Abstract
The translational value of preclinical models of methamphetamine abuse depends in large part on the degree to which the drug regimens used in animals produce methamphetamine exposure patterns similar to those experienced by human methamphetamine abusers. To approximate one common form of methamphetamine abuse, we studied the effects of a schedule of intravenous methamphetamine administration in rats which included 2 weeks of progressively more frequent drug injections (0.125 mg/kg/injection) followed by 40 maintenance days during which animals received 40 daily injections (at 15-min intervals), with the dose gradually increasing (0.125–0.25 mg/kg per injection) every 5–10 days. This treatment produced an emerging behavioral profile characterized by gradually more continuous periods of activation consisting of progressively more intense, focused stereotypy interrupted by episodic bursts of locomotion. We also assessed markers of dopamine neurotransmission (dopamine transporter, vesicular monoamine transporter, and dopamine D1 and D2 receptors) at 15 min and (including dopamine levels) at 6 and 30 days following cessation of methamphetamine treatment. All dopamine components measured in caudate–putamen were significantly reduced at 15 min and 6 days after the final methamphetamine injection. Dopamine D1 and D2 receptors fully recovered after 30 days of drug abstinence, whereas dopamine and the dopamine transporter exhibited significant but incomplete recovery by this time point. In contrast, only the vesicular monoamine transporter exhibited no evidence of recovery over the 30-day withdrawal period. These data are discussed in terms of damage to dopamine terminals and compensatory adjustments in mechanisms maintaining functional dopaminergic transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence from animal studies indicates that at least some methamphetamine (METH) treatment regimens may produce enduring neurochemical alterations (Ricaurte et al. 1980; Schmidt et al. 1985b; Bowyer and Holson 1995; Davidson et al. 2001; Harvey et al. 2000). However, the relevance of these findings to the effects in human METH abusers may be limited by substantial differences between typical patterns of abuse and the protocols most often used to study METH toxicity in animals (Segal and Kuczenski 1997b; Cho et al. 2001; Segal et al. 2003; Davidson et al. 2001). For example, we have argued that an escalation in dosage typically precedes higher dose maintenance (Simon et al. 2002) or binge regimens in METH abusers, and therefore, a similar dosing procedure should be used in animal models (Segal and Kuczenski 1997a, 1999b, 2000). Recently, we demonstrated the significant impact of such a METH pretreatment schedule on the effects of an acute high-dose “binge” treatment, including tolerance to METH-induced hyperthermia (Segal et al. 2003).
In addition to dose escalation, another important difference between most animal treatment models and human drug exposure patterns is the pronounced species differences in METH pharmacokinetics. Thus, whereas the elimination half-life of METH is about 1 h in rats (Melega et al. 1995; Cho et al. 2001; Rivière et al. 1999; Gilles et al. 2000; Kitaichi et al. 2003), it is estimated to be ∼12 h in humans (Cook et al. 1993; Harris et al. 2003). We recently discussed the potential consequences of such a difference, both with respect to variations in time of exposure to the drug and cumulative alterations in drug concentration resulting from multiple injections (Cho et al. 2001). Since both plasma level and duration of drug exposure are known to significantly impact the neurochemical and behavioral changes associated with chronic drug treatment (Unterwald et al. 2001; Segal and Kuczenski 1997a; Nielsen et al. 1980; Davidson et al. 2001), the differences in elimination half-life must be considered in developing animal treatment models that are used to investigate changes relevant to those which occur in human METH abusers. We recently suggested that the human plasma METH profile may be approximated in the rat by increasing METH injection frequency, and on the basis of kinetic modeling, we determined that intravenous injections every 15 min in the rat would achieve plasma levels similar to those estimated to occur in humans who inject the same dose of METH every 3 h (Cho et al. 2001). In the present study, we used such an escalating dose-maintenance procedure to assess selected behavioral and neurochemical consequences of long-term exposure to METH in rats. Our results indicate that this METH administration paradigm produced a unique behavior profile and that most, but not all, dopamine (DA) markers assessed exhibited partial or complete recovery by 30 days after treatment.
Materials and methods
Subjects
Male Sprague–Dawley rats (Harlan Labs, Gilroy, CA) weighing 325–350 g at the beginning of drug treatment were housed for at least 2 weeks prior to surgery in groups of two or three animals, in wire mesh cages, with ad libitum access to food and water. The room was temperature- (20°C) and humidity-controlled (55±5%) and maintained on a reversed 12 h dark (from 9:00 a.m. to 9:00 p.m.), 12 h light cycle to allow for treatment and testing during the normal active phase of the rat’s awake/sleeping cycle. During the dark period, all facilities were illuminated with red light to facilitate observation of the animals. These studies adhered to animal welfare guidelines (National Research Council, Guide for the Care and Use of Laboratory Animals, 1996).
Drugs
Methamphetamine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in a saline vehicle (see below) and administered intravenously. Doses represent the free base.
Surgery
After 2 weeks of acclimation, animals were implanted with i.v. catheters under halothane anesthesia. Catheters were constructed by fitting a 13 cm length of silastic tubing to a guide cannula, bent at a right angle. The guide cannula was embedded in dental cement and attached to a 2.5 cm circle of Marlex mesh and mounted on the animal’s back. The silastic tubing was passed subcutaneously from the rat’s back to the right external jugular vein. A Tygon cap was inserted over the guide cannula to maintain a closed system. Animals were singly housed after surgery, and on a daily basis prior to experimental testing, the catheter was flushed with sterile saline (0.15 ml) containing 30 USP units heparin and 3.75 mg disodium ticarcillin, potassium clavulanate.
Drug administration
Remote drug delivery was accomplished with a Med Associates Inc. syringe pump. Tubing from the syringe pump was attached to the catheter via a liquid swivel and a commercially available cannula connector (Plastic Products). Drug was administered during the animals’ awake cycle (from 9:00 a.m. to 9:00 p.m.), beginning each day at 11:00 a.m. METH was dissolved in 0.9% saline containing 3.0 units/day heparin and 3.75 mg/day disodium ticarcillin, potassium clavulanate, and control animals received a comparable number of vehicle infusions (disodium ticarcillin, potassium clavulanate was discontinued 10 days after connection to catheter). Drug or vehicle was administered in 0.125 ml, over a 4 s interval. An 80 dB, 2.2 kHz tone (over a background noise level of ∼62 dB) preceded by 5 s and accompanied each 4-s METH infusion. The audible tone was used to provide rats with a drug-predictive cue, shown in recent studies to be a potentially significant factor in stimulant-induced neuronal alterations (Ghitza et al. 2003). Observation of our animals during tone presentation indicated that all rats initially exhibited orienting responses to the stimulus which, in saline-treated rats, habituated after several tone-saline pairings. In contrast, rats that received drug continued to orient to the tone, and in fact, most displayed some ambulation in the 5 s prior to drug infusion. It appears, therefore, that the tone alerted the rats to subsequent METH administration and thus allowed them to behaviorally, and perhaps physiologically, anticipate the drug effect. Since “lack of expectancy” (Jacobs et al. 2003) may be a particularly important factor with respect to differences observed between contingent and noncontingent drug administration, the use of the tone cue may minimize such differences. However, other significant distinguishing components may exist between active and passive procedures, and ultimately, self-administration of METH using our treatment exposure pattern will be necessary to address this issue.
General procedures
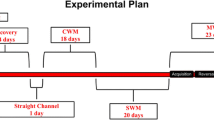
Behavior was monitored in custom-designed activity chambers as previously described in detail (Segal et al. 2003). About 2 weeks after surgery, animals were placed in individual experimental chambers, connected to the syringe tubing, and remained in the chambers for the duration of the experiment. Each morning throughout the study, at the beginning of the dark phase, the behavioral chambers were serviced and the animals were weighed. Following a 3-day acclimation period, during which animals received single daily infusions of saline, the escalating dose (ED) phase was initiated. During this phase, animals received an increasing number of daily injections of saline or 0.125 mg/kg METH, at progressively shorter intervals for 17 days (Table 1).
On the day following the ED pretreatment, maintenance phase (MP) treatment was initiated. During this phase, animals received 40 infusions/day at 15-min intervals. Over the 37-day maintenance phase, the METH dose was progressively increased from 0.125 to 0.25 mg/kg/infusion (Table 2). During the final segment of the MP (days 28–37), animals received a total dose of 10 mg/kg/day.
Throughout the experiment, the saline-treated animals continued to gain weight (from ∼335 to ∼435 g). The weights of the drug-treated animals declined to about 88% of controls by the end of the ED phase and to about 83% of controls by the end of the MP. After cessation of drug administration, the weights of the METH-treated animals rapidly recovered to about 90% of controls by the sixth withdrawal day, and by the 20th withdrawal day, there were no significant weight differences between the two groups. In addition, throughout the experiment, none of the animals exhibited evidence of severe METH-induced hyperthermia (e.g., excess salivation, nasal discharge, dehydration, or lying prone), thus obviating the need for cold-room or ice-bath treatment. No METH-treated animals died.
Pharmacokinetic calculations
Theoretical plasma METH concentrations were estimated from an equation for an open two-compartment pharmacokinetic model:
The rate constant α describes the initial tissue uptake and elimination processes and β describes the elimination process. The coefficients A and B were estimated from earlier studies to be 12.54 and 8.6 μM, respectively, for a dose of 10 mg/kg (Cho et al. 2001) and were adjusted for dose.
Neurochemical characterization
Groups of drug-treated (N=34) and saline-treated (N=16) animals were sacrificed for tissue analyses of DA and metabolites, for [3H]WIN 35,428, [3H]dihydrotetrabenazine, [3H]raclopride, and [3H]SCH 23390 binding, and for tissue and plasma levels of METH and metabolites at 15 min, 6 days, and 30 days after the last METH infusion as indicated in Results. Brains were hemisected along the mid-sagittal plane; one half brain was prepared for autoradiography, while a sample of caudate tissue (Segal and Kuczenski 1974) was obtained from the second half brain for analysis of METH and AMPH at the 15-min time point or for DA and metabolites at the 6- and 30-day time points. Thus, with the exception of the 15-min time point, all dopamine components were assessed in all animals.
Tissue transmitter levels
For tissue levels of DA and metabolites, samples were prepared according to Schmidt et al. (1990) and were analyzed using HPLC with electrochemical detection as previously described (Kuczenski et al. 1995).
Autoradiography
Coronal sections (20 μm) of fresh frozen hemisected rat brain were cut in a cryostat (−14 EC; Leica CM 3050) and thaw-mounted in triplicate onto glass slides (Fisherbrand Superfrost Plus). The slides were vacuum-desiccated overnight at 4°C and stored at −70°C until use. Nissl-stained sections containing the striatum, corresponding to AP 1–1.6 mm from the rat brain atlas of Paxinos and Watson (1986), were used to identify matching sections for radioligand binding. On the day of the assay, these sections were thawed to room temperature under vacuum. The following studies were conducted according to previously published protocols with minor modifications stated:
-
[3H]WIN 35,428 binding: 10 nM [3H]WIN 35,428 (86 Ci/mmol; New England Nuclear), nonspecific binding with 60 μM cocaine (Coulter et al 1995)
-
[3H]Dihydrotetrabenazine binding: 10 nM [3H]DTBZ (18.8 Ci/mmol; American Radiolabeled Chemicals), nonspecific binding with 20 μM tetrabenazine (Darchen et al. 1989)
-
[3H]SCH 23390 binding: 6 nM [3H]SCH 23390 (86 Ci/mmol; New England Nuclear); 40 nM ketanserin to prevent binding to 5-hydroxytryptamine2A receptors, nonspecific binding with 30 μM SKF 38393 (Shi et al. 1999)
-
[3H]Raclopride binding: 10 nM [3H]raclopride (71.2 Ci/mmol; New England Nuclear), nonspecific binding with 300 μM sulpiride
Following the last rinse for all binding studies, the slides were air-dried, vacuum-desiccated overnight, and then apposed to Kodak MR film for 4–6 weeks at room temperature. Films were developed using a Kodak X OMAT 2000A Processor. Autoradiograms were digitized on a digital flatbed scanner (HP ScanJet 5300C) and quantified using a computerized image analysis system (MCID, Imaging Research). Coexposed tritiated standard strips (Amersham) were used to convert optical density values to fmol/mg. Measurements were determined for striatal regions defined according to the rat brain atlas of Paxinos and Watson (1986). Each region was analyzed in three brain sections for each animal for total and nonspecific binding. The means of these triplicate values were used to calculate specific binding (total minus nonspecific binding). Mean values for each region were then obtained for the METH-treated and control striata.
Brain and plasma METH and metabolites
The concentrations of METH, AMPH, and 4-OH-METH were determined by a gas chromatography/mass spectroscopy procedure (Melega et al. 1995).
Data analysis
Data were statistically analyzed using repeated-measures ANOVA and t-tests with Bonferroni corrections for specific group/time comparisons.
Results
Behavior
Escalating dose phase
During the course of the escalating dose (ED) treatment, the dose (0.125 mg/kg) was unchanged, but the number of injections (all administered during the dark/active period) progressively increased (Table 1). Behavioral activation (increased vertical and horizontal locomotion) appeared rapidly, achieving peak levels within 5–10 min after each i.v. injection (Fig. 1). By about day 10, the response no longer returned to saline control levels between successive injections (data not shown), and by day 17 (19 min between injections), behavior remained at a relatively constant, high level of activation after about 2–3 h following the start of the day’s treatment. In addition to changes in the magnitude of locomotor activity, two qualitative behavioral alterations were also apparent during the ED phase. For one, observation of the rats indicated that their locomotion occurred progressively more often in the form of episodic bursts of activity (see Segal and Kuczenski 1997b for a detailed description of this behavior), and there was also a gradual appearance of focused stereotypies, primarily in the form of repetitive head movements and oral behaviors (licking and biting). Oral stereotypies became more prominent during the final days of this treatment phase.
Locomotor response to the intravenous administration (arrows) of saline or METH (0.125 mg/kg/injection) on representative days of the escalating dose (ED) phase of the ED–MP treatment. Drug was administered during the dark/active phase (represented by shading in the figure), beginning each day at 11:00 a.m. Values represent means±SEM
Both the quantitative and qualitative behavioral changes observed were likely influenced significantly by the pattern of drug accumulation that resulted as a function of interjection interval. Predicted plasma profiles for the injection intervals on days 1, 7, and 17, based on pharmacokinetic modeling we previously described (Cho et al. 2001), are displayed in Fig. 2. These theoretical plasma levels, although potentially different from actual patterns by virtue of possible changes in various pharmacokinetic factors (see Discussion), do reveal that from day 1 to day 17, the maximum peak drug plasma levels achieved may have increased by greater than threefold. It is equally important to note that the drug concentration nadir also correspondingly increased as the interval between successive injections of the same METH dose (0.125 mg/kg) decreased, so that by day 17, the lowest plasma drug level estimated to occur through most of the day (between 0.5 and 0.6 μM) is markedly higher than the peak levels achieved after a single injection (0.26 μM). Thus, by the end of the ED phase, there was a relatively sustained, although fluctuating, high level of drug maintained through most of the treatment period (dark/active cycle). Furthermore, the greatest percentage change occurred within the first 2 h after the initial injection, and both the peak and nadir levels exhibited proportionally little increase during the remainder of the treatment period.
Theoretical plasma METH concentrations (μM) during the intravenous administration (arrows) of METH (0.125 mg/kg/injection) on representative days of the escalating dose phase of treatment. Corresponding behavioral responses are presented in Fig. 1. See Materials and methods for details of pharmacokinetic calculations
It appears, however, that cumulative drug levels within each treatment day are not solely responsible for all the behavioral alterations that were observed during the ED phase since profound differences occurred in the responses to the first injection across successive days (Fig. 3). The locomotor response to the initial daily injection exhibited a progressive increase up to about day 7, then gradually declined by the last day of the ED phase (Fig. 3, top). A similar pattern of increase and decline occurred for the repetitive head movement stereotypy (Fig. 3, bottom), although this behavior was still present on day 17, whereas on day 1, no focused stereotypy of any type was apparent. By contrast, oral stereotypies (Fig. 3, bottom) became progressively more prominent after the first injection of each day throughout the ED phase, so that by day 17, this relatively intense form of perseverative behavior appeared during more than 50% of the observational interval, and locomotion was correspondingly diminished.
a Locomotor responses and b stereotypy responses to the first intravenous injection of METH (0.125 mg/kg) on representative days of the escalating dose phase treatment. Values are means±SEM. Stereotypy responses represent the percent time of the indicated interval during which animals were engaged in repetitive head movements or oral stereotypies. ***p<0.001 compared to the response on day 1. ++p<0.01 and +++p<0.001 compared to response on day 7
Maintenance phase
During this phase, the number (40 injections) and interval (15 min) between successive injections remained constant, although the dose was gradually increased by 0.025-mg/kg increments (Table 2), when the behavioral activity profile was observed to remain relatively unchanged for at least two consecutive days at each dose level. Through the maintenance treatment, automated monitoring of locomotor patterns and observations of representative animals revealed progressive behavioral changes within and between days. Even at the highest dose used (0.25 mg/kg/injection), significant qualitative differences in the oral and locomotor responses were apparent through the course of 10 days of maintenance injections (Fig. 4). On both the first and tenth days of this highest maintenance dose, locomotor activity rapidly increased over the first three to four injections and occurred primarily in the form of brief episodic bursts which interrupted relatively persistent oral stereotypies (data not shown). The greatest change in behavior over successive days appeared between the eighth and 40th injections (from 1:00 p.m. to 9:00 p.m.) and was most apparent as a progressive decline in total locomotor activity during this time interval across days (Fig. 4, inset 1). This decrease from the first to the tenth day reflected the presence of gradually longer intervals of intense oral stereotypy and correspondingly fewer locomotor bursting episodes. This difference in the behavioral profile persisted for the first hour after the last injection, as reflected in the comparison between days 1 and 10 (Fig. 5).
Locomotor responses to intravenous METH (0.25 mg/kg/injection) administration (arrows) on the first (day 1) and final (day 10) days of the maintenance phase at this dose level. Drug was administered during the dark/active phase (shaded area) of the light–dark cycle. Values are means±SEM. Crossovers on representative days of the maintenance phase (inset). Values represent crossovers cumulated over the indicated interval encompassing 32 METH (0.25 mg/kg) injections and are presented as means±SEM. ***p<0.001 compared to the response on day 1. +p<0.05, +++p<0.001 compared to the response on day 4
Behavioral responses to the last (40th) intravenous METH (0.25 mg/kg) injection on the first (day 1) and final (day 10) days of the maintenance phase at this dose level. a Temporal profile of the oral stereotypy responses. Values (means±SEM) represent the percent of each 15 min interval during which animals were engaged in oral behaviors. b Oral stereotypy and crossovers cumulated over the initial 60 min following METH administration. **p<0.01 compared to the response on day 1
Pharmacokinetics
Theoretical plasma levels of METH for the last day of the maintenance treatment are similar in shape to the right panel in Fig. 2, though plateau levels would be higher, fluctuating around 1.75 μM. To determine actual plateau levels of METH, eight of the animals were sacrificed 15 min following their last METH injection of the day-long exposure, about 4–5 h after steady-state plasma drug levels are presumed to have been achieved (Cho et al. 2001). Plasma and brain tissue levels of METH and its metabolites, AMPH and p-OH-METH, are summarized in Table 3. Although significant regional differences in levels of METH and its metabolites were observed (Table 3), in general, brain levels of METH (e.g., caudate–putamen METH, 11.19±0.77 nmol/g) were about tenfold higher than plasma levels (1.070±0.081 nmol/ml), a ratio typically observed following acute METH administration (Melega et al. 1995; Gilles et al. 2000).
Dopamine nerve terminal markers
To assess the consequences of this pattern of METH exposure on molecular elements of the striatal dopamine system, we determined radioligand binding to the DA nerve terminal components, DAT and VMAT2, to D1 and D2 DA receptors and levels of DA and its metabolites as a function of time after the last METH injection.
In caudate–putamen, the DA metabolites DOPAC and HVA were significantly elevated by about 15–25% compared to control values at 6 days after cessation of drug administration and remained elevated at the 30-day time point (Fig. 6). In contrast, all other dopaminergic measures (Fig. 6) were significantly decreased at 6 days following drug exposure, with the magnitude of decrease ranging from ∼10% for radioligand binding to VMAT2 and the D1 and D2 receptors to ∼25% for radioligand binding to DAT and for tissue DA levels; the measures that were assessed at 15 min exhibited decreases similar to the 6-day time point. However, by 30 days after METH treatment, radioligand binding to D1 and D2 DA receptors had recovered to control levels, and both tissue DA levels and radioligand binding to DAT exhibited significant recovery from the 6-day levels, though both markers still remained significantly attenuated by about 10% compared to saline controls. In contrast, radioligand binding to VMAT2 showed no evidence of recovery, remaining decreased by ∼10%.
Effects of METH administration on components of synaptic dopamine as a function of time following cessation of treatment. Values are means±SEM, expressed as percent of saline-treated controls. In all cases, the n values were 8–11 for each measure. *p<0.05, **p<0.01, ***p<0.001 compared to saline-treated controls. +p<0.05 compared to 15-min values
A somewhat similar profile of effects was also evident in nucleus accumbens core and shell; that is, following METH exposure, the DA markers exhibited relatively small decrements which generally appeared to recover during the 30-day interval prior to final testing (mean values for radioligand binding to D1, D2, and DAT at the 30-day time point were all 100% of control or higher). However, only the decrease in radioligand binding to DAT (in both NA core and shell) achieved statistical significance at the 15 min and 6 day time points (data not shown).
Discussion
We have argued that the translational value of animal research is enhanced by the degree to which the METH protocols used simulate the exposure patterns experienced by METH abusers. In the present study, we used a drug administration paradigm to at least partially compensate for the profound species difference in METH half-life (Cho et al. 2001).
Behavior
Both quantitative and qualitative changes in behavior were found to occur during the ED phase of the experiment. As the interinjection interval decreased, drug-induced behavioral activation became progressively more continuous, eventually persisting throughout the entire treatment period, as well as into the early portion of the light phase. In addition, profound qualitative changes in the behavioral profile were observed, including the emergence of progressively more intense and persistent focused stereotypy episodes, interrupted by correspondingly shorter intervals of locomotor bursting (Segal and Kuczenski 1997b). This mixture of stereotypy and periodic bursts of locomotion predominated by the end of the ED phase, when the total dose during each treatment period was 4.0 mg/kg, spread out in 32, 0.125-mg/kg injections over 10 h. Importantly, since acute injection of doses even considerably higher than 4.0 mg/kg does not produce this behavioral profile, it appears that the continuous exposure to increasing levels of METH within each day (see Fig. 2) was largely responsible for the occurrence of this unique behavior (Segal and Kuczenski 1997a, b). Although sensitization mechanisms may also contribute to the progressively enhanced behavioral effects that occurred during the ED phase, the qualitative changes in the behavioral profile are difficult to explain by any simple sensitization model (Segal 1975; Segal and Kuczenski 1987, 1994). We suggest that by the end of the ED phase, neurochemical changes produced by the relatively prolonged exposure to METH were largely responsible for the qualitatively distinct behavioral profile that emerged (see below).
It should also be noted that during maintenance treatment, by 1–2 h after the start of each day’s drug administration, the behavioral profile became relatively constant until subsiding within about 2 h after the last injection. This generally sustained, high state of behavioral activation is in marked contrast to the distinct behavioral fluctuations which occur with the series of successive subcutaneous injections in “binge” protocols we and others have used previously. We suggest that the more continuous exposure to METH and the corresponding high levels of sustained activation, which result from the present injection procedure, more closely simulates METH effects in humans than does the successive subcutaneous injection regimens used in most animal binge studies. Therefore, the neurochemical consequences of more continuous METH exposure may more closely reflect those that occur in METH abusers.
Pharmacokinetics
Plasma levels of METH at the end of the maintenance phase were near 1.0 μM. Based on extrapolations from pharmacokinetic analyses of intravenous METH in humans (Cook et al. 1993), levels near 1.0 μM would be anticipated following administration of a dose of about 40 mg in a 70-kg human, a dose which has been suggested to be within the range used by METH abusers (McCann et al. 1998; Angrist 1994). In addition, recent data from 70 arrested ongoing METH abusers revealed mean plasma levels near 1.4 μM (Melega et al., unpublished). Furthermore, based on the limited amount of data available, brain levels of METH in our rats also appear to be in the range achieved by human METH abusers. For example, Kalasinsky et al. (2001) characterized regional brain levels in autopsied METH abusers and found median values in frontal cortex, caudate, and cerebellum of 10.7, 9.8, and 9.1 nmol/g, respectively, similar to values we obtained (Table 3). It appears, therefore, that our ED–MP treatment protocol resulted in plasma and brain levels of the drug that may be relevant to some patterns of METH abuse.
However, it is important to note that the plasma METH levels in our rats were below values we predicted from our previous modeling (1.07 vs 1.64 μM at 15 min after the last injection) (Cho et al. 2001). One possible explanation for this difference is that pharmacokinetic adaptations, including changes in METH disposition or metabolism, may have occurred as a consequence of the ED–MP treatment paradigm and thus could have contributed to the lower than predicted plasma METH levels. However, in previous studies, we have not observed changes in METH pharmacokinetics following a subcutaneous escalating dose pretreatment (O’Neil et al, in preparation). Furthermore, when METH pharmacokinetic alterations have been observed in other laboratories, increases, rather than decreases, in plasma METH concentrations have been reported (Schmidt et al. 1985a; Gygi et al. 1996; Kitaichi et al. 2003); some of these earlier studies (Schmidt et al. 1985a; Gygi et al. 1996) also found substantial changes in brain/plasma METH ratios. In contrast, the near tenfold difference in brain and plasma concentrations observed in the present study was similar to the ratio typically observed following acute administration of the drug (Melega et al. 1995; Gilles et al. 2000), suggesting that the prolonged chronic METH pretreatment in our study did not substantially alter METH penetration and accumulation in the brain.
Differences between observed and predicted plasma METH concentrations may also stem from our selection of specific pharmacokinetic parameters for purposes of modeling (Cho et al. 2001) since dose-dependent physiological/hemodynamic changes may affect actual pharmacokinetic values. In particular, peak concentrations in response to each METH injection and plateau levels during steady-state conditions would be particularly sensitive to variations in volume of distribution, for which a wide range of values has been reported (Melega et al. 1995; Hutchaleelaha and Mayersohn 1996; Rivière et al. 1999; Gilles et al. 2000; Kitaichi et al. 2003). It is important to note, however, that regardless of the actual volume of distribution, the temporal features of the plasma concentration profile remain similar.
Neurochemistry
The ED–MP treatment resulted in a number of changes in striatal dopaminergic components which exhibited distinguishable temporal profiles of recovery, suggesting potentially different underlying mechanisms. First, both D1 and D2 dopamine receptors were significantly decreased by about 10% for at least 6 days following cessation of drug administration, and both had fully recovered by the 30 day time point. Acute administration of moderate to high doses of amphetamine-like stimulants results in a rapid desensitization of the DA D1 receptor-stimulated adenylate cyclase (Barnett and Kuczenski 1986; Roberts-Lewis et al. 1986), and this drug-induced desensitization process appears to be facilitated by repeated stimulant pretreatment (Barnett et al. 1987). Similar to these animal data, Tong et al. (2003) reported decreased DA-stimulated adenylate cyclase activity in the striatum of METH users. In addition, consistent with our D2 receptor results, Volkow et al (2001a) reported a 16% decrease in caudate D2 DA receptors in METH abusers. That the D1 and D2 decreases had fully recovered suggests that these changes in DA receptor function may reflect adaptive mechanisms in response to the prolonged increase in dopaminergic transmission during METH exposure.
The ED–MP treatment also resulted in pronounced decrements in DAT and DA levels (∼25%) which were evident for at least 6 days, but which exhibited partial recovery by the 30-day time point. The decreases in DA and DAT, like the changes in DA receptors, may also reflect mechanisms involved in compensatory down regulation in response to the prolonged exposure to METH, with more gradual restoration of predrug functioning after cessation of drug administration. Furthermore, with a more prolonged drug-free interval, DA and DAT levels could exhibit full recovery. However, an alternative explanation for the absence of full recovery is suggested by our VMAT2 data, which exhibited no recovery from the 10% decrement that was evident 15 min after cessation of METH exposure. In fact, by the 30-day time point, all three DA nerve terminal markers were diminished by about 10% relative to controls. Because it has been argued that the vesicular transporter may be used as an index of DA terminal integrity (Wilson and Kish 1996; Naudon et al. 1994; Vander Borght et al. 1995; Frey et al. 1997; Guilarte 2001; Wilson et al. 1996; Guilarte et al. 2003), the decrement in VMAT2 and correspondingly reduced DAT and DA levels may reflect METH-induced damage to DA nerve terminals. In their studies of postmortem tissue samples from METH abusers, Wilson et al. (1996) found no significant decrement in VMAT2. However, it may be important to note that their VMAT2 values were in fact reduced by about 6% compared to controls, although detection of statistical significance may have been limited by the relatively large variability and small number of samples.
We suggest, therefore, that repeated, sustained increases in synaptic DA produce reversible compensatory adaptations in components of both presynaptic (DA, DAT) and postsynaptic (D1, D2 DA receptors) DA function which recover with cessation of METH exposure. However, because the METH treatment used in the present study may also promote damage to DA terminals, as perhaps reflected in the VMAT2 decrement, recovery of DA and DAT levels by the 30-day time point is limited to the remaining DA nerve terminals. It is important to note, however, that relatively small decreases in DA nerve terminal markers may not necessarily involve a decrement in synaptic DA function. For example, corresponding to the reductions in DA and DAT, caudate DOPAC and HVA levels were significantly elevated at both the 6 and 30 day time points, which may reflect a compensatory increase in DA synthesis in response to the loss of DA terminals.
In contrast to our results, most previous preclinical studies, particularly those that have used an acute “neurotoxic binge” protocol, have found more pronounced and relatively persistent decrements in many DA terminal markers (Bowyer and Holson 1995; Fleckenstein et al. 2000; Eisch et al. 1992; Cass 1997; Cass and Manning 1999; Seiden and Ricaurte 1987; Ricaurte et al. 1980). Our inclusion of an ED regimen may have attenuated the effects of subsequent higher dose administration (Segal et al. 2003), and it is conceivable that the pattern of repeated, prolonged periods of continuous METH exposure during the maintenance phase of the present study also contributes to the more transient and less severe neurotoxic effects. However, it is also important to note that, although our animals received a relatively high total amount of drug (>300 mg/kg) over 54 days of treatment, the highest daily dose was 10 mg/kg, whereas the total dose used in most acute “neurotoxic binge” protocols ranges from 16 to 40 mg/kg or higher. Therefore, the differences in either the pattern of drug exposure and/or peak levels may be responsible for differences in the magnitude and persistence of DA terminal changes. In this regard, although peak METH levels have been shown to affect the degree of neurotoxicity, several studies which examined the long-term effects of METH abuse have also suggested a relationship between the cumulative dose and/or duration of exposure and the changes associated with METH abuse (Volkow et al. 2001c; Chang et al. 2002; Ernst et al. 2000).
The procedure used in the present study was designed to at least partially compensate for the species differences in METH elimination half-life, thereby approximating the relatively more sustained drug exposure associated with human METH administration. However, several potentially important differences should be noted. For one, the 15 min injection interval in rats was selected to simulate the METH levels expected from injections of the same dose every 3 h in humans (Cho et al. 2001). However, the more frequent injections result in correspondingly more frequent fluctuations in METH levels, although the magnitude of these oscillations is considerably less than what occurs with the repeated administration of METH using the “neurotoxic binge” protocol. In addition, because of the differences in elimination half-life, the decline in METH level after the last dose is far more gradual in humans than in rats. Nevertheless, although potentially important differences exist, we suggest that the procedure used in the present study more closely simulate the human METH exposure patterns than do other commonly used preclinical models. Importantly, the use of this ED-maintenance procedure has revealed dynamic changes in dopamine nerve terminal markers that may provide insight into the mechanisms contributing to the long-term consequences of METH abuse.
In summary, our ED-maintenance paradigm resulted in periods of prolonged behavioral activation, characterized by progressively more intense stereotypy episodes, interrupted by bursts of locomotion. After discontinuation of treatment, all the markers of striatal DA function measured were significantly reduced. By 30 days following the last METH injection, there was full or partial recovery of all the markers except VMAT2, which remained at about 90% of control levels throughout the withdrawal period. These results suggest that the frequent injection paradigm, designed to more closely simulate at least one human METH exposure pattern, may have produced some damage to caudate DA nigrostriatal terminals in addition to compensatory adjustments in various mechanisms involved in maintaining normal levels of striatal DA transmission.
References
Angrist B (1994) Amphetamine psychosis: clinical variations of the syndrome. In: Cho AK, Segal DS (eds) Amphetamine and its analogues. Academic, San Diego, pp 387–414
Barnett JV, Kuczenski R (1986) Desensitization of rat striatal dopamine-stimulated adenylate cyclase after acute amphetamine administration. J Pharmacol Exp Ther 237:820–825
Barnett JV, Segal DS, Kuczenski R (1987) Repeated amphetamine pretreatment alters the responsiveness of striatal dopamine-stimulated adenylate cyclase to amphetamine-induced desensitization. J Pharmacol Exp Ther 242:40–47
Bowyer JF, Holson RR (1995) Methamphetamine and amphetamine neurotoxicity. In: Chang LW, Dyer RS (eds) Handbook of neurotoxicology. Marcel Dekker, Inc., New York, pp 845–870
Cass WA (1997) Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther 280:105–113
Cass WA, Manning MW (1999) Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci 19:7653–7660
Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN (2002) Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res Neuroimaging 114:65–79
Cho AK, Melega WP, Kuczenski R, Segal DS (2001) Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse 39:161–166
Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M (1993) Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos 21:717–723
Coulter CL, Happe HK, Bergman DA, Murrin LC (1995) Localization and quantification of the dopamine transporter: comparison of [3H]WIN 35,428 and [125I]RTI-55. Brain Res 690:217–224
Darchen F, Masueo Y, Vial M, Rostene W, Scherman D (1989) Quantitative autoradiography of the rat brain vesicular monoamine transporter using the binding of [3H]dihydrotetrabenazine and 7-amino-8-[125I]iodoketanserin. Neuroscience 33:341–349
Davidson C, Gow AJ, Lee TH, Ellinwood EH (2001) Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev 36:1–22
Eisch AJ, Gaffney M, Weihmuller FB, O’Dell SJ, Marshall JF (1992) Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res 598:321–326
Ernst T, Chang L, Leonido-Yee M, Speck O (2000) Evidence for long-term neurotoxicity associated with methamphetamine abuse—a 1H MRS study. Neurology 54:1344–1349
Fleckenstein AE, Gibb JW, Hanson GR (2000) Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol 406:1–13
Frey KA, Kilbourn MR, Robinson TE (1997) Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol 334:273–279
Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO (2003) Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci 23:7239–7245
Gilles J, Rivière GJ, Gentry WB, Owens SM (2000) Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther 292:1042–1047
Guilarte TR (2001) Is methamphetamine abuse a risk factor in parkinsonism? Neurotoxicology 22:725–731
Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience 122:499–513
Gygi MP, Gygi SP, Johnson M, Wilkins DG, Gibb JW, Hanson GR (1996) Mechanisms for tolerance to methamphetamine effects. Neuropharmacology 35:751–757
Harris DS, Boxenbaum H, Everhart ET, Sequeira G, Mendelson JE, Jones RT (2003) The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther 74:475–486
Harvey DC, Lacan G, Tanious SP, Melega WP (2000) Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 871:259–270
Hutchaleelaha A, Mayersohn M (1996) Influence of activated charcoal on the disposition kinetics of methamphetamine enantiomers in the rat following intravenous dosing. J Pharm Sci 85:541–545
Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer ANM (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24:566–573
Kalasinsky KS, Bosy TZ, Schmunk GA, Reiber G, Anthony RM, Furukawa Y, Guttman M, Kish SJ (2001) Regional distribution of methamphetamine in autopsied brain of chronic human methamphetamine users. Forensic Sci Int 116:163–169
Kitaichi K, Morishita Y, Doi Y, Ueyama J, Matsushima M, Zhao YL, Takagi K, Hasegawa T (2003) Increased plasma concentration and brain penetration of methamphetamine in behaviorally sensitized rats. Eur J Pharmacol 464:39–48
Kuczenski R, Segal DS, Cho AK, Melega WP (1995) Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci 15:1308–1317
McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA (1998) Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18:8417–8422
Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK (1995) Pharmacokinetic and pharmacodynamic analysis of the actions of d-amphetamine and d-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther 274:90–96
Naudon L, Leroux-Nicollet I, Costentin J (1994) Short-term treatments with haloperidol or bromocriptine do not alter the density of the monoamine vesicular transporter in the substantia nigra. Neurosci Lett 173:1–4
Nielsen EN, Lee TH, Ellison G (1980) Following several days of continuous administration d-amphetamine acquires hallucinogen-like properties. Psychopharmacology 68:197–200
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, Sydney
Ricaurte GA, Schuster CR, Seiden LS (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193:153–163
Rivière GJ, Byrnes KA, Gentry WB, Owens SM (1999) Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther 291:1220–1226
Roberts-Lewis JM, Roseboom PH, Iwaniec LM, Gnegy ME (1986) Differential down-regulation of D1-stimulated adenylate cyclase activity in rat forebrain after in vivo amphetamine treatments. J Neurosci 6:2245–2251
Schmidt CJ, Gehlert DR, Peat MA, Sonsalla PK, Hanson GR, Wamsley JK, Gibb JW (1985a) Studies on the mechanism of tolerance to methamphetamine. Brain Res 343:305–313
Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW (1985b) Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 233:539–544
Schmidt D, Roznoski M, Ebert MH (1990) Qualitative and quantitative high performance liquid chromatographic analysis of monoamine neurotransmitters and metabolites in cerebrospinal fluid and brain tissue using reductive electrochemical detection. Biomed Chromatogr 4:215–220
Segal DS (1975) Behavioral and neurochemical correlates of repeated d-amphetamine administration. In: Mandell AJ (ed) Advances in biochemical psychopharmacology. Raven Press, New York, pp 247–266
Segal DS, Kuczenski R (1974) Tyrosine hydroxylase activity: regional and subcellular distribution in brain. Brain Res 68:261–266
Segal DS, Kuczenski R (1987) Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther 242:917–926
Segal DS, Kuczenski R (1994) Behavioral pharmacology of amphetamine. In Cho AK, Segal DS (eds) Amphetamine and its analogues: psychopharmacology, toxicology and abuse. Academic, San Diego, pp 115–150
Segal DS, Kuczenski R (1997a) An escalating dose “binge” model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci 17:2551–2566
Segal DS, Kuczenski R (1997b) Repeated binge exposure to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther 282:561–573
Segal DS, Kuczenski R (1999b) Escalating dose/binge stimulant exposure: relationship between emergent behavioral profile and differential caudate-putamen/nucleus accumbens dopamine responses. Psychopharmacology 142:182–192
Segal DS, Kuczenski R (2000) Escalating dose-binge exposure to amphetamine and methamphetamine: behavioral and neurochemical characterization. In: Contemporary neuropsychiatry (Proceedings of the 3rd International Congress of Neuropsychiatry). Springer-Verlag, Tokyo
Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2003) Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology 28:1730–1740
Seiden LS, Ricaurte GA (1987) Neurotoxicity of methamphetamine and related drugs. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven Press, New York, pp 359–366
Shi X, Yin R, Dow-Edwards D (1999) Chronic haloperidol alters dopamine receptors: effects of cocaine exposure during the preweaning period. Eur J Pharmacol 370:241–249
Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W (2002) A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 21:35–44
Tong JC, Ross BM, Schmunk GA, Peretti FJ, Kalasinsky KS, Furukawa Y, Ang LC, Aiken SS, Wickham DJ, Kish SJ (2003) Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase activity in human methamphetamine users. Am J Psychiatry 160:896–903
Unterwald EM, Kreek MJ, Cuntapay M (2001) The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res 900:103–109
Vander Borght TM, Kilbourn MR, Desmond T et al (1995) The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 294:577–583
Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N (2001a) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021
Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN (2001c) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382
Wilson JM, Kish SJ (1996) The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci 16:3507–3510
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Acknowledgements
This research was supported by NIH Grants DA-01568 and DA-02854 and a fellowship (DA 14449) to M.L.O.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Segal, D.S., Kuczenski, R., O’Neil, M.L. et al. Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology 180, 501–512 (2005). https://doi.org/10.1007/s00213-005-2188-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2188-4