Abstract
Background
Abnormalities of membrane phospholipid metabolism have been described in Alzheimer’s disease (AD). We investigated, with the aid of 31P magnetic resonance spectroscopy, the in vivo intracerebral availability of phosphomonoesters (PME) and phosphodiesters (PDE) in patients with AD.
Methods
Eighteen outpatients with mild or moderate probable AD and 16 nondemented elderly volunteers were assessed with the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) and its cognitive subscale of the CAMDEX schedule (CAMCOG). Scans were performed on a 1.5 T magnetic resonance imager addressing a 40-cm3 voxel in the left prefrontal cortex. Main outcome measures were mean relative peak areas of PME and PDE, which provide an estimate of membrane phospholipid metabolism.
Results
PME resonance and the PME/PDE ratio were increased in AD patients as compared to controls (p<0.05). PME was negatively correlated with global cognitive performance as shown by the Mini-Mental State Examination (rs=−0.36, p=0.05) and CAMCOG scores (rs=−0.49, p=0.007), as well as with discrete neuropsychological functions, namely, memory (rs=−0.53, p=0.004), visual perception (rs=−0.54, p=0.003), orientation (rs=−0.36, p=0.05), and abstract thinking (rs=−0.48, p=0.01).
Conclusions
We provide evidence of reduced membrane phospholipid breakdown in the prefrontal cortex of mild and moderately demented AD patients. These abnormalities correlate with neuropsychological deficits that are characteristic of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormalities in the lipid composition have been identified in different regions of the brain of Alzheimer’s disease (AD) patients. Postmortem studies showed lower docosahexanoate levels in the parietal cortex (Skinner et al. 1989) and reduced total free fatty acid content in prefrontal areas (Talbot et al. 2000). Anisotropy studies have additionally demonstrated abnormal membrane fluidity in hippocampal synaptosomes (Eckert et al. 2000).
Phosphatidylcholine (PC) represents ∼40% of the phospholipid contents of eukaryotic membranes (Klein 2000). In cholinergic neurons, PC is converted into lyso-PC, releasing arachidonic acid and substrates for the subsequent synthesis of acetylcholine (Blusztajn et al. 1987). The catabolic enzymes implicated in this pathway belong mostly to the phospholipase A2 (PLA2) family (Dennis 1994). In AD, abnormal enzymatic activity of different PLA2 subtypes has been consistently reported. Gattaz et al. (1995a,b) demonstrated that calcium-dependent cytosolic PLA2 (cPLA2) displays reduced enzymatic activity in the frontal and parietal cortex, the latter finding being further associated with higher counts of senile plaques and neurofibrillary tangles (Gattaz et al. 1996). Ross et al. (1998) showed that PLA2 activity is significantly decreased in the temporoparietal cortex and hippocampus. Finally, lower calcium-independent PLA2 (iPLA2) activity was identified in the prefrontal cortex of frontal-variant AD patients (Talbot et al. 2000). Taken together, such findings suggest that aberrations in the synthesis and degradation of membrane phospholipids are diffuse metabolic events that occur in AD brains.
The intracerebral availability of phospholipid precursors and metabolites, as well as the occurrence of high-energy phosphates, can be estimated by the analysis of the 31P spectral curve within a discrete brain area with the aid of magnetic resonance spectroscopy (MRS). Phosphomonoesters (PME) and phosphodiesters (PDE) are the precursors and breakdown products of membrane phospholipids, respectively, and the ratio between both provides information on membrane phospholipid turnover. 31P MRS studies of AD patients reported altered PME and PDE resonance in temporoparietal areas, which reflects changes in the biophysical state of membrane phospholipids (Pettegrew et al. 1988, 1995; Brown et al. 1989; Smith et al. 1993). Pettegrew et al. (1988) found elevated PME levels in cortical areas of patients with mild AD and increased PDE levels in severe AD, hypothesizing that PME changes occur early in the disease, whereas PDE changes occur later. The authors further proposed that changes in membrane phospholipid and high-energy phosphate metabolism might precede the onset of dementia (Pettegrew et al. 1995). Brown et al. (1989) showed that PME, PME/PDE ratio, and inorganic phosphate levels were elevated in temporoparietal areas of AD patients as compared to controls, although in another series addressing brain energy metabolism, no significant difference between AD and controls was observed for either PME or PDE (Brown et al. 1993). Gonzalez et al. (1996) reported a 50% increase in PME/PDE ratio, but no changes in phosphocreatine, beta-nucleoside triphosphate (beta-NTP), and inorganic phosphate levels in AD brains. Bottomley et al. (1992) and Murphy et al. (1993), however, found no significant differences in any 31P indexes, addressing whole axial sections through the lateral cerebral ventricles of the brain.
Correlations with morphologic data further suggested that elevations in PME levels precede the appearance of senile plaques in early stages of AD, whereas elevations in PDE levels, reflecting degeneration of neural membranes, correlate with the appearance of plaques in late stages of the disease (Pettegrew et al. 1988). In autopsy specimens of parietal gray matter from subjects with severe AD, spectroscopy abnormalities correlated with the density of neurofibrillary tangles (Smith et al. 1993).
The objective of the present study was to investigate, with the aid of 31P MRS, the in vivo cerebral phospholipid metabolism in a sample of patients with mild and moderate AD. In contrast to previous MRS studies, we addressed, in a larger sample, the 31P spectrum within a voxel located in the prefrontal cortex, rather than temporoparietal, taking into account the available evidence drawn from biochemical studies of abnormal phospholipid metabolism also in frontal areas. Our data corroborate earlier indications of abnormal phospholipid metabolism in the AD brain and further suggest that this biological finding is a more widespread phenomenon than previously thought. Based on the data from the literature exposed above, we hypothesized that a reduced phospholipid breakdown in AD brain could be detected as an increment of PME and a decrement of PDE.
Methods and materials
Our sample comprised 18 patients with probable AD according to NINCDS-ADRDA (McKhann et al. 1984) and 16 age-matched nondemented healthy volunteers. AD patients had Mini-Mental State Examination (MMSE; Folstein et al. 1975) scores of at least 18 so that only patients with mild and moderate AD were included in the test group. Illiterate subjects were not included in the study. Patients and controls were submitted to physical and neurological examinations including laboratory tests and brain imaging, i.e., computed tomography (CT) or magnetic resonance imaging (MRI), with the purpose of excluding non-Alzheimer dementias. In order to rule out vascular contributions to cognitive decline, subjects with a Hachinski score higher than 3 (Hachinski et al. 1975) or those with CT or MRI evidence of leukoaraiosis were not included in the study. Clinical data and cognitive performance were assessed by the Brazilian version (Bottino et al. 1999) of the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) interview (Roth et al. 1986) and its cognitive subscale CAMCOG. Briefly, the CAMDEX interview encompasses the Mini-Mental State Examination (Folstein et al. 1975), the Abbreviated Mental Test (AMT; Hodkinson 1972), part of the Blessed Dementia Scale (Blessed et al. 1969), and the Hachinski score (Hachinski et al. 1975). The cognitive domains ascertained by the CAMCOG schedule include memory, orientation, language, attention, praxis, calculation, abstract thinking, and visual perception subtests. The Clinical Dementia Rating Scale (CDR) score (Hughes et al. 1982) was used to ascertain the severity of dementia. Controls were evaluated with the same battery in order to ensure healthy cognitive functions. There were no significant differences between the two groups regarding age, gender, years of schooling, and the Hachinski score (Table 1). Patients and controls were right-handed and were drug-free for at least 2 weeks, except for non-CNS-acting medicines and benzodiazepines, which probably do not affect brain phospholipid metabolism, as opposed to neuroleptics and possibly antidepressants (Gattaz et al. 1987). Thus, subjects undergoing treatment with cholinesterase inhibitors, antidepressants, or neuroleptics were not included in the sample. Informed consent was provided prior to enrolment.
Spectroscopy determinations
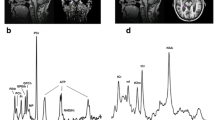
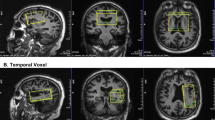
Magnetic resonance spectroscopy (MRS) scans were performed on a Philips Gyroscan ACSIII system operating at 1.5 T and addressing a 40-cm3 voxel (28×28×50 mm) in the left prefrontal cortex (Fig. 1). A body coil was used to obtain T1-weighted axial, coronal, and sagittal localizer images, and a 31P-dedicated head coil was used to obtain the spectra of defined volumes of interest in the prefrontal lobe. In spite of the fact that the body coil produces images with a lower signal-to-noise ratio, it was preferred in this case for imaging rather than a conventional proton birdcage head coil, which would require changing and recalibrating coils during each exam. After a localizer scan, 16 slices were done in transversal, sagittal, and coronal planes. Anatomical images were used for planning the volumes of interest in the left prefrontal cortex. Spectroscopy scans were performed with a 28-cm-diameter quadrature transmit/receive birdcage head coil. The method we used was an image-selected in vivo spectroscopy (ISIS) pulse-acquire sequence (Ordidge et al. 1986) with one volume selection. Repetition time was 3,000 ms. The free induction decay (FID) sampling rate was 2,000 Hz. The number of sample points was 1,024 and the average of each spectrum was 768 measurements. The magnetic field homogeneity was shimmed to a range that contains the volume. Shimming resulted in line widths<10 Hz. The spectra were processed by means of the interactive public-domain software Magnetic Resonance User Interface (MRUI, Java version), which is a graphical user interface that yields time-domain analysis of in vivo MR spectroscopy data and has been shown to be adequate for similar purposes in previous studies conducted by our group (Yacubian et al. 2002). The average FID signal was zero-filled to 2,048 data points and processed with a convolution difference procedure (80 Hz) and exponential multiplication (8 Hz). After Fourier transformation, a zero-order and a linear-phase correction was applied, which enabled spectral peaks obtained in the time domain to be transformed into a frequency domain curve. For chemical shift adjustment, the phosphocreatine (PCr) resonance was used, which was set to 0 parts per million (ppm) in accordance with conventions. To reduce the background signal, a baseline correction was applied, which calculates an average value for each point in a resonance free part of the spectrum (noise). This average was then subtracted from the original spectrum and the result was reverse Fourier-transformed to obtain a time-domain signal for quantification purposes. The spectra were not proton-decoupled because such resource was not available with the equipment we used. Spectral curves of 31P-containing molecules were obtained, and the respective peaks of PME, PDE, PCr, adenosine-triphosphate (α-, β-, and γ-ATP), and inorganic phosphate (Pi) were determined. Peaks of any given spectrum were selected by applying the noise amplitude for discrimination between peaks and noise. Following manual determination of eligible peaks by the operator, underpeak areas (amplitudes in the time domain) were automatically calculated by the MRUI software using the Advanced Method for Accurate, Robust and Efficient Spectral Fitting (AMARES; Vanhamme et al. 1997) algorithm in the time domain. Relative values were obtained for each phosphometabolite peak in terms of percent areas, which represented the ratio between the underpeak area of any given metabolite peak to the sum of all underpeak areas within the same spectrum (Fig. 2). The analysis of spectroscopy data was blind to proband status and was made by a senior radiologist (C.C.C.).
31P-MRS spectrum. From bottom to top: original 31P spectrum obtained in the frequency domain in parts per million (ppm), assuming that phosphocreatine (PCr)=0 ppm, in accordance with conventions: estimated spectrum generated after MRUI/AMARES processing; individual components of the 31P spectrum, representing peaks of phosphomonoesters (PME) (7), inorganic phosphorus (Pi) (6), phosphodiesters (PDE) (5), phosphocreatine (PCr) (4), and γ-, α-, and β-fractions of adenosine triphosphate (ATP) (3, 2, 1); residual curve after subtracting the estimated curve from the original curve
Statistical analysis
PME, PDE, and the PME/PDE ratio were the primary variables with respect to membrane phospholipid metabolism. Means were obtained for the relative underpeak areas of PME and PDE for patients and controls and were compared with the aid of two-tailed t tests for independent samples. Pearson’s correlation coefficients were used to test the association between PME and five a priori defined neuropsychological variables related to AD, namely, the scores on total CAMCOG and subscales memory, visual perception, orientation, and abstract thinking). Statistical analyses were performed using SPSS Version 10.0.1 (SPSS Inc., Chicago, IL).
Because a considerable amount of broad signal is removed from the quantified spectra during the transformation of raw into estimated curves by the MRUI/AMARES protocol, it is necessary to certify that the overall quality of data is equivalent in the two groups. Thus, we compared PME and PDE line widths in controls and patients and no significant differences were found, which demonstrate that no significant group bias was introduced as a consequence of data processing (Table 1).
Results
Alzheimer’s disease patients, as expected, had lower MMSE and CAMGOG scores (Table 1). We found a significant increase in PME resonance in AD as compared to controls (AD 8.7±3.0, controls 6.8±2.1, t=−2.05, p<0.05) and in the PME/PDE ratio (AD 0.3±0.1, controls 0.2±0.01, t=−2.10, p<0.05). PDE values in AD (33.1±4.2) were lower than in controls (36.5±5.7), but marginally above significant limits (t=1.89, p=0.06; Fig. 3). No significant differences were observed regarding PCr, Pi, and alpha-, beta-, and gamma-ATP spectral values (Table 2). No differences in PME and PDE values were found between male and female within both AD and control groups (data not shown).
Spectroscopy data of patients with Alzheimer’s disease (AD) and controls: phosphomonoesters (PME) and phosphodiesters (PDE) values represent percent areas calculated in terms of the ratio between PME or PDE under-peak area to the sum of all under-peak areas within the same spectral curve. a, b Distribution of Alzheimer’s disease and control subjects according to percent areas of PME and PDE resonance, respectively. c PME/PDE ratio
PME and PDE resonances were negatively correlated (r=−0.42, p<0.02). In the whole sample (18 AD and 16 controls), we found negative correlations between PME values and global cognitive performance, namely, total MMSE (Pearson’s r=−0.36, p=0.05) and total CAMCOG (r=−0.49, p=0.007) scores. PME/PDE ratio was also negatively correlated with the CAMCOG score (r=−0.43, p=0.01). Higher PME was associated with a worse performance in discrete subtests of the CAMCOG schedule, such as memory (r=−0.53, p=0.004), visual perception (r=−0.54, p=0.003), orientation (r=−0.36, p=0.05), and abstract thinking (r=−0.48, p=0.01). The magnitude of these correlation coefficients remained similar within the subgroups AD and controls analyzed separately, but significance was lost (Table 3). No correlations were found between PME and PDE values with the scores on attention (r=−0.04, p=0.82), language (r=−0.28, p=0.16), arithmetic (r=−0.24, p=0.23), and executive functions (r=−0.15, p=0.44).
Discussion
Our data corroborate previous 31P spectroscopy evidence of abnormal cerebral phospholipid metabolism in AD. The findings of increased PME levels and increased PME/PDE ratio suggest either increased synthesis in membrane phospholipids, or a reduction of the metabolic pathway, or perhaps a combination of the two mechanisms. Since PME and PDE are the precursors and metabolites of membrane phospholipids, respectively, an increased PME resonance, associated with a decreased PDE, suggests a reduced phospholipid turnover affecting neuronal membranes. An increase in PME might as well be interpreted as a consequence of higher precursor levels, in which case, however, PDE levels would be expected to be higher.
The lack of significant differences between patients and controls regarding the values of PCr, Pi, alpha-, beta-, and gamma-ATP in this sample suggests that brain energy stores are not depleted in prefrontal areas at early stages of AD. Pettegrew et al. (1994) identified severity-dependent alterations in high-energy phosphates and membrane phospholipids metabolism in mild probable AD patients. In accordance with our findings on membrane phospholipids, the authors propose that mildly demented AD patients display increased levels of PME, which tend to decrease with the progression of the dementing process, as a cumulative consequence of nerve terminal degeneration (Pettegrew et al. 1994). This important observation possibly explains the inconsistency of findings derived from studies that included patients at different stages of AD.
Such abnormality may not be restricted to the parietal cortex, as demonstrated by previous studies (Brown et al. 1989). The present study rather supports the assumption that abnormal phospholipid metabolism is observed in prefrontal areas as well. Smith et al. (1995) also used the 31P MRS technique to address the metabolism of phosphate-containing molecules in the frontal lobe of 17 AD patients. In that sample, the reduction in the PME/PDE ratio was predominantly in female AD patients, whereas in our study, findings were observed in both male and female.
A recent publication by Mulder et al. (2003) showed a significant reduction in the concentration of lysophosphatidylcholine (lyso-PC) in the CSF of AD patients, as measured by tandem mass spectrometry. Because total phosphatidylcholine (PC) concentrations were unchanged among patients and controls, the authors concluded that the lower lyso-PC/PC ratio in patients with AD was a consequence of a decreased breakdown of PC. Such biological finding in the CSF of AD patients is highly consistent with our 31P MRS evidence of reduced phospholipid turnover in the AD brain.
In our total sample, higher PME was correlated with severity of cognitive decline, as shown by lower total CAMCOG scores, and with the degree of impairment on discrete neuropsychological functions, namely, memory, orientation, abstract thinking, and visual perception (which, in the CAMCOG schedule, refers to recognition and naming skills). The negative association between PME and total CAMCOG, as well as between PME and memory, and between visual perception and abstract thinking subscores, remained within significant levels after Bonferroni’s correction. Verbal and nonverbal (visual) memory processing are cognitive abilities typically affected in mild and moderate AD, indicating temporoparietal damage. On the other hand, impairment on abstract thinking suggests some degree of frontal lobe dysfunction. The direction of these correlations was also observed in the subsamples of AD patients and controls, but with loss of significance probably due to the reduction of samples size.
Other studies have tentatively addressed correlation between 31P spectroscopy parameters and the degree of cognitive decline and dementia in AD. Smith et al. (1995) found a negative correlation between the phosphocreatine/inorganic phosphate (PCr/Pi) ratio, which was lower in AD, and the severity of dementia as assessed by dementia rating scale. Cuenod et al. (1995) found a significant increase in the phosphomonoester/total phosphorus ratio in patients with AD as compared to controls. Although in their sample the use of a ratio above 11% as a threshold yielded 83.3% sensitivity and 73.3% specificity test for AD, no metabolic parameter correlated with the cognitive state as assessed by the Mini-Mental State Examination (Cuenod et al. 1995).
There are methodological limitations that could not be overcome with the available 1.5-T equipment. It was not our intention to provide localizable information in this study, and we were unable to quantify the proportion of white and gray matter in the voxel, which would enable us to control for a possible asymmetry in voxel composition between the two groups. Regarding the contribution from grey and white matter to the present findings, it should be noticed that although one subgroup of PLA2 (cytosolic) is only present in grey matter (Stephenson et al. 1994), there are no localizable findings regarding the calcium-independent form of the enzyme (iPLA2), which is responsible for about 75% of PLA2 activity in the brain.
In addition, given the fact that in the MR spectrum only molecules which have enough mobility (long T2) are visible, one cannot rule out that the different PME/PDE ratio between patient and control is due to the visibility of PME and/or PDE signals rather than to the change of the amount of PME or PDE compounds.
Nevertheless, the metabolic abnormalities that were ascertained with the aid of the 31P MRS method did correlate with the expected neuropsychological deficits of mild and moderately demented AD patients. However, it is noteworthy that our 31P MRS determinations addressed the prefrontal cortex, which is relatively spared in early stages of AD. This suggests that abnormal phospholipid metabolism is a widespread phenomenon within the AD brain. Such abnormality can be detected in the initial stages of the dementing illness, in a brain area that is not severely affected by plaque and tangle pathology, as compared to medial temporal lobe structures. Thus, we speculate that increased PME, which is suggestive of abnormal membrane phospholipid metabolism, might be regarded as an intracerebral metabolic marker of mild AD.
Intracerebral phospholipid turnover relies on the integrity of membrane metabolism and its biochemical regulation. If elevated PME levels were due to an increased synthesis in membrane products, which might be interpreted in AD as a compensatory mechanism to the massive loss of synapses, one would expect to identify an increment in the activity of the related enzymes. However, in AD brains, PLA2 activity shifts to the opposite direction, with consistent evidence of reduced catabolic activity in the hippocampus and in the frontal, temporal, and parietal cortex (Gattaz et al. 1996; Ross et al. 1998; Talbot et al. 2000).
This is particularly interesting in the pursuit of alternative AD disease mechanisms. Animal experiments showed that brain PLA2 activity is involved with the formation of short- and long-term memory (Schaeffer and Gattaz, in press). Studies in cell cultures have implicated PLA2 in the regulation of amyloid precursor protein (APP) processing (Emmerling et al. 1993). Arachidonic acid, one of the main products of PC metabolism, also participates in the polymerization of hyperphosphorylated tau protein into neurofibrillary tangles, a critical step in the pathophysiology of Alzheimer’s disease (Lukiw and Bazan 2000). Thus, reduced PLA2 activity may be implicated in the reduction of intracerebral phospholipid turnover and may contribute to the cholinergic loss and the pathological cascades in AD.
In the search for peripheral correlates of cerebral enzymatic abnormalities, several studies have focused on PLA2 activity in platelet membranes (Gattaz et al. 1995a, b), where a reduction in PLA2 activity can also be detected in AD (Gattaz et al. 1996). In a recent study conducted in our group, lower platelet PLA2 activity was correlated with the severity of cognitive decline, in a sample of nondemented memory-impaired subjects and AD patients (Gattaz et al. 2004).
Conclusion
Changes in phospholipid metabolism can be detected in vivo in early stages of AD with the aid of 31P spectroscopy. Discrepancies in the literature may be due to differences in technical setting or in subject population types. Our data reinforce the hypothesis that disordered cerebral phospholipid metabolism is a broad intracerebral phenomenon, which may play an etiological role in Alzheimer’s dementia. We further support the contention that alterations in brain membrane abnormalities can be noninvasively detected by 31P MRS at early stages of the disease, yielding additional diagnostic implications. Further research is needed in order to define whether this finding should be regarded as an etiological factor, or rather as an epiphenomenon of the neurodegenerative process.
References
Blessed G, Tomlinson BE, Roth M (1969) The association between quantitative measures and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Blusztajn JK, Liscovitch M, Richardson UI (1987) Synthesis of acethylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A 84(15):5474–5477
Bottino CMC, Almeida OP, Tamai S, Forlenza OV, Scalco MZ, Carvalho IAM (1999) Entrevista estruturada para diagnóstico de transtornos mentais em idosos—CAMDEX The Cambridge Examination for Mental Disorders of the Elderly. Cambridge University Press (Brazilian version, translated and adapted on behalf of the editors)
Bottomley PA, Cousins JP, Pendrey DL, Wagle WA, Hardy CJ, Eames FA et al (1992) Alzheimer dementia: quantification of energy metabolism and mobile phosphoesters with P-31 NMR spectroscopy. Radiology 183:695–699
Brown GG, Levine SR, Gorell JM, Pettegrew JW, Gdowski JW, Bueri JA et al (1989) In vivo 31P NMR profiles of Alzheimer’s disease and multiple subcortical infarct dementia. Neurology 39(11):1423–1427
Brown GG, Garcia JH, Gdowski JW, Levine SR, Helpern JÁ (1993) Altered brain energy metabolism in demented patients with multiple subcortical ischemic lesions. Working hypotheses. Arch Neurol 50(4):384–8
Cuenod CA, Kaplan DB, Michot JL, Jehenson P, Leroy-Willig A, Forette F et al (1995) Phospholipid abnormalities in early Alzheimer’s disease. In vivo phosphorus 31 magnetic resonance spectroscopy. Arch Neurol 52(1):89–94
Dennis EA (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269:13057–13060
Eckert GP, Cairns NJ, Maras A, Gattaz WF, Muller WE (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11(4):181–186
Emmerling MR, Moore CJ, Doyle PD, Carroll RT, Davis RE (1993) Phospholipase A2 activation influences the processing and secretion of the amyloid precursor protein. Biochem Biophys Res Commun 197(1):292–297
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gattaz WF, Köllisch M, Thuren T, Virtanen JA, Kinnunen PKJ (1987) Increased plasma phospholipase A2 activity in schizophrenic patients: reduction after neuroleptic therapy. Biol Psychiatry 22:421–426
Gattaz WF, Maras A, Cairns NJ, Levy R, Förstl H (1995a) Decreased phospholipase A2 activity in Alzheimer brains. Biol Psychiatry 37:13–17
Gattaz WF, Steudle A, Maras A (1995b) Increased platelet phospholipase A2 in schizophrenia. Schizophr Res 16:1–6
Gattaz WF, Cairns NJ, Levy R, Forstl H, Braus DF, Maras A (1996) Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 246(3):129–131
Gattaz WF, Forlenza OV, Talib LL, Barbosa NR, Bottino CM (2004) Platelet phospholipase A2 activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm 111(5):591–601
Gonzalez RG, Guimaraes AR, Moore GJ, Crawley A, Cupples LA, Growdon JH (1996) Quantitative in vivo 31P magnetic resonance spectroscopy of Alzheimer disease. Alzheimer Dis Assoc Disord 10(1):46–52
Hachinski VC, Iliff LD, Phil M, Zilhka E, Du Boullay GH, Mcallister VI et al (1975) Cerebral blood flow in dementia. Arch Neurol 32:632–637
Hodkinson HM (1972) Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1:233–238
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Klein J (2000) Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm 107(8–9):1027–1063
Lukiw WJ, Bazan NG (2000) Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem Res 25(9–10):1173–1184
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944
Mulder C, Wahlund LO, Teerlink T, Blomberg M, Veerhuis R, Van Kamp GJ et al (2003) Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. J Neural Transm 110(8):949–955
Murphy DG, Bottomley PA, Salerno JA, DeCarli C, Mentis MJ, Grady CL et al (1993) An in vivo study of phosphorus and glucose metabolism in Alzheimer’s disease using magnetic resonance spectroscopy and PET. Arch Gen Psychiatry 50(5):341–349
Ordidge RJ, Connely A, Lohman JÁ (1986) Image-selected in vivo spectroscopy (ISIS). A new technique for spatially selective NMR spectroscopy. J Magn Reson 66:283–294
Pettegrew JW, Kanagasabai P, Moossy J, Martinez J, Rao G, Boller F (1988) Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer’s disease. Arch Neurol 45:1093–1096
Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR (1994) Alterations of cerebral metabolism in probable Alzheimer’s disease: a preliminary study. Neurobiol Aging 15(1):117–132
Pettegrew JW, Klunk WE, Kanal E, Panchalingam K, McClure RJ (1995) Changes in brain membrane phospholipid and high-energy phosphate metabolism precede dementia. Neurobiol Aging 16(6):973–975
Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer’s disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70(2):786–793
Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R (1986) CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 149:698–709
Schaeffer E, Gattaz WF (in press) Inhibition of calcium-independent phospholipase A2 activity in rat hippocampus impairs short- and long-term memory formation. Psychopharmacology
Skinner ER, Watt C, Besson JAO, Best PV (1989) Lipid composition of different regions of the brain in patients with Alzheimer’s disease. Biochem Soc Trans 17:213–214
Smith CD, Gallenstein LG, Layton WJ, Kryscio RJ, Markesbery WR (1993) 31P magnetic resonance spectroscopy in Alzheimer’s and Pick’s disease. Neurobiol Aging 14(1):85–92
Smith CD, Pettigrew LC, Avison MJ, Kirsch JE, Tinkhtman AJ, Schmitt FA et al (1995) Frontal lobe phosphorus metabolism and neuropsychological function in aging and in Alzheimer’s disease. Ann Neurol 38(2):194–201
Stephenson DT, Manetta JV, White DL, Chiou XG, Cox L, Gitter B, May PC, Sharp JD, Kramer RM, Clemens JA (1994) Calcium-sensitive cytosolic phospholipase A2 (cPLA2) is expressed in human brain astrocytes. Brain Res 637:97–105
Talbot K, Young RA, Jolly-Tornetta C, Lee VM, Trojanowski JQ, Wolf BA (2000) A frontal variant of Alzheimer’s disease exhibits decreased calcium-independent phospholipase A2 activity in the prefrontal cortex. Neurochem Int 37(1):17–31
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129(1):35–43
Yacubian J, de Castro CC, Ometto M, Barbosa E, de Camargo CP, Tavares H, Cerri GG, Gattaz WF (2002) 31P-spectroscopy of frontal lobe in schizophrenia: alterations in phospholipid and high-energy phosphate metabolism. Schizophr Res 58(2–3):117–122
Acknowledgements
The present study was supported by Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP, Projects 97/11083-0, 99/00740-5, and 02/13633-7). The Laboratory of Neuroscience receives financial support from the Associação Beneficente Alzira Denise Herzog da Silva (ABADHS).
We are indebted to Dr. Eduardo Simão, M.D., for the contribution to data collection and conduction of MRS scans.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forlenza, O.V., Wacker, P., Nunes, P.V. et al. Reduced phospholipid breakdown in Alzheimer’s brains: a 31P spectroscopy study. Psychopharmacology 180, 359–365 (2005). https://doi.org/10.1007/s00213-005-2168-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2168-8