victor et al. 1989), which is commonly heralded by Wernicke’s encephalopathy (WE) and resolves to render victims with global amnesia (Butters and Cermak 1980). The neuropathological lesions marking WE are in thalamus, mammillary bodies, and inferior colliculus. In addition to these sites of pathology, KS patients commonly have cerebellar involvement. The amnesia of KS has traditionally been attributed to lesions of the thalamus and mammillary bodies (Squire et al. 1990), but more recently was related to hippocampal volume loss (Sullivan and Marsh 2003) and may even involve the cholinergically rich medial septum/diagonal band (Sullivan et al. 2004a).
MRI studies have also revealed temporal lobe white matter deficits (Sullivan et al. 1996), particularly in patients with a history of seizure; significant mammillary body (Davila et al. 1994; Shear et al. 1996; Sullivan et al. 1999) and anterior hippocampal (Sullivan et al. 1995) shrinkage in amnesic and nonamnesic alcoholics; and a higher incidence of substantial white matter hyperintensities than matched controls (Gallucci et al. 1989; Jernigan et al. 1991; but see Pfefferbaum et al. 1992, 2005b; Pfefferbaum and Sullivan 2005a). Volume shrinkage in thalamus (Sullivan et al. 2003b), caudate, and putamen (Sullivan et al. in press) occurs in alcoholics. Of particular relevance to the identification of neural substrates of addiction, we have observed significant volume shrinkage in the nucleus accumbens of recently drinking alcoholics relative to ones who had been sober for at least a month (Sullivan et al. in press), and the volume shrinkage is even greater than that observed in KS patients (Sullivan et al. 2004a).
Both alcoholic men and women are vulnerable to alcoholism’s untoward effect on brain structure, although the sex-related pattern of brain structural involvement is controversial. For example, one series of studies reported more severe ventricular expansion and tissue volume deficits in cortex (Hommer et al. 2001), corpus callosum (Hommer et al. 1996), and hippocampus (Agartz et al. 1999) in alcoholic women than men. By contrast, another series of studies revealed virtually the opposite pattern, where men had greater abnormalities than women in size of the lateral ventricles, cortical gray matter (Pfefferbaum et al. 2001b) and white matter, corpus callosum, and pons (Pfefferbaum et al. 2002).
In vivo MRI studies are consistent with the postmortem studies of gross brain morphology, which report white matter abnormalities in brainstem and subcortical structures, including the cerebellar vermis (Phillips et al. 1987), mammillary bodies (Alling and Bostrom 1980), hippocampus (Harding et al. 1997), and corpus callosum, which thins (Harper and Kril 1988) and atrophies (Tarnowska-Dziduszko et al. 1995). Although white matter pathology is reported more often than gray matter pathology, neuronal loss does occur but is restricted to the superior frontal cortex (Harper and Kril 1989; Harper et al. 1987; Kril et al. 1997).
Longitudinal studies
Our controlled longitudinal MRI studies of alcoholics in recovery or relapse have revealed that with short-term (about 1 month) abstinence from alcohol cortical gray matter increases in volume. With longer-term abstinence (about 1 year), the third ventricle shrinks, but with relapse it expands and white matter shrinks (Pfefferbaum et al. 1995). Over a 5-year interval, the degree of excessive drinking in alcoholics is related to the degree of cortical gray matter loss, especially in the frontal lobes (Pfefferbaum et al. 1998). Although the amount of alcohol consumed over a lifetime has not usually been a reliable predictor of quantitative measures of the condition of the brain, estimations of recency and frequency of heavy drinking have served as better indicators (e.g., Parsons et al. 1987). In support of the recency–frequency index, we observed that the more alcohol consumed during a 5-year period of relapse, the greater the cortical gray matter volume loss alcoholics sustained (Pfefferbaum et al. 1998). Additional studies suggest that cortical white matter volume may be particularly amenable to recovery with abstinence (O’Neill et al. 2001; Shear et al. 1994) or vulnerable to further decline with continued drinking (Pfefferbaum et al. 1995). Possible dehydration or rehydration of white matter tissue, particularly to account for volume changes with abstinence, has been considered but has received little support (Harper et al. 1988; Mann et al. 1992, 1995).
Although the mechanism for either volume loss or restoration with abstinence remains unclear, it probably involves changes in both myelination and axonal integrity in white matter and glial and dendritic changes in cortical neuropil. MR spectroscopy (MRS) (Adalsteinsson et al. 2002) provides an in vivo method to quantifying brain biomarkers of factors (e.g., Martin et al. 1995; Meyerhoff et al. 2004; Seitz et al. 1999), including neuronal integrity (N-acetyl aspartate, NAA), glial activity (myo-Inositol, mI), and cell membrane turnover (choline, Cho). Longitudinal studies using MRS have revealed significant increases in NAA in selective brain regions, providing quantitative, within-subject biochemical evidence for improvement in the condition of neuronal integrity (Parks et al. 2002; but see O’Neill et al. 2001).
Observed brain structural and biochemical recovery is testimony to the neuronal regenerative possibilities (cf. Nixon and Crews 2002 and 2004), and improvement in cognitive and motor abilities with abstinence from alcohol (reviewed below) supports the functional value of such regeneration. Indeed, a growing number of longitudinal neuropsychological studies report significantly better scores on tests of working memory, visuospatial abilities, and gait and balance with abstinence from alcohol and further decline or lack of improvement with resumption of drinking. Some components of these functional domains recover faster (Rosenbloom et al. 2004) or more fully than others (e.g., Becker et al. 1983; Brandt et al. 1983; Mann et al. 1999; Nixon and Glenn 1995; Parsons et al. 1987; Sullivan et al. 2000c), but at least a measurable degree of recovery typically accompanies prolonged sobriety.
In vivo evidence for brain microstructural compromise in chronic alcoholism
Structural MRI enables quantification of bulk volume and some aspects of tissue quality of brain but does not directly assess the microstructure of brain tissue and its components, such as axons, microtubules, and myelin. By contrast, diffusion tensor imaging (DTI) is especially suited for the study of white matter and can reveal quantitative information about its microstructural integrity through examination of coherence and connectivity of white matter tracts (Moseley et al. 1990; for reviews on the method and application in human studies, see Kubicki et al. 2002; Le Bihan 2003; Lim and Helpern 2002; Pfefferbaum and Sullivan 2005b; Rosenbloom et al. 2003; Sullivan and Pfefferbaum 2003).
Diffusion tensor imaging
The intrinsic properties of water diffusion in human brain tissue can provide information about the structural characteristics of the tissue. The diffusion of water molecules in tissue with unconstrained microstructure (e.g., CSF) is characterized by Brownian motion. The resultant molecular displacement has a gaussian distribution, and the diffusion is, therefore, “isotropic”—the molecules move equally in all directions. In tissue with a regular and orderly microstructure, such as brain white matter, the water molecules behave in a more constrained fashion with a preponderant motion in a given orientation; the diffusion is “anisotropic.” Tissue can also be characterized in terms of the amount of its water diffusion, expressed as “diffusivity.”
White matter is highly organized in fiber bundles, which restrict diffusion of water, and the orientation of the diffusion depends on the orientation of the specific fiber tracts observed (Waxman et al. 1995). The axon’s cytoskeleton consists of axoplasm and organelles, including neurofilaments, mitochondria, and microtubules. The gross morphology of axons resembles bundles of cable wiring organized as fasciculi, commissures, and fibers that link brain regions. With trauma or disease, the cytoskeleton, including the linear orientation of neurofilaments that lends to high anisotropy in healthy white matter, can be perturbed and result in diminished anisotropy (Arfanakis et al. 2002) and reactive glia can form (Sandvig et al. 2004). In addition to intracellular space, extracellular spaces between fibers sequester fluid and provide an avenue for water movement in white matter, contributing to anisotropic diffusivity.
Anisotropy is calculated on a within-voxel basis and is commonly expressed as a percent or fraction, i.e., fractional anisotropy (FA) (Pierpaoli and Basser 1996). FA of CSF is near 0 and can approach 1 in the corpus callosum. Some regions of white matter normally have considerably lower FA than others, even though they are fully volumed, that probably represents architectural differences in fiber track organization at the intravoxel level, i.e., intact fibers crossing within an image voxel. Processes that cause changes at the microstructural level, such as demyelination or aberrant accumulation of interstitial fluid, can cause a significant measurable decrease in FA of white matter.
White matter in normal aging and alcoholism
White matter in normal aging and alcoholism
To determine the effects of alcoholism on the brain, the effects of normal aging must first be characterized because the possibility of the interaction of age and alcoholism occurring at the microstructural level. Several DTI studies have demonstrated variability of white matter anisotropy across brain regions in healthy individuals, depending on the linearity and homogeneity of the local fiber structure (Pfefferbaum et al. 2005c in press; Pierpaoli et al. 2001; Virta et al. 1999). Normal aging and certain neurological and psychiatric conditions also modulate regional FA and diffusivity (for reviews, see, Kubicki et al. 2002; Moseley 2003; Pfefferbaum and Sullivan 2005b; Sullivan and Pfefferbaum 2003). A series of studies by Peters and colleagues in monkey models of aging revealed cross-sectional evidence for fluctuations in the condition of myelin, including increase in number of myelin lamellae with age (Peters et al. 2001), occasional splits in the myelin sheaths permitting formation of blisters of fluid (Peters and Sethares 2002), age-related lysing of the myelin sheath, and abnormally thin sheaths of myelin, which is a sign of remyelination (Peters and Sethares 2003). DTI is likely to provide sensitive in vivo markers of such age-related, degenerative, and restorative alterations in the structural integrity of axonal constituents.
Our initial studies using DTI in uncomplicated alcoholism reported abnormally low anisotropy in regionally defined white matter of alcoholic men (Pfefferbaum et al. 2000) and women (Pfefferbaum and Sullivan 2002). Both men and women show deficits in the callosal genu and centrum semiovale, and men showed additional deficits in the callosal splenium; pericallosal white matter is also affected (Pfefferbaum et al. 2000). In women, the white matter abnormality identified with DTI went undetected with structural MRI (Pfefferbaum et al. 2002; Pfefferbaum and Sullivan 2002). Figure 1 provides examples of averaged DTI images of anisotropy (FA) and bulk mean diffusivity (〈D〉) in alcoholics and controls.
Averaged images at two levels in 15 alcoholics and 19 controls for FA (top) and bulk mean diffusivity (bottom). Taken from Pfefferbaum and Sullivan (2005a)
Age–alcoholism interactions have been observed in callosal macrostructure, postmortem (Wiggins et al. 1988) and in vivo (Pfefferbaum et al. 1996). Recently we showed an age–alcoholism interaction in both FA and diffusivity, where older alcoholics had greater abnormalities for their age than younger ones (Pfefferbaum et al. 2005b). Diffusivity, which is strikingly higher in alcoholic men and women than controls and shows regionally nonspecific, substantial correlations, provides additional clues for explaining the alcohol-related erosion of white matter constituents. Alcoholism disrupts cytoskeletal integrity (Harper 1998; Putzke et al. 1998) and may reduce intracellular structural complexity so that it is less obstructive to water movement. Given low anisotropy and high diffusivity, we have speculated that intravoxel diffusivity in alcoholism arises from both intracellular and extracellular compartments (Pfefferbaum and Sullivan 2005a).
As with the MRI studies of the brain’s macrostructure, in vivo DTI studies of white matter’s microstructure in aging and alcoholism are consistent with postmortem findings (Kemper 1994). Small connecting fibers of the anterior corpus callosum are especially vulnerable in aging and likely contribute to deficits in cognitive processes dependent on prefrontal circuitry (Craik et al. 1990; Gunning-Dixon and Raz 2003; Raz 1999). Degradation of myelin and microtubules, and even axon deletion, also accompany normal aging (Aboitiz et al. 1996; Meier-Ruge et al. 1992). Brain white matter is especially affected in alcoholism (De la Monte 1988; Harper et al. 2003) in both sexes (Harper et al. 1990). In addition, callosal and other supratentorial white matter sustains demyelination (Lewohl et al. 2000; Tarnowska-Dziduszko et al. 1995), microtubule disruption (De la Monte 1988; Mayfield et al. 2002; Paula-Barbosa and Tavares 1985; Putzke et al. 1998; Wiggins et al. 1988), and axonal deletion, possibly arising from regional neuronal loss (Alling and Bostrom 1980; Badsberg-Jensen and Pakkenberg 1993; Courville 1955; De la Monte 1988; Harper and Kril 1991, 1993; Kril et al. 1997; Lancaster 1993).
Functional concomitants of alcoholism: implications for neural circuitry disruption
A significant percentage of recovering chronic alcoholics exhibit mild to moderate, transient to enduring deficits in complex cognitive processes. Typically, the processes affected are visuospatial abilities, executive functions, and gait and balance (for reviews, see Fein et al. 1990; Moselhy et al. 2001; Oscar-Berman 2000; Sullivan 2000), evidenced in both alcoholic men (Sullivan et al. 2000d) and women (Sullivan et al. 2002b). The executive functions affected include working memory, problem solving, temporal ordering, response inhibition, and psychomotor speed (Moselhy et al. 2001; Nixon et al. 2002; Oscar-Berman and Hutner 1993; Sullivan 2000). Sobriety can effectively reverse or at least reduce impairment in visuospatial abilities, gait and balance, and selective executive functions, but difficulties in components of working memory (Brandt et al. 1983; Parsons 1983; Sullivan et al. 2000c) and postural stability can linger, the latter especially in women (Rosenbloom et al. 2004).
Brain structure–function correlations have been difficult to establish using volumetric MRI measures of brain structures. Even attempts to correlate selective component processes of executive functions with prefrontal volumes in alcoholics have been largely unsuccessful, despite consistently observed significant behavioral deficits and volume deficits in the target variables. A few correlations based on brain structural volumes, however, have been forthcoming, and most were based on relationships with sensory or motor functions that may be indicative of the more focal nature of such processes relative to cognitive processes, which draw on multiple brain regions for successful performance. In particular, olfactory discrimination ability was correlated with thalamic volumes in one study (Shear et al. 1992), and two other studies showed that postural stability correlated selectively with anterior superior cerebellar vermian volumes (Sullivan et al. 2000a, Butler et al. 2005).
The brain structural mechanism underlying alcohol-related cognitive compromise may arise from degradation of selective neural circuitry rather than frank lesions or complete disconnections (Estruch et al. 1997; Sullivan 2003). That is, performance may be impaired by disruption, rather than severing, of white matter tracts providing connectivity between cortical sites. The white matter systems substrate of cognitive deficits is particularly relevant in individuals with an impaired, but not lost, function. Supporting this concept, we have shown that DTI measures have functional relevance in alcoholism. For example, the brain microstructure–function relationships have comported with the systematic organization of the corpus callosum with its topographically compartmentalized tracts, where the genu connects lateralized frontal sites and the splenium connects lateralized parietal and occipital sites (de Lacoste et al. 1985; Purves and Seltzer 1986). In two separate samples of alcoholic men and a sample of alcoholic women (Pfefferbaum et al. 2000, in review, 2005b; Pfefferbaum and Sullivan 2002), we observed that the level of performance on tests of attention, working memory, and visuospatial ability related selectively to regional microstructural integrity of the corpus callosum in alcoholics. In one study (Pfefferbaum et al., in review, 2005b), we calculated a working memory composite score based on Backward Digit Span and Block Spans from the Wechsler Memory Scale-Revised (Wechsler 1987) and Trail Making Part B (Lezak 1995), which was hypothesized to be related to DTI measures of frontal white matter integrity, and also assessed visuospatial ability with the Matrix Reasoning subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999), performance on which is selectively impaired by lesions of the parietal cortex (Villa et al. 1990). A series of multiple regression analyses identified a double dissociation in alcoholics: low scores on the working memory composite correlated with high diffusivity (i.e., increase freely moving water molecules) in the genu, whereas low scores on matrix reasoning correlated with high diffusivity in the splenium.
Another source of evidence for the importance of circuitry to performance by alcoholics derives from tasks requiring callosal transfer (Schulte et al. in press), whether for tasks naturally requiring processing contributions from both hemispheres or for tasks demanding bihemispheric processing because of neurological compromise. Regarding the first instance, cognitive and motor tasks typically invoke multiple functions of both hemispheres and require callosal integrity for interhemispheric information exchange (Ellis and Oscar-Berman 1989; Schulte et al. 2003). In groups of controls and alcoholics, we tested whether DTI indices of callosal integrity would predict reaction time measures of interhemispheric processing. The paradigms used were the crossed–uncrossed difference (CUD), testing visuomotor interhemispheric transfer, and the redundant targets effect (RTE), testing parallel processing of visual information by each cerebral hemisphere; a large CUD and a small RTE each is indicative of slower interhemispheric transfer. We found that in controls a large CUD correlated with low FA and high diffusivity in the genu and splenium, and that in alcoholics, a small RTE correlated with low FA in genu and splenium and high diffusivity in the callosal body. These results suggest that even subtle degradation of callosal fiber coherence can result in “mild yet detectable disturbance in interhemispheric processing” (Schulte et al. in press). Regarding the second instance, even when only one cerebral hemisphere is needed for task execution, elderly and compromised individuals (Cabeza et al. 2002), including alcoholics (Pfefferbaum et al. 2001a), commonly draw on both hemispheres.
Cerebellar circuitry disruption: a mechanism of functional impairment in alcoholism
Neuropsychological behaviors characteristic of chronic alcoholism include impaired judgment, blunted affect, poor insight, social withdrawal, reduced motivation, distractibility, and attentional deficits (for reviews, Oscar-Berman and Hutner 1993; Parsons et al. 1987; Sullivan 2000) and are typical of frontal lobe dysfunction (Cummings 1993; Fuster 1999). As noted earlier, quantitative testing has consistently shown alcoholics to be impaired in these frontal executive functions (e.g., Becker et al. 1983; Brandt et al. 1983; Nixon et al. 1992; Noel et al. 2001; Oscar-Berman and Hutner 1993; Parsons 1993; Sullivan et al. 1993, 1997). In vivo structural neuroimaging studies have confirmed the presence in frontal lobes of substantial volume shrinkage (Fein et al. 2002; Kubota et al. 2001; Pfefferbaum et al. 1997), proton metabolite abnormality (Martin et al. 1995; Parks et al. 2002; Schweinsburg et al. 2003; Seitz et al. 1999), glucose metabolic and perfusion impairment (Gansler et al. 2000; Gilman et al. 1990) in alcohol abusing and dependent individuals (for reviews, Fein et al. 1990; Moselhy et al. 2001; Oscar-Berman 2000), but in only some instances have frontal abnormalities predicted impaired executive function (Adams et al. 1995; Dao-Castellana et al. 1998; Rosse et al. 1997).
It is now recognized that the cerebellum and its extensive circuitry supports functions classically associated with the frontal lobes, including verbal associate learning, word production, problem solving, cognitive planning, attentional set shifting, and working memory (e.g., Courchesne et al. 1994; Schmahmann 2000). These findings are relevant to alcoholism because of the significant structural compromise sustained following chronic alcohol exposure by selective regions of cerebellum. In vivo neuroimaging studies have demonstrated significant volume deficits of the cerebellum that are profound in the anterior superior vermis (Sullivan et al. 2000a). These findings are consistent with postmortem reports of shrinkage, prominent in large neurons of the anterior superior vermis (Harper 1998; Phillips et al. 1987; Torvik and Torp 1986), and with fetal and adult animal models of alcoholism, identifying cerebellar volume loss and cell dysmorphology (Dlugos and Pentney 1997, 2000; Green et al. 2002; Pentney 1991; Pentney and Dlugos 2000). Taken together with dysmorphology of the prefrontal cortex and its underlying white matter, these observations point to frontocerebellar endpoint and circuitry disruption as a candidate mechanism of both transient and enduring behavioral impairments characteristic of alcoholism (Schmahmann 1997). Recently, we found that selective regions of cerebellar volume shrinkage (Fig. 2) were better predictors of the three principal neuropsychological signs of alcoholism—executive, visuospatial, and balance impairment—than frontal lobe volumes (Sullivan 2003). Furthermore, the possibility of recovery suggests that either a brain lesion is incomplete and damage reparable or alternative brain systems can compensate for damage (Filley 2001; Sullivan 2000).
Midsagittal view of an MRI of the brain of an alcoholic, showing severely shrunken folia of the anterior superior vermis compared with an age-matched control man. Taken from Sullivan et al. (2003a)
Alcohol’s untoward effect on the cerebellum is macroscopically and microscopically selective, affecting the anterior superior lobules of the vermis, the Purkinje cell structure (Pentney 1993), and granular and molecular cell layers (Phillips et al. 1987), notable in alcoholic patients with a history of thiamine deficiency (Baker et al. 1999). Frontal feedback endpoints of these damaged systems include dorsolateral prefrontal cortex, which is selectively affected, and motor cortex, which remains relatively spared by alcoholism (Harper and Kril 1989). Complementing this dissociation of alcohol’s selective effect within the frontal lobes is the discovery in nonhuman primates of parallel closed-loop cerebellar circuits. Each loop has feedforward and feedback components between cerebellar and frontal sites, and each has functional correlates (Kelly and Strick 2003). In one loop, the motor cortex (M1) receives input from Purkinje cells of cerebellar lobules IV–VI and sends projections to the granule cells there. In the other loop, prefrontal cortical area 46 receives Purkinje cell output from Crus II of the inferior posterior cerebellum and projects to granule cells there. Behavioral studies accompanying these circuitry studies revealed that the motor loop supports performance on a motor tracking test, whereas the cognitive loop supports performance on a motor sequencing task. Establishment of an analogous brain structure–function double dissociation in alcoholics awaits testing but if observed would support the existence of such parallel systems in humans and confirm the selective nature of alcoholism’s action on brain circuitry. We further speculate that pathology affecting single nodes (e.g., cerebellum) of such a brain system may impair function of a spatially distant node (e.g., problem solving subserved by prefrontal cortex), and pathology affecting multiple nodes (e.g., prefrontal and cerebellar) may exert a compounded effect on the selective functions of each neural node of the system (e.g., problem solving and balance; cf. Sullivan 2003).
fMRI evidence for process-demanding compensatory shifts in alcoholism
Functional MRI (fMRI) enables the detection of blood flow response to the activation of specific brain regions by motor, sensory, or cognitive processes. Our findings based on fMRI suggest the importance in alcoholics of cerebellar activation in otherwise frontal lobe functions. This additional activation enabled alcoholics to achieve normal levels of coordinated motor performance despite evidence for cerebellar dysmorphology but at a cost to processing capacity. This functional style observed in alcoholics, while perhaps compensatory, has been characterized as inefficient (Nixon and Parsons 1991). As noted by Nixon (1993), traditional concepts of processing inefficiency derive from conditions engendering altered speed/accuracy trade-offs. Alcoholics move slower to attain normal accuracy, as we observed in a quantified version of the finger-to-nose test, in which alcoholics achieved equivalent or even smaller trajectory deviations than controls (Sullivan et al. 2002a). This performance is symptomatic of cerebellar hemisphere dysfunction, characterized by deliberation of otherwise automatic movements. When automatic processing becomes effortful, it calls on limited processing capacity, which is then unavailable for other tasks. Consistent with this model is an fMRI study, demonstrating that finger tapping output of alcoholics was less efficient than controls in terms of tapping rate/pixel number activation (Parks et al. 2003). Evidence for “disorganized inefficient brain functioning” also derives from electrophysiological studies of event-related potentials (ERPs). One such study recorded ERPs to rare nontargets and observed that alcoholics had smaller-than-normal P3a amplitudes to rare nontargets and a less coherent pattern of brain activation than controls (Hada et al. 2000). Another study focused on the ERP at N400, which is prominent when semantic expectancies are not fulfilled (e.g., “I take my coffee with horse”). Detoxified alcoholics produced lower N400 amplitudes than controls across all cranial electrode sites examined (Nixon et al. 2002). Taken together, these divergent phenomena suggest a common psychological mechanism—processing inefficiency—and perhaps a neural mechanism—degraded white matter microstructure—as underlying these possible instances of impaired neural transmission.
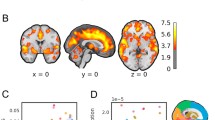
Our fMRI study of verbal working memory provides evidence for the role of the cerebellum in augmenting or compensating for functional impairment of the prefrontal cortex in alcoholics. Using a Sternberg paradigm in two-condition (low vs high memory load) block design (Desmond et al. 1997), subjects were given either one or six letters to keep in mind for 5 s, after which subjects respond as quickly and accurately as possible whether a test letter was previously presented. Despite equivalent reaction time and accuracy performance by controls and alcoholics, a region-of-interest analysis revealed greater activations in the alcoholics than controls in left prefrontal cortex and right superior cerebellum (Desmond et al. 2003) (Fig. 3). Primate studies have shown that these two distant brain regions are connected (Schmahmann 1996; Schmahmann and Pandya 1997). These brain regions are notably affected in alcoholics, whom we hypothesize recruit more far-reaching brain areas within the frontocerebellar system than controls to perform tasks at normal levels. The advantage of recruitment of brain regions in addition to those normally used to complete a task is to overcome functional impairment; the potential disadvantage is reduction of cognitive capacity or reserve to perform other tasks simultaneously.
This region-of-interest analysis reveals greater activation in alcoholics relative to matched controls in left prefrontal cortex (two left columns of images) and right superior cerebellar cortex, specifically Larsell’s lobule HVI (two right columns of images) (Larsell and Jansen 1972). Numbers along x (top row), y (middle row), and z (bottom row) axes refer to MNI coordinates. Note that this figure is a composite set of representative images taken from Figs. 4 and 5 of Desmond et al. (2003)
Nonalcoholism factors contributing to the condition of brain structure and function
It seems undeniable that chronic alcoholism takes a toll on brain structure and function, but myriad social, genetic, behavioral, environmental, and nutritional factors have the potential of compounding the alcoholism effects. Factors that may predate alcoholism’s onset and perhaps contribute to its development have also been identified and include below-average regional brain volumes and behavioral problems commonly accompanying alcoholism [including attention-deficit/hyperactivity disorder (Castellanos et al. 2003); antisocial personality disorder (Ceballos et al. 2003); electrophysiological signs (Begleiter and Porjesz 1984; Porjesz et al. 1998); and psychiatric comorbidity (Grant et al. 2004a,b; Sullivan et al. 2000b, 2003b). Furthermore, abuse or dependence on nonalcohol addicting substances often accompany alcoholism and must be considered on their own right as neurotoxins and as additive or interactive agents to alcohol’s neurotoxic effects (Bjork et al. 2003; Di Sclafani et al. 1998; Nixon et al. 1998). Nutritional deficiencies, which are common concomitants of chronic abusive drinking, have notorious deleterious effects on brain structure and cognitive and motor performance and may interact with the untoward effects of alcoholism to produce particularly profound and devastating sequelae, as can accompany conditions like central pontine myelinolysis, Wernicke’s encephalopathy, and Marchiafava–Bignami disease (e.g., Martin et al. 2003; Victor et al. 1989). Indeed, a series of neuropathological studies, which used retrospective, symptom criteria to verify Wernicke’s encephalopathy found that only alcoholics with antemortem signs of such thiamine deficiency (Caine et al. 1997) showed decreased Purkinje cell density and molecular layer volume in the cerebellar vermis and flocculi (Baker et al. 1999), mammillary bodies (Harding et al. 2000; Sheedy et al. 1999), and medial dorsal thalamic nuclei (Harding et al. 2000). Because of the difficult in obtaining accurate accounts of nutritional histories, prospective studies of alcoholism–nutritional interactions may best be conducted in animal models (Pfefferbaum et al. 2005a). These significant cofactors, typically noted as exclusionary variables, need to be considered in any rigorous study of alcoholism, and their inclusion in future studies should serve to enrich and broaden our understanding of this complex, devastating addiction.
Conclusion
Alcoholism has profound untoward effects on the cerebrum and cerebellum. Because of their far-reaching circuitry, disruption of selective cerebellar loci can have significant effects on remote brain regions, including the prefrontal cortex. To date, our guiding hypothesis that disruption of frontocerebellar circuitry is a principal neural mechanism underlying alcoholism’s salient, enduring, and debilitating deficits—ataxia, executive dysfunction, and visuospatial impairment—has been consistently supported by our MRI structural–neuropsychological studies and fMRI experiments. Given the possibility of structural and functional repair and recovery in sober alcoholics, at least a portion of the neuropathology must be transient (Carlen et al. 1986; Harper and Kril 1990) and the lesions incomplete (Filley 2001; Sullivan 2000). The transience of certain aspects of brain pathology may belie the problem of finding specific brain structural volume–functional relationships in alcoholics. Indeed, the dynamic course of alcoholism presents an important and challenging neuroscience model for understanding mechanisms of functional recovery, compensation, and processing limitations that should be applicable to any neurological condition characterized by a fluctuating course.
References
Aboitiz F, Rodriguez E, Olivares R, Zaidel E (1996) Age-related changes in fibre composition of the human corpus callosum: sex differences. NeuroReport 7:1761–1764
Adalsteinsson E, Sullivan EV, Pfefferbaum A (2002) Biochemical, functional and microstructural magnetic resonance imaging (MRI). In: Liu Y, Lovinger DM (eds) Methods in alcohol-related neuroscience research. CRC Press, Boca Raton, FL, pp 345–372
Adams KM, Gilman S, Koeppe R, Kluin K, Junck L, Lohman M, Johnson-Greene D, Berent S, Dede D, Kroll P (1995) Correlation of neuropsychological function with cerebral metabolic rate in subdivisions of the frontal lobes of older alcoholic patients measured with [18F]Fluorodeoxyglucose and positron emission tomography. Neuropsychology 9:275–280
Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW (1999) Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 56:356–363
Alling C, Bostrom K (1980) Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathologica (Berl) 50:77–80
Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME (2002) Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 23:794–802
Badsberg-Jensen G, Pakkenberg B (1993) Do alcoholics drink their neurons away? Lancet 342:1201–1204
Baker K, Harding A, Halliday G, Kril J, Harper C (1999) Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91:429–438
Becker JT, Butters N, Hermann A, D’Angelo N (1983) A comparison of the effects of long-term alcohol abuse and aging on the performance of verbal and nonverbal divided attention tasks. Alcohol Clin Exp Res 7:213–219
Begleiter H, Porjesz B (1984) Event-related brain potentials in boys at risk for alcoholism. Science 225:1493–1496
Bjork JM, Grant SJ, Hommer DW (2003) Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. Am J Psychiatry 160:2038–2045
Brandt J, Butters N, Ryan C, Bayog R (1983) Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry 40:435–442
Butler EE, Druizin M, Sullivan EV (2005) Gait changes and adaptions in adulthood: pregnancy, aging, and alcoholism. In: Rose J, Gamble JG (eds) Human walking, 3rd edn. Lippincott Williams and Wilkins, Baltimore, MD, Chapter 8 in press
Butters N, Cermak LS (1980) Alcoholic Korsakoff’s syndrome: an information processing approach to amnesia. Academic, New York
Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002) Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17:1394–1402
Caine D, Halliday GM, Kril JJ, Harper CG (1997) Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry 62:51–60
Carlen PL, Penn RD, Fornazzari L, Bennett J, Wilkinson DA, Wortzman G (1986) Computerized tomographic scan assessment of alcoholic brain damage and its potential reversibility. Alcohol Clin Exp Res 10:226–232
Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL (2003) Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. Am J Psychiatry 160:1693–1696
Ceballos NA, Nixon SJ, Phillips JA, Tivis R (2003) Semantic processing in alcoholics with and without antisocial symptomatology. J Stud Alcohol 64:286–291
Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L (1994) Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci 108:848–865
Courville CB (1955) Effects of alcohol on the nervous system of man. San Lucas Press, San Diego, CA
Craik FIM, Morris LW, Morris RG, Loewen ER (1990) Relations between source amnesia and frontal lobe functioning in older adults. Psychol Aging 5:148–151
Cummings JL (1993) Frontal–subcortical circuits and human behavior. Arch Neurol 50:873–880
Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A (1998) Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med 28:1039–1048
Davila MD, Shear PK, Lane B, Sullivan EV, Pfefferbaum A (1994) Mammillary body and cerebellar shrinkage in chronic alcoholics: an MRI and neuropsychological study. Neuropsychology 8:433–444
De la Monte SM (1988) Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol 45:990–992
de Lacoste M, Kirkpatrick J, Ross E (1985) Topography of the human corpus callosum. J Neuropathol Exp Neurol 44:578–591
Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH (1997) Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci 17:9675–9685
Desmond JE, Chen SHA, De Rosa E, Pryor MR, Pfefferbaum A, Sullivan EV (2003) Increased fronto-cerebellar activation in alcoholics during verbal working memory: an fMRI study. NeuroImage 19:1510–1520
Di Sclafani V, Clark HW, Tolou-Shams M, Bloomer CW, Salas GA, Norman D, Fein G (1998) Premorbid brain size is a determinant of functional reserve in abstinent crack-cocaine and crack-cocaine-alcohol-dependent adults. J Int Neuropsychol Soc 4:559–565
Dlugos CA, Pentney RJ (1997) Morphometric evidence that the total number of synapses on Purkinje neurons of old F344 rats is reduced after long-term ethanol treatment and restored to control levels after recovery. Alcohol Alcohol 32:161–172
Dlugos CA, Pentney RJ (2000) Effects of chronic ethanol consumption on SER of Purkinje neurons in old F344 rats. Alcohol 20:125–132
Ellis RJ, Oscar-Berman M (1989) Alcoholism, aging, and functional cerebral asymmetries. Psychol Bull 106:128–147
Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, Urbano-Marquez A (1997) Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci 146:145–151
Fein G, Bachman L, Fisher S, Davenport L (1990) Cognitive impairments in abstinent alcoholics. West J Med 152:531–537
Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ (2002) Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res 26:558–564
Filley CM (2001) The behavioral neurology of white matter. Oxford University Press, New York
Fuster J (1999) Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand 99:51–57
Gallucci M, Amicarelli I, Rossi A, Stratta P, Masciocchi C, Beomonte-Zobel B, Casacchia M, Passariello R (1989) MR imaging of white matter lesions in uncomplicated chronic alcoholism. J Comput Assist Tomogr 13:395–398
Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, Achong D (2000) Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: a pilot SPECT study. J Stud Alcohol 61:32–37
Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA (1990) Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol 28:775–785
Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K (2004a) Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiological Survey on alcohol and related conditions. Arch Gen Psychiatry 61:807–816
Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP (2004b) Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 61:361–368
Green JT, Tran T, Steinmetz JE, Goodlett CR (2002) Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res 956:302–311
Gunning-Dixon FM, Raz N (2003) Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia 41:1929–1941
Hada M, Porjesz B, Begleiter H, Polich J (2000) Auditory P3a assessment of male alcoholics. Biol Psychiatry 48:276–286
Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM (1997) Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7:78–87
Harding A, Halliday G, Caine D, Kril J (2000) Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123:141–154
Harper C (1998) The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? Neuropathol Exp Neurol 57:101–110
Harper CG, Kril JJ (1988) Corpus callosal thickness in alcoholics. Br J Addict 83:577–580
Harper C, Kril JJ (1989) Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci 92:81–89
Harper CG, Kril JJ (1990) Neuropathology of alcoholism. Alcohol Alcohol 25:207–216
Harper C, Kril J (1991) If you drink your brain will shrink. Neuropathological considerations. Alcohol Alcohol Suppl 1:375–380
Harper CG, Kril JJ (1993) Neuropathological changes in alcoholics. In: Hunt WA, Nixon SJ (eds) Alcohol induced brain damage. NIAAA Research Monograph No. 22. National Institute of Health, Rockville, MD, pp 39–69
Harper CG, Kril JJ, Daly JM (1987) Are we drinking our neurones away? Br Med J 294:534–536
Harper CG, Kril JJ, Daly JM (1988) Brain shrinkage in alcoholics is not caused by changes in hydration: a pathological study. J Neurol Neurosurg Psychiatry 51:124–127
Harper CG, Smith NA, Kril JJ (1990) The effects of alcohol on the female brain—a neuropathological study. Alcohol Alcohol 25:445–448
Harper C, Dixon G, Sheedy D, Garrick T (2003) Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuro-psychopharmacol Biol Psychiatry 27:951–961
Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M (1996) Decreased corpus callosum size among alcoholic women. Arch Neurol 53:359–363
Hommer DW, Momenan R, Kaiser E, Rawlings RR (2001) Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158:198–204
Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak L (1991) Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res 15:418–427
Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444
Kemper TL (1994) Neuroanatomical and neuropathological changes during aging and dementia. In: Albert ML, Knoefel JE (eds) Clinical neurology of aging. Oxford University Press, New York, pp 3–67
Kril JJ, Halliday GM, Svoboda MD, Cartwright H (1997) The cerebral cortex is damaged in chronic alcoholics. Neuroscience 79:983–998
Kubicki M, Westin C-F, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, Kikinis R, Jolesz FA, McCarley R, Shenton ME (2002) Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatr 10:324–336
Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T (2001) Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry 71:104–106
Lancaster FE (1993) Ethanol and white matter damage in the brain. In: Hunt WA, Nixon SJ (eds) Alcohol-induced brain damage. NIAAA Research Monograph No. 22. National Institute of Health, Rockville, MD, pp 387–399
Larsell O, Jansen J (1972) The human cerebellum, cerebellar connections, and cerebellar cortex. University of Minnesota Press, Minneapolis
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4:469–480
Lewohl J, Wang L, Miles M, Zhang L, Dodd P, Harris R (2000) Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res 24:1873–1882
Lezak MD (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, New York
Lim KO, Helpern JA (2002) Neuropsychiatric applications of DTI—a review. NMR Biomed 15:587–593
Mann K, Mundle G, Strayle M, Schroth G (1992) The reversibility of alcoholic brain damage is not due to rehydration: a controlled MRI and CT study (abstr). Alcohol Clin Exp Res 16:80
Mann K, Mundle G, Strayle M, Wakat P (1995) Neuroimaging in alcoholism: CT and MRI results and clinical correlates. J Neural Transm (Gen Sect) 99:145–155
Mann K, Gunther A, Stetter F, Ackermann K (1999) Rapid recovery from cognitive deficits in abstinent alcoholics: a controlled test–retest study. Alcohol Alcohol 34:567–574
Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR (1995) Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clin Exp Res 19:1078–1082
Martin PR, Singleton CK, Hiller-Sturmhofel S (2003) The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health 27:134–142
Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA (2002) Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem 81:802–813
Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E (1992) Age-related white matter atrophy in the human brain. Ann N Y Acad Sci 673:260–269
Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW (2004) Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res 28:650–661
Moseley M (2003) Diffusion tensor imaging and aging—a review. NMR Biomed 15:553–560
Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D (1990) Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 176:439–445
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36:357–368
Nixon SJ (1993) Application of theoretical models to the study of alcohol-induced brain damage. In: Hunt W, Nixon SJ (eds) Alcohol induced brain damage. NIAAA monograph. National Institute of Health, Rockville, MD, pp 213–228
Nixon K, Crews FT (2002) Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 83:1087–1093
Nixon K, Crews FT (2004) Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci 24:9714–9722
Nixon SJ, Glenn SW (1995) Cognitive, psychosocial performance and recovery in female alcoholics. In: Galanter M (ed) Recent developments in alcoholism, vol 12. Alcoholism and women. Plenum Press, New York, pp 287–308
Nixon SJ, Parsons OA (1991) Alcohol-related efficiency deficits using an ecologically valid test. Alcohol Clin Exp Res 15:601–606
Nixon SJ, Tivis R, Parsons OA (1992) Interpersonal problem-solving in male and female alcoholics. Alcohol Clin Exp Res 16:684–687
Nixon SJ, Paul R, Phillips M (1998) Cognitive efficiency in alcoholics and polysubstance abusers. Alcohol Clin Exp Res 22:1414–1420
Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J (2002) Neurophysiological efficiency in male and female alcoholics. Prog Neuro-Psychopharmacol Biol Psychiatry 26:919–927
Noel X, Paternot J, Van der Linden M, Sferrazza R, Verhas M, Hanak C, Kornreich C, Martin P, De Mol J, Pelc I, Verbanck P (2001) Correlation between inhibition, working memory and delimited frontal area blood flow measure by 99mTc-Bicisate SPECT in alcohol-dependent patients. Alcohol Alcohol 36:556–563
O’Neill J, Cardenas VA, Meyerhoff DJ (2001) Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res 25:1673–1682
Oscar-Berman M (2000) Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K (eds) Review of NIAAA’s neuroscience and behavioral research portfolio. NIAAA Research Monograph No. 34. National Institute of Health, Rockville, MD, pp 437–472
Oscar-Berman M, Hutner N (1993) Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ (eds) Alcohol-induced brain damage. NIAAA Research Monographs #22. National Institutes of Health, Rockville, MD, pp 121–156
Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR (2002) Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res 26:1368–1380
Parks MH, Morgan VL, Pickens DR, Price RR, Dietrich MS, Nickel MK, Martin PR (2003) Brain MRI activation associated with self-paced finger-tapping in chronic alcohol dependent patients. Alcohol Clin Exp Res (in press)
Parsons OA (1983) Cognitive dysfunction and recovery in alcoholics. Subst Alcohol Actions/Misuse 4:175–190
Parsons O (1993) Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ (eds) Alcohol induced brain damage. NIAAA Research Monograph No. 22. National Institutes of Health, Rockville, MD, pp 173–194
Parsons OA, Butters N, Nathan PE (1987) Neuropsychology of alcoholism: implications for diagnosis and treatment. Guilford Press, New York
Paula-Barbosa MM, Tavares MA (1985) Long term alcohol consumption induces microtubular changes in the adult rat cerebellar cortex. Brain Res 339:195–199
Pentney RJ (1991) Remodeling of neuronal dendritic networks with aging and alcohol. Alcohol Alcohol Suppl 1:393–397
Pentney RJ (1993) Alterations in the structure of the cerebellum after long-term ethanol consumption. In: Hunt WA, Nixon SJ (eds) Alcohol-induced brain damage. NIAAA Research Monograph No. 22. National Institute of Health, Rockville, MD, pp 249–276
Pentney RJ, Dlugos CA (2000) Cerebellar Purkinje neurons with altered terminal dendritic segments are present in all lobules of the cerebellar vermis of ageing, ethanol-treated F344 rats. Alcohol Alcohol 35:35–43
Peters A, Sethares C (2002) Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442:277–291
Peters A, Sethares C (2003) Is there remyelination during aging of the primate central nervous system? J Comp Neurol 460:238–254
Peters A, Sethares C, Killiany RJ (2001) Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol 435:241–248
Pfefferbaum A, Sullivan EV (2002) Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage 15:708–718
Pfefferbaum A, Sullivan EV (2005a) Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology 30:423–432
Pfefferbaum A, Sullivan EV (2005b) Diffusion MR imaging in psychiatry and aging in physiological MR in clinical neuroscience. In: Gillard J, Waldman A, Barker P (eds) Physiological magnetic resonance imaging in clinical neuroscience. Cambridge University Press, Cambridge, pp 558–578
Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Lane B, Ha CN, Rosenbloom MJ, Sullivan EV (1992) Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res 16:1078–1089
Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO (1995) Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 19:1177–1191
Pfefferbaum A, Lim KO, Desmond J, Sullivan EV (1996) Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res 20:752–757
Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO (1997) Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 21:521–529
Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO (1998) A controlled study of cortical gray matter and ventricular changes in alcoholic men over a five year interval. Arch Gen Psychiatry 55:905–912
Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M (2000) In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res 24:1214–1221
Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV (2001a) Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. NeuroImage 14:7–20
Pfefferbaum A, Rosenbloom MJ, Deshmukh A, Sullivan EV (2001b) Sex differences in the effects of alcohol on brain structure. Am J Psychiatr 158:188–197
Pfefferbaum A, Rosenbloom MJ, Serventi K, Sullivan EV (2002) Corpus callosum, pons and cortical white matter in alcoholic women. Alcohol Clin Exp Res 26:400–405
Pfefferbaum A, Adalsteinsson E, Bell RL, McBride WJ, Sullivan EV (2005a) An in vivo rat model of Wernicke’s Encephalopathy imaged on a human 3T scanner (abstr). Presented at Miami, FL, Annual meeting of the International Society for Magnetic Resonance in Medicine, 2005, May 8–14
Pfefferbaum A, Adalsteinsson E, Sullivan EV (2005b) Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging (in revision)
Pfefferbaum A, Adalsteinsson E, Sullivan EV (2005c) Frontal circuitry degradation marks healthy adult aging: evidence from diffusion tensor imaging. Neuroimage (in press)
Phillips SC, Harper CG, Kril J (1987) A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain 110:301–314
Pierpaoli C, Basser PJ (1996) Towards a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix L, Virta A, Basser P (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 13:1174–1185
Porjesz B, Begleiter H, Reich T, Vaneerdewegh P, Edenberg H, Foroud T, Goate A, Litke A, Chorlian D, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer L, Kuperman S, O’Connor S, Rohrbaugh J (1998) Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary-results from the COGA project. Alcohol Clin Exp Res 22:1317–1323
Purves P, Seltzer B (1986) The topography of commissural fibers. Alan R. Liss, New York
Putzke J, De Beun R, Schreiber R, De Vry J, Tolle T, Zieglgansberger W, Spanagel R (1998) Long-term alcohol self-administration and alcohol withdrawal differentially modulate microtubule-associated protein 2 (MAP2) gene expression in the rat brain. Brain Res Mol Brain Res 62:196–205
Raz N (1999) Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA (eds) Handbook of aging and cognition II. Erlbaum, Mahwah, NJ, pp 1–90
Rosenbloom MJ, Sullivan EV, Pfefferbaum A (2003) Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health 27:146–152
Rosenbloom MJ, Pfefferbaum A, Sullivan EV (2004) Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology 18:589–597
Rosse RB, Riggs RL, Dietrich AM, Schwartz BL, Deutsch SI (1997) Frontal cortical atrophy and negative symptoms in patients with chronic alcohol dependence. J Neuropsychiatry Clin Neurosci 9:280–282
Sandvig A, Berry M, Barrett LB, Butt A, Logan A (2004) Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia 46:225–251
Schmahmann JD (1996) From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 4:174–198
Schmahmann J (1997) The cerebellum and cognition. Academic Press, San Diego, CA
Schmahmann J (2000) The role of the cerebellum in affect and psychosis. J Neurolinguist 13:189–214
Schmahmann JD, Pandya DN (1997) Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 17:438–458
Schulte T, Pfefferbaum A, Sullivan EV (2003) Parallel interhemispheric processing in aging and alcoholism: relation to corpus callosum size. Neuropsychologia 42:257–271
Schulte T, Sullivan EV, Mueller-Oehring EM, Adalsteinsson E, Pfefferbaum A (2005) Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex: available on line January 5, in press in print
Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I (2003) Effects of alcoholism and gender on brain metabolism. Am J Psychiatr 160:1180–1183
Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W (1999) Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin Exp Res 23:158–163
Shear PK, Butters N, Jernigan TL, DiTraglia GM, Irwin M, Schuckit MA, Cermak LS (1992) Olfactory loss in alcoholics: correlations with cortical and subcortical MRI indices. Alcohol 9:247–255
Shear PK, Jernigan TL, Butters N (1994) Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res 18:172–176
Shear PK, Sullivan EV, Lane B, Pfefferbaum A (1996) Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res 20:1489–1495
Sheedy D, Lara A, Garrick T, Harper C (1999) Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcohol Clin Exp Res 23:1624–1628
Squire LR, Amaral DG, Press GA (1990) Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci 10:3106–3117
Sullivan EV (2000) Human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K (eds) Review of NIAAA’s neuroscience and behavioral research portfolio. NIAAA Research Monograph No. 34. National Institutes of Health, Bethesda, MD, pp 473–508
Sullivan EV (2003) Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res 27:1409–1419
Sullivan EV, Pfefferbaum A (2001) Magnetic resonance relaxometry reveals central pontine abnormalities in clinically asymptomatic alcoholic men. Alcohol Clin Exp Res 25:1206–1212
Sullivan EV, Marsh L (2003) Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology 61:1716–1719
Sullivan EV, Pfefferbaum A (2003) Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol 45:244–255
Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A (1993) Factors of the Wisconsin Card Sorting Test as a test of dorsolateral prefrontal cortical function in schizophrenia and alcoholism. Psychiatry Res 46:175–199
Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A (1995) Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res 19:110–122
Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A (1996) Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res 20:348–354
Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A (1997) Patterns of content, contextual, and working memory impairment in schizophrenia and nonamnesic alcoholism. Neuropsychology 11:195–206
Sullivan EV, Lane B, Rosenbloom MJ, Deshmukh A, Desmond J, Lim KO, Pfefferbaum A (1999) In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res 23:1629–1636
Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000a) Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 14:341–352
Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A (2000b) Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry 57:894–902
Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A (2000c) Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology 14:178–188
Sullivan EV, Rosenbloom MJ, Pfefferbaum A (2000d) Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24:611–621
Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A (2002a) Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin Exp Res 26:705–713
Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A (2002b) A profile of neuropsychological deficits in alcoholic women. Neuropsychology 16:74–83
Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A (2003a) Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res 27:301–309
Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A (2003b) The effects of alcohol dependence comorbidity and anti-psychotic medication on volumes of the thalamus and pons in schizophrenia. Am J Psychiatry 160:1110–1116
Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A (2004a) Striatal volume deficits in alcoholism with and without Korsakoff’s Syndrome (abstr). Neuropsychopharmacology 29(Supplement 1):S188
Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A (2005) Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry (in press)
Tarnowska-Dziduszko E, Bertrand E, Szpak G (1995) Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathol 33:25–29
Torvik A, Torp S (1986) The prevalence of alcoholic cerebellar atrophy: a morphometric and histological study of an autopsy material. J Neurol Sci 75:43–51
Victor M, Adams RD, Collins GH (1989) The Wernicke–Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition, 2nd edn. F.A. Davis, Philadelphia
Villa G, Gainotti G, De Bonis C, Marra C (1990) Double dissociation between temporal and spatial pattern processing in patients with frontal and parietal damage. Cortex 26:399–407
Virta A, Barnett A, Pierpaoli C (1999) Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MR. Magn Reson Imaging 17:1121–1133
Waxman SG, Kocsis JD, Stys PK (1995) The axon: structure, function and pathophysiology. Oxford University Press, New York
Wechsler D (1987) Wechsler Memory Scale-revised. The Psychological Corporation, San Antonio, TX
Wechsler D (1999) The Wechsler Abbreviated Scale of intelligence. The Psychological Corporation, The Psychological Corporation
Wiggins RC, Gorman A, Rolsten C, Samorajski T, Ballinger WE, Freund G (1988) Effects of aging and alcohol on the biochemical composition of histologically normal human brain. Metab Brain Dis 3:67–80
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA10723, AA05965, AA12388, AA12999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, E.V., Pfefferbaum, A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology 180, 583–594 (2005). https://doi.org/10.1007/s00213-005-2267-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2267-6