Abstract
Rationale
Topiramate, an anticonvulsant medication, may be effective as a treatment for alcohol and cocaine addiction. While a recent clinical study has demonstrated the potential utility of topiramate for smoking cessation in alcohol-dependent smokers, the effects of topiramate on tobacco addiction have not been systematically examined in humans.
Objectives
To determine topiramate’s effects on acute physiological and subjective responses to intravenous (IV) nicotine in overnight abstinent smokers.
Methods
Seven male and five female smokers participated in a double-blind, placebo-controlled, crossover study, which consisted of one adaptation and three experimental sessions. Before each session, participants were treated orally with either a single 25 or 50 mg topiramate dose or with placebo. Starting 2 h following the medication treatment, participants received an IV saline injection, followed by 0.5 and 1.0 mg/70 kg IV nicotine.
Results
Topiramate treatment at 50 mg, compared to 25 mg or placebo, attenuated heart rate increases induced by nicotine. Topiramate, compared to placebo, enhanced the ratings of subjective effects from nicotine including “drug strength,” “good effects,” “head rush,” and “drug liking.” Topiramate treatment did not affect performance on the Stroop test.
Conclusions
These results suggest that topiramate may enhance the subjective effects of nicotine and attenuate the heart rate response to nicotine. While the exact mechanisms are unclear, enhancement of the dopaminergic system and attenuation of the noradrenergic system may mediate the topiramate’s effects on the subjective and cardiovascular responses to nicotine, respectively. The utility of topiramate for smoking cessation needs to be examined further in controlled clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco addiction, the chief preventable cause of death in developed countries, causes over 430,000 deaths annually in the United States alone (CDC 2005). Currently, the first-line pharmacotherapies to aid smoking cessation include nicotine replacement therapy (NRT) and sustained release bupropion (Fiore 2000). NRT or bupropion treatments achieve abstinence rates between 15–20% at 1 year, approximately two times better than placebo (Fiore 2000; Silagy et al. 2004). These figures emphasize the need for more effective pharmacotherapies for tobacco addiction. Several medications have been examined as potential medications for tobacco addiction. A recent human study provided preliminary evidence that topiramate may be effective for smoking cessation in alcohol-dependent smokers (Johnson et al. 2005). Among participants of a clinical trial examining the effects of topiramate for alcohol dependence, those who were assigned to topiramate were more likely to quit smoking than those assigned to placebo (19.4 vs 6.9% at week 12). While this study had a number of limitations, these findings raised an intriguing possibility that topiramate may be effective as a pharmacological aid to smoking cessation. These promising findings warrant further studies specifically designed to examine topiramate’s effects on tobacco addiction.
The goal of this study was to characterize topiramate’s effects on subjective and physiological responses to intravenous (IV) nicotine in abstinent smokers. Topiramate has multiple pharmacological effects including inhibition of Na+ and Ca++ currents, inhibition of carbonic anhydrase, increased dopamine and serotonin release, blockade of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate subtypes of glutamate receptors, and potentiating gamma-aminobutyric acid (GABA) effects (Gibbs et al. 2000; Gordey et al. 2000; Herrero et al. 2002; Kuzniecky et al. 2002; Okada et al. 2005; White et al. 2000). The potential therapeutic effects of topiramate for addictive disorders have been hypothesized to be due to the inhibition of dopamine release in mesolimibic dopaminergic pathways. Topiramate appears to enhance GABA and inhibit glutamate receptors, both of which inhibit dopamine release (Johnson 2004). More recently, the direct effects of topiramate on dopamine and serotonin release in the brain were demonstrated (Okada et al. 2005). Topiramate, at doses comparable to therapeutic levels in humans, enhanced the basal and Ca++-induced dopamine levels in the prefrontal cortexes of rats. Thus, topiramate might attenuate or enhance the subjective effects of nicotine, based either on its indirect attenuation of the dopaminergic system or its potentiation of dopamine release, respectively (Johnson 2004; Okada et al. 2005). This study was designed to test these alternative mechanisms and examine topiramate’s potential for treating tobacco addiction.
Materials and methods
Participants
Seven male and five female nontreatment-seeking smokers were recruited from the New Haven area (seven African-Americans, three Caucasians, one Hispanic, and one Asian-American). Two additional participants were enrolled but dropped out of the study before receiving medication treatment and were not included in the analysis. The average age (SD) of the participants was 35.5 (10.6). On average, participants smoked 18.7 (8.0) cigarettes/day, and had a Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991) score of 7.1 (2.2). Participants had normal results to physical, laboratory, and psychiatric examinations. None of the participants were dependent on alcohol or on any drugs other than nicotine. All participants gave signed informed consent prior to their entry into the study. Experimental sessions were conducted in the Biostudies Unit located at the Veterans Affairs (VA) Connecticut Healthcare System (West Haven campus), and the participants were paid for participation. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
Procedures
This outpatient, double-blind, placebo-controlled, crossover study had one adaptation and three experimental sessions. On the experimental days, participants were instructed not to smoke after midnight. Smoking abstinence was verified by obtaining breath carbon monoxide levels (<10 ppm). Before the sessions started, an indwelling IV catheter was placed in an antecubital vein of each participant for nicotine infusion, blood drawing, and as a safety precaution. After baseline measures were obtained, participants received the study medication followed by a light meal. Starting 2 h after medication administration, when peak levels of topiramate were expected, participants were given saline followed by two escalating doses of nicotine (0.5 and 1.0 mg/70 kg) intravenously. The injections were given 30 min apart, over 60 s. Cardiac rhythm was monitored continuously during sessions, and 12-lead electrocardiograms were obtained before and at the end of the session. The sessions started at 8 a.m. and were 3 to 9 days apart to minimize the carryover effects from topiramate or nicotine.
Drugs
Nicotine and topiramate administration
Nicotine bitartrate was obtained from Interchem (Paramus, NJ). Nicotine samples were prepared by the research pharmacy at the VA Connecticut Healthcare System in a 5-cc volume of saline. An investigational new drug application was submitted to the Food and Drug Administration for IV nicotine. Quantitative analysis (performed at the University of California, San Francisco, Clinical Pharmacology Laboratory) indicated 100% purity of the nicotine solution. To examine the dose–response effects of nicotine, participants were administered placebo (saline) followed by two ascending doses of nicotine (0.5 and 1.0 mg/70 kg). The nicotine doses selected were within the nicotine dose range that produces robust and reproducible subjective and physiological effects (Henningfield et al. 1985; Jones et al. 1999).
Topiramate (Topramax) was obtained from Ortho McNeil Pharmaceutical, (Raritan, NJ). Topiramate was given as a 25- or 50-mg single dose at the start of the sessions after completion of the baseline measures. To minimize any possible adverse events, 25 mg topiramate preceded the 50 mg topiramate dose and placebo treatment was randomly inserted into the sequence. To place the doses of topiramate administered in this study in context, the typical prescribed starting dosage of topiramate is 25–50 mg/day orally, gradually increased by 25–50 mg/week until the usual maintenance dosage of 100–400 mg/day is reached (Medical Economics Data 2005). Following oral administration of topiramate, peak effects are reached within 1.5 to 4 h.
Outcome measures
The outcome measures consisted of biochemical, physiological, subjective, and cognitive performance measures. The biochemical measures were plasma nicotine and cotinine concentrations to verify abstinence from smoking and level of smoking, respectively. For these measures, blood samples were obtained at the beginning of each session.
The physiological measures were systolic and diastolic blood pressure and heart rate. These measures were taken before medication treatment and every 20 min for 2 h afterwards. Additional physiological measures were taken 5 min before and 1, 2, 3, 5, 8, 10, and 15 min after saline or nicotine injections.
The three subjective measures were: the Drug Effects Questionnaire (DEQ), a Visual Analog Scale (VAS) questionnaire for nicotine withdrawal, and the Profile of Mood States (POMS). The DEQ, used to assess the acute subjective effects of nicotine, consists of five items: “drug strength,” “good effects,” “bad effects,” “head rush” and “like the drug.” Participants rated these items on a 100-mm scale, from 0, equal to “not at all,” to 100, equal to “extremely.” The DEQ was given before medication treatment and every 30 min afterwards for 2 h. DEQ was also given 5 min before and 1, 3, 5, 8, and 10 min after saline or nicotine injections.
The VAS questionnaire was used to assess nicotine withdrawal and included eight items adapted from the nicotine withdrawal symptoms checklist (Hughes 1996). The items were “cigarette craving,” “irritability/anger,” “anxiety,” “difficulty concentrating,” “restlessness,” “increased appetite,” “depressed mood,” and “insomnia” (Hughes and Hatsukami 1986; Hughes and Hatsukami 1997). Participants were asked to rate each symptom on a scale from 0 (not present) to 100 (severe). The VAS withdrawal scale was given three times: at the beginning of each session, 5 min before saline and nicotine doses, and 20 min after the second nicotine administration.
The POMS is a 72-item rating scale used to measure the effects of medication treatments on mood (McNair et al. 1971). The POMS has six subscales: (1) composed–anxious; (2) agreeable–hostile; (3) elated–depressed; (4) confident–unsure; (5) energetic–tired; (6) clear headed–confused. The POMS was given three times: at the beginning of the session, before saline and nicotine administration, and at the end of each experimental session.
The classical Stroop test, which measures response inhibition, was given 1 h and 45 min after topiramate or placebo treatment. Participants first responded to a series of red, blue, and green XXXX’s to practice associating the particular color with the corresponding key on the computer. In the congruent condition, the words “red,” “blue,” and “green” are shown to the participant in the same color as the presented word. For example, the word “red” is shown in red ink. The interference condition involves presenting the person with the words “red,” “blue,” and “green” written in an incongruent ink color (i.e., the word “red” written in blue ink). In all conditions, the participant is asked to name the ink color of the printed word.
We used the Automated Neuropsychological Assessment Matrics version of a computerized classical Stroop test (Reeves et al. 2001), which is modeled after Golden’s clinical version (Golden 1978). Stimuli were presented every 1 s and had an interstimulus interval of 350 ms. The Stroop design consisted of one 3-min block of congruent stimuli and one 3-min block of incongruent stimuli. The outcome measures for the Stroop test were reaction time and interference score, which reflect slowing on the incongruent task relative to the congruent one (Reeves et al. 2001). The Stroop test has been shown to be sensitive to tobacco deprivation and nicotine replacement treatments (Mancuso et al. 1999; Provost and Woodward 1991).
Data analysis
To determine treatment effects on selected measures, we conducted a mixed-effect repeated-measures crossover analysis using SAS Proc Mixed, version 9. The model included a fixed main effect for treatment (placebo, 25, or 50 mg topiramate), dose (saline, 0.5 and 1 mg/70 kg), time of measurement, and the interactions between these three effects. We also included a random effect for participant and a blocking factor for treatment sequence. For heart rate, blood pressure, and DEQ, which had frequent measurements, three a priori questions were addressed in separate analyses for each measure. The first analysis examined medication (25 or 50 mg topiramate or placebo) effects alone and included measurements taken before and up to 2 h after medication treatment. The second analysis examined medication and saline interactions and included measurements taken before and up to 25 min after saline administration. The third analysis examined medication and nicotine (0.5 and 1.0 mg/70 kg) interactions and included measurements taken before and up to 25 min after two nicotine injections. For the analysis of the Stroop data, the model also included main effects for the Stroop block (congruent or incongruent). Significant treatment or treatment-by-time interactions (p<0.05) were followed by post hoc group comparisons using Fisher’s least significant difference test.
Results
Physiological response
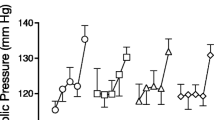
For IV nicotine administration, there was a significant treatment effect for heart rate [F(2,518)=21.9; p<0.0001], with lower heart rate responses under the 50-mg-topiramate condition (Fig. 1). Pairwise comparisons indicated significant difference between 50 mg topiramate and placebo conditions (p<0.05). For systolic or diastolic blood pressure, no treatment effects were observed. There was also a significant effect of time for heart rate [F(7,517)=41.5; p<0.0001] and systolic blood pressure [F(7,517)=2.6; p<0.001], but there was no significant treatment-by-time interaction. For heart rate, a significant main effect [F(1,517)=4.4; p<0.05] for dose was observed, indicating a greater heart rate increase for the second delivery of nicotine. Topiramate treatment did not affect the heart rate or the systolic or diastolic blood pressure responses to saline. Topiramate also did not affect baseline heart rate or the systolic or diastolic blood pressure.
Topiramate treatment effect on the heart rate response to saline and nicotine administration. Measurements were taken just before and 1, 2, 3, 5, 8, 10, and 15 min after saline and nicotine (0.5 and 1.0 mg/70 kg) administrations. Significant treatment effects, relative to placebo, were observed for 50 mg topiramate (p<0.05)
Subjective response
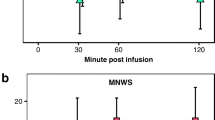
As shown in Fig. 2, nicotine administration had a significant treatment effect on the rating of “drug strength” [F(2,350)=6.2; p<0.001], “good effects” [F(2,319)=8.2; p<0.001], “head rush” [F(2,319)=4.3; p<0.005], and “drug liking” [F(2,319)=7.6; p<0.001]. All five items showed significant time effects (p<0.001) but no significant treatment-by-time interactions (p>0.05). A significant dose effect was observed for the rating of “drug strength” [F(1,319)=17.9; p<0.0001], “good effects” [F(1,319)=12.9; p<0.001], and “head rush” [F(2,350)=10.9; p=0.001], indicating a greater response for the second nicotine dose. Pairwise comparisons indicated that ratings of “drug strength,” “good effects,” and “drug liking” were greater for both 50 and 25 mg topiramate than for the placebo (p<0.05). The rating of “head rush” was greater for 50 mg topiramate than for placebo (p<0.05). Topiramate treatment did not affect the subjective response to saline (p<0.05).
Topiramate treatment effect on the DEQ responses to nicotine administration. Measurements were taken just before and 1, 3, 5, 8, and 10 min after saline and nicotine (0.5 and 1.0 mg/70 kg) administrations. Significant treatment effects, relative to placebo, were observed for “head rush” at 50 mg topiramate and for “drug strength,” “good effects,” and “drug liking,” at both 25 and 50 mg topiramate treatments (p<0.05)
For the average withdrawal severity, no significant treatment or treatment-by-time interaction was observed (p<0.05). A significant time effect was observed indicating decreased tobacco withdrawal severity in response to nicotine administration (p<0.05). The average withdrawal severity (with its SEM) was 25.6 (3.3) at baseline, 29.8 (4.1) after 2 h, 26.3 (4.1) after saline, 22.1 (3.3) after 0.5 mg nicotine, and 18.5 (2.8) after 1.0 mg nicotine.
Because sedation is one of the possible side effects of topiramate, the energetic–tired subscale of POMS was of particular interest, and it showed a significant time effect [F(2,88)=9.4; p<0.001], but no treatment or treatment-by-time interactions (p>0.05). Similarly, the other subscales of the POMS showed only significant time effects (p<0.05).
Plasma nicotine and cotinine measurements
Baseline cotinine levels (SD) were 167 ng/mL (30) for the placebo, 199 ng/mL (43) for the 25-mg-dose topiramate, and 181 ng/mL (37) for the 50-mg-dose topiramate treatment days (p>0.05). The baseline plasma nicotine levels were <5 ng/mL for all three treatment conditions.
Cognitive measures
Neither the reaction time nor interference scores on the Stroop test showed any treatment effects (p>0.05). The average reaction time was 946 ms (68) following placebo, 902 ms (70) following 25 mg topiramate and 936 ms (83) following 50 mg topiramate treatment.
Discussion
In our study, topiramate treatment enhanced the subjective effect of IV nicotine. Topiramate mainly enhanced the pleasurable (e.g., “drug liking” and “good effects”), but not the aversive (e.g., “bad effects”) effects of nicotine. These subjective effects could not be attributed to nonspecific mood changes by topiramate, because topiramate alone did not affect any measures of mood. The mechanisms by which topiramate enhances subjective nicotine effects are not clear. Given the proposed role of the dopaminergic system activation in mediating the rewarding effects of nicotine, one plausible mechanism may be that topiramate enhances dopamine release (Okada et al. 2005), rather than the proposed inhibition of the dopaminergic system activity (Johnson 2004). Interestingly, bupropion, one of the first-line treatments for smoking cessation, also increases the synaptic dopamine levels by inhibiting its presynaptic reuptake (Meyer et al. 2002). This increase in baseline dopamine levels by bupropion may prevent decreases in the reward threshold associated with withdrawal from nicotine and, as a result, prevent relapse to smoking (Cryan et al. 2003). Whether topiramate’s effects on the brain’s reward system are similar to bupropion needs to be determined in future studies.
Topiramate treatment decreased the nicotine-induced heart rate increases. This effect was dose-dependent such that only the 50 mg topiramate was effective. We are not aware of any previous studies documenting that topiramate lowers heart rate. However, topiramate decreases blood pressure (Wilding et al. 2004). While the mechanisms underlying topiramate’s cardiovascular effects are not clear, nicotine’s cardiovascular effects are noradrenergically mediated, and topiramate may dampen this activation. In particular, topiramate blocks AMPA-type glutamate receptors in the locus coeruleus, thereby decreasing noradrenergic activity (Cheseaux et al. 2003; Zullino et al. 2002). Topiramate’s inhibition of noradrenergic activity may also mediate its reported attenuation of benzodiazepine and opioid withdrawal symptoms in humans (Cheseaux et al. 2003; Zullino et al. 2002). This same noradrenergic inhibition may contribute to topiramate’s efficacy for smoking cessation. Another noradrenergic inhibitor, clonidine, is also effective for smoking cessation (Fiore 2000).
In addition to its anticonvulsant use, topiramate is also approved for migraine prophylaxis, and it may treat alcohol and cocaine dependence (Johnson et al. 2003; Kampman et al. 2004). As mentioned before, a preliminary study suggested that topiramate may be a potential treatment for alcohol-dependent smokers (Johnson et al. 2005). These preliminary findings have to be replicated in future controlled clinical trials. Smoking in alcohol dependent individuals is a serious problem with a prevalence rates of 80% for alcoholics compared to 23% for the general population (Hughes 1996). In fact, alcoholics are more likely to die from smoking-related illnesses than from alcohol related illnesses (Hurt et al. 1996). Thus, the development of specific pharmacological treatments for dually addicted individuals will have great public health implications.
An important question is the relevance of the IV nicotine injection used in this study for the amount of nicotine intake during cigarette smoking. In previous human studies, these two routes of nicotine administration showed similar pharmacokinetics, although cigarette smoking achieved peak plasma levels faster than the IV route, 20 vs 30 s (Gourlay and Benowitz 1997; Rose et al. 1999). Smokers inhale on average 1–2 mg of nicotine per cigarette, extended over 5 min (Hukkanen et al. 2005), compared to the 0.5 and 1.0 mg nicotine infused over 60 s in our study. Although we did not collect plasma nicotine levels following nicotine administration, previous studies suggest that the peak plasma or arterial nicotine levels following the 0.5 and 1.0 mg IV nicotine administrations would be higher than those achieved with smoking (Gourlay and Benowitz 1997). Whether topiramate treatment would also affect nicotine effects with a slower rate of delivery, as in smoking, needs to be examined in future studies.
In our study, smokers tolerated topiramate treatment without any adverse events, including sedation. In addition, topiramate treatment did not affect the participants’ cognitive performance on the Stroop test. In this study, we used the usual starting dose of topiramate (25 to 50 mg). Topiramate daily doses range from 100 mg for migraine disorders to 100–400 mg for seizure disorders. In previous clinical trials, long-term topiramate treatment has had limited clinical usefulness because of cognitive slowing, memory problems, and speech problems (Tatum et al. 2001). These side effects may be minimized with a low initial dose, slow titration, and low maintenance doses of topiramate (Lee et al. 2003; Salinsky et al. 2005).
In conclusion, topiramate may enhance the subjective effects of nicotine and attenuate the heart rate response to nicotine. While the exact mechanisms are unclear, enhancement of the dopamine system and attenuation of the noradrenaline system may mediate topiramate’s effects on the subjective and cardiovascular responses to nicotine, respectively. The proposed utility of topiramate for smoking cessation needs to be examined further in controlled clinical trials.
References
CDC (Center for Disease Control) (2005) Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR Morb Mortal Wkly Rep 54(25):625–628
Cheseaux M, Monnat M, Zullino DF, Cottier AC, Besson J (2003) Topiramate in benzodiazepine withdrawal. Hum Psychopharmacol 18(5):375–377
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168(3):347–358
Fiore M (2000) Tobacco use and dependence guideline panel. Treating tobacco use and dependence. U.S. Department of Health and Human Services, USA
Gibbs JW III, Sombati S, DeLorenzo RJ, Coulter DA (2000) Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia 41(Suppl 1):S10–S16
Golden C (1978) Stroop color and word test: a manual for clinical and experimental uses. Stoelting Company, Wood Dale
Gordey M, DeLorey TM, Olsen RW (2000) Differential sensitivity of recombinant GABA(A) receptors expressed in Xenopus oocytes to modulation by topiramate. Epilepsia 41(Suppl 1):S25–S29
Gourlay SG, Benowitz NL (1997) Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther 62(4):453–463
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Henningfield JE, Miyasato K, Jasinski DR (1985) Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther 234(1):1–12
Herrero AI, Del Olmo N, Gonzalez-Escalada JR, Solis JM (2002) Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology 42(2):210–220
Hughes JR (1996) Treating smokers with current or past alcohol dependence. Am J Health Behav 20(5):286–290
Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43(3):289–294
Hughes JR, Hatsukami DK (1997) Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse 9151–9159
Hukkanen J, Jacob P III, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57(1):79–115
Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ III (1996) Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA 275(14):1097–1103
Johnson BA (2004) Topiramate-induced neuromodulation of cortico-mesolimbic dopamine function: a new vista for the treatment of comorbid alcohol and nicotine dependence? Addict Behav 29(7):1465–1479
Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ (2003) Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361(9370):1677–1685
Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA (2005) Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med 165(14):1600–1605
Jones HE, Garrett BE, Griffiths RR (1999) Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther 288(1):188–197
Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP (2004) A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend 75(3):233–240
Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, Hetherington H (2002) Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology 58(3):368–372
Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, Gates J, Penovich P, Al-Asmi A, Dubeau F, Jones-Gotman M (2003) The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia 44(3):339–347
Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E (1999) Selective effects of nicotine on attentional processes. Psychopharmacology (Berl) 146(2):199–204
McNair D, Lorr M, Dropperman L (1971). Manual for profile of mood states. Educational and Industrial Testing Services, San Diego
Medical Economics Data (2005) Physicians desk reference. Medical Economics Data, Montvale
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S (2002) Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 163(1):102–105
Okada M, Yoshida S, Zhu G, Hirose S, Kaneko S (2005) Biphasic actions of topiramate on monoamine exocytosis associated with both soluble N-ethylmaleimide-sensitive factor attachment protein receptors and Ca(2+)-induced Ca(2+)-releasing systems. Neuroscience 134(1):233–246
Provost SC, Woodward R (1991) Effects of nicotine gum on repeated administration of the Stroop test. Psychopharmacology (Berl) 104(4):536–540
Reeves D, Winter K, Kane R, Elsmore T, Bleiberg J (2001) ANAM. User’s manual. National Cognitive Recovery Foundation, San Diego
Rose JE, Behm FM, Westman EC, Coleman RE (1999) Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend 56(2):99–107
Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB (2005) Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology 64(5):792–798
Silagy C, Lancaster T, Stead L, Mant D, Fowler G (2004) Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 3 (http://DOI.10.1002/14651858.CD000146.pub2)
Tatum WO IV, French JA, Faught E, Morris GL III, Liporace J, Kanner A, Goff SL, Winters L, Fix A (2001) Postmarketing experience with topiramate and cognition. Epilepsia 42(9):1134–1140
White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH (2000) Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia 41(Suppl 1):S17–S20
Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M (2004) A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord 28(11):1399–1410
Zullino DF, Cottier AC, Besson J (2002) Topiramate in opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 26(6):1221–1223
Acknowledgements
This research was supported by the National Institute on Drug Abuse grants P50-DA12762, K05-DA0454 (TRK), R01-DA 14537, and K12 00167 (MS). We would like to thank the Biostudies nursing staff for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sofuoglu, M., Poling, J., Mouratidis, M. et al. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology 184, 645–651 (2006). https://doi.org/10.1007/s00213-005-0296-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0296-9