Abstract
Rationale
The progesterone metabolite 5α-pregnane-3α-ol-20-one (3α,5α-THP) is an important modulator of the hypothalamic–pituitary–adrenal axis and stress-induced corticosterone response. Typically, 3α,5α-THP levels are increased in response to acute stress, which may then reduce corticosterone release from the adrenals. Early postnatal stimulation is a developmental stressor that can produce pervasive endocrine effects.
Objectives
The present studies investigated the effects of early postnatal stimulation on plasma progestin and corticosterone levels and hippocampal progestin levels of rats.
Methods
On postnatal days 9 and 10, rats were either left in their home cage undisturbed or injected intraperitoneally as a means of early stimulation (ES). Tissues were collected on either postnatal day 10 (6 h after last handling experience) or adulthood. Plasma corticosterone, progesterone, and 3α,5α-THP and hippocampal progesterone and 3α,5α-THP were measured by radioimmunoassay.
Results
On postnatal day 10, plasma, but not hippocampal, levels of progesterone and 3α,5α-THP were significantly lower among rats exposed to ES than control rats. These effects occurred concomitant with a tendency for plasma corticosterone to be higher among ES compared to control rats. In adulthood, hippocampal 3α,5α-THP was significantly lower among ES vs control rats.
Conclusions
Together, these data suggest that ES may influence immediate secretion of 3α,5α-THP and corticosterone and have pervasive effects in adulthood on the biosynthesis and/or metabolism of progestins in the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral and endocrine responses to acute stress are influenced by early postnatal experience (Ader 1965; Denenberg et al. 1963; Meaney et al. 1996). Postnatal manipulations can have disparate effects to alter hypothalamic–pituitary–adrenal (HPA) response to stress. Two classes of postnatal manipulations, handling and maternal separation, are commonly utilized to investigate effects on stress-responsiveness. Handling involves removing pups from the dam for a constant, but brief, period of time (usually more than 2, but less than 15 min per day; Madruga et al. 2005; Maldonado and Kirstein 2005). Much longer periods of removal from the dam are typically referred to as maternal separation (Madruga et al. 2005). Although these stressors vary in the degree of disruption of factors that are important for normal development, both can produce salient alterations in behavioral and/or neuroendocrine stress responses.
Handling typically reduces HPA-responsiveness. For example, handling on postnatal days 1–21 decreases anxiety, increases exploration, enhances emotional learning, and reduces corticosterone and adrenocorticotropin (ACTH) response to acute stress as adults (Ader 1970; Levine et al. 1967; Meaney et al. 1996; Nunez et al. 1996). This chronic early postnatal handling paradigm also results in long-term increases in glucocorticoid receptors in the hippocampus (Meaney and Aitken 1985). In addition, repetitive, brief periods of early postnatal handling protect against age-related hippocampal neuronal death and cognitive impairments (Meaney et al. 1988). Thus, early exposure to handling stress can have profound effects on neuroendocrine and behavioral systems, which can persist into adulthood.
Maternal isolation typically increases later HPA-responsiveness (Macri et al. 2004). Maternal separation increases vocalization of pups, as well as corticosterone secretion, and dopamine turnover in the hypothalamus (Kehoe et al. 2000; McCormick et al. 2002). The increase in corticosterone produced by maternal separation on postnatal days 2–9 persists until postnatal days 26–30 and occurs concomitant with elevations in whole brain levels of the progesterone metabolite 5α-pregnane-3α-ol-20-one (3α,5α-THP) (Kehoe et al. 2000; McCormick et al. 2002). Furthermore, pups that are separated from dams daily on postnatal days 2–6 or 15–21 show poorer learning as juveniles and more anxiety behavior as adults (Frisone et al. 2002; Zimmerberg and Kajunski 2004). Thus, maternal separation can also have acute and pervasive effects on neuroendocrine and/or behavioral response to stress.
One way in which early stimulation (ES), such as handling and/or maternal separation, may influence behavioral and/or neuroendocrine response to stressors is through effects at the γ-aminobutyric acid (GABA) system. For example, ES can alter GABAA receptor binding and expression of GABAA receptor subunits. Repeated, prolonged bouts of maternal separation decrease GABAA receptor binding (Caldji et al. 2000). As few as two short-term episodes of ES alter stress-responsiveness and decrease GABAA receptor α1 subunit expression in adulthood (Hsu et al. 2003). Levels of, and/or response to, 3α,5α-THP, a potent positive modulator of GABAA receptors, are altered by stress (Barbaccia et al. 2001; Paul and Purdy 1992; Purdy et al. 1991). Acute increases in 3α,5α-THP in the hippocampus produce anxiolytic, antistress, and antiseizure effects akin to that of other GABAA receptor agonists (Bitran et al. 1991, 1999; Finn et al. 2003; Frye et al. 2000; Majewska et al. 1986; Rhodes and Frye 2001). 3α,5α-THP is expressed across a variety of mammalian and amphibian species (Barbaccia et al. 2001; Mensah-Nyagan et al. 2001). Among rodents, 3α,5α-THP is secreted as early in development as embryonic day 18 (Kellogg and Frye 1999; Kellogg et al. 2005). Rat pups’ distress vocalizations produced by maternal separation on postnatal day 7 are attenuated by 3α,5α-THP administration (Zimmerberg et al. 1999). These findings, in conjunction with reports that 3α,5α-THP dampens HPA responses (Patchev and Almeida 1996; Patchev et al. 1996), have led to the hypothesis that ES may have proximate and/or pervasive effects on 3α,5α-THP that may underlie ES-mitigated differences in neuroendocrine and/or behavioral response to stress (Barbaccia et al. 2001). Thus, the present experiment examined whether ES produced effects on 3α,5α-THP levels in the circulation and/or hippocampus of male rats in early development and/or later in adulthood.
Methods and materials
Animals and handling
Experiment 1
Rats were generated at the Children’s Hospital of Philadelphia from pregnant Sprague–Dawley dams (n=6) received on gestational day 17 from Charles River Laboratories. Dams were housed one per cage on a 12-h light/dark cycle with free access to food and water. After delivery, pups remained with the dam until postnatal day 9, when females were culled from the litters. Four to eight males from each of the six litters were randomly assigned to receive ES or be nonmanipulated controls. Controls were never separated from the dam. Rats exposed to ES were either injected on postnatal day 9 with lithium chloride [3 meq/kg, intraperitoneal (IP); six litters] or saline (0.1 ml, IP; five litters), or not injected and were placed in a clean cage separate from the dam for 30 min. On postnatal day 10, all ES groups were injected with saline and placed in a clean cage separate from the dam for 6 h. As such, there were three ES treatments: (1) removal from the dam for 30 min on day 9, followed by removal from the dam for 6 h on day 10; (2) saline injection and removal from the dam for 30 min on day 9, followed by removal from the dam for 6 h on day 10; (3) lithium chloride injection and removal from the mother for 30 min on day 9, followed by removal from the dam for 6 h on day 10. Previous studies on the effects of these different ES on GABAA receptor subunit expression reveal each ES produced effects similar to other ES that were different from controls (Hsu et al. 2003). Furthermore, one-way analyses of variance (ANOVAs) were used to ascertain if there were any differences between the three ES manipulations on any measure; there were none, and these groups were combined. Some rats (n=2–3) from each ES group and from the control group were killed on postnatal day 10, while other rats were allowed to grow to adulthood (postnatal day 90). This resulted in four groups: ES postnatal day 10 rats (n=10), control postnatal day 10 rats (n=20), ES postnatal day 90 (n=10), and control postnatal day 90 rats (n=8).
Experiment 2
Rats in experiment 2 were bred and raised in the Laboratory Animal Care Facility at SUNY-Albany from stock from Taconic Farms (Germantown, NY). As in experiment 1, pregnant dams were housed one per cage with free access to food and tap water in their home cages. However, in this experiment, a reversed 12-h light/dark cycle was used. After delivery, both male and female pups remained with the dam. As in experiment 1, potential litter effects were minimized by using a few pups per litter in each experimental condition. On postnatal day 9, male pups from six litters were randomly assigned to be controls that were never separated from the dam or exposed to ES with saline or no injections on postnatal day 9, and placed in a clean cage separate from the dam for 30 min. On postnatal day 10, each ES pup was placed in a clean arena separate from the dam for 6 h. As such, in this experiment, there were only two ES treatments: (1) removal from the dam for 30 min on day 9, followed by removal from the dam for 6 h on day 10; (2) saline injection and removal from the dam for 30 min on day 9, followed by removal from the dam for 6 h on day 10. As ANOVAs indicated that there were no differences between the two ES conditions, they were combined. Some rats from each group were killed on postnatal day 10, while other rats were allowed to grow to adulthood (postnatal day 60). This resulted in four groups: ES postnatal day 10 (n=10), control postnatal day 60 (n=7), ES postnatal day 60 (n=10), and control postnatal day 60 (n=7).
Tissue collection for progestin measurement
Rats from each group were killed on postnatal day 10 or in adulthood by rapid decapitation. Trunk blood was collected and remained on ice until refrigerated centrifugation (4°C at 3,000×g for 8 min). Serum was aliquoted and stored at −70°C until radioimmunoassay for corticosterone, progesterone, and 3α,5α-THP. Brains were rapidly removed, placed in dry ice, and stored at −70°C until the hippocampus was dissected bilaterally for radioimmunoassay for P and 3α,5α-THP.
Radioimmunoassay for corticosterone, progesterone, and 3α,5α-THP
Corticosterone was extracted from plasma by heating at 80°C for 30 min. Samples were incubated for 60 min at room temperature with 3H CORT [NET 182: specific activity=48.2 Ci/mmol; New England Nuclear (NEN), Boston, MA] and a 1:20,000 dilution of antibody (Endocrine Sciences: #B3-163; Frye et al. 1996; Frye and Bayon 1999). This antibody cross-reacts slightly with deoxycorticosterone (approximately 4%) but not with cortisol, aldosterone, or progesterone (<1%). Dextran-coated charcoal was used to separate bound and free following a 15-min incubation on ice and centrifugation at 3,000×g for 10 min. The supernatant was decanted into a glass scintillation vial with scintillation cocktail. Unknowns were interpolated from the standard curve using Assay Zap. The minimum level of detection with the assay is 15 pg/tube, and the inter- and intra-assay reliability coefficients were 0.05 and 0.08, respectively.
Plasma and hippocampal progesterone were determined by radioimmunoassay according to previously published methods (Frye and Vongher 1999). Briefly, progesterone was extracted from plasma samples by diethyl ether; the solvent was removed using a speed drier, and samples were resuspended in assay buffer (pH 7.4). For brains, hippocampal tissue was homogenized with a glass/Teflon homogenizer in distilled water. Steroids were extracted from the homogenate with ether, dried down in an evaporator drier, and the pellet was reconstituted in trimethyl pentane (TMP) to half the homogenate volume. Extracts were chromatographed using Celite column chromatography. The progestin fraction was collected using a 100% TMP wash. Fractions were dried using a speed drier and then reconstituted in phosphate assay buffer. Radioimmunoassay was performed using [3H] P (NET-208, specific activity 48.4 Ci/mmol, NEN) and antisera (P#337 from Dr. G.D. Niswender, Colorado State University). The progesterone antibody was used in a 1:30,000 dilution and bound between 30 and 50% of [3H] P. The standard curve was prepared in duplicate to give a range of nine concentrations from 50 to 8,000 pg/ml, total volume 800 μl. Incubation (4°C for 24 h) was terminated by the addition of charcoal. Following a 15-min incubation on ice, samples were centrifuged at 1,200×g for 10 min. Sample concentrations were calculated using the logit-log method (Rodbard and Hutt 1974). The minimum detectable limit of the assay was 10 pg, and the intra-assay and inter-assay coefficients of variance were 0.10 and 0.09, respectively.
3α,5α-THP was measured according to previously established methods (Finn and Gee 1994; Frye et al. 1998; Frye and Bayon 1999; Smythe et al. 1994). Briefly, steroids were extracted from plasma samples using diethyl ether. Steroids were extracted from homogenized brain samples in 50% MeOH, 1% acetic acid; homogenate was then centrifuged at 4°C, 1,200×g for 10 min. The homogenate was filtered through Sepak cartridges and washed with increasing concentrations of methanol (50 and 100%). Three hundred microliters of 0.1 M phosphate assay buffer (pH 7.4) was added to test tubes containing steroid extracts and equilibrated. The antibody, purchased from Dr. Robert Purdy (Veterans Medical Affairs, La Jolla, CA), is very specific to 3α,5α-THP (Finn and Gee 1994). The 1:5,000 dilution of this antibody bound between 40 and 60% of [3H] 3α,5α-THP (NET-1047, 51.3 Ci/mmol; NEN). Although there is cross-reactivity of this 3α,5α-THP antibody with 3α-hydroxypregn-4en-20-one (84%), dihydroprogesterone (11%), its β isomer (7%), and P (6%), there is negligible cross-reactivity (<2%) with pregnenolone and corticosterone, as well as other progestins. There is also extensive cross-reactivity with the 5α-reduced metabolites of corticosterone and deoxycorticosterone. However, this antibody has been deemed suitable for measurement of 3α,5α-THP without high-performance liquid chromatography purification (Finn and Gee 1994).The standard curve was prepared in duplicate with a range of nine concentrations from 50 to 8,000 pg/ml, total volume 950 μl. Incubation at 4°C for 24 h was terminated by charcoal separation of bound and free. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt (1974), interpolation of the standards, and correction for recovery. The minimum detectable limit of the assay was 50 pg. The intra- and inter-assay coefficients of variance were 0.12 and 0.15, respectively.
Statistical analyses
Two-way analyses of variance were utilized to examine effects of ES and age on plasma and hippocampal steroid concentrations. There were no differences among ES groups (as has been previously reported) in either experiment; therefore, these groups were combined to comprise the handled group. The alpha level for significance was p<0.05. Where appropriate, Fishers post hoc tests were utilized to assess group differences.
Results
Experiment 1
Effects on postnatal day 10
Corticosterone
Plasma corticosterone levels on postnatal day 10 were altered by ES. On postnatal day 10, ES rats had a tendency to have higher plasma corticosterone levels than did controls [F(1,28)=3.06, P= 0.09; see Table 1].
Progesterone
There were significant differences between groups in plasma progesterone levels on postnatal day 10. Rats that received ES had significantly lower plasma progesterone levels compared with control rats on postnatal day 10 [F(1,28)=10.29, P=0.003; see Fig. 1, left]. There were no differences between groups in hippocampal progesterone levels on postnatal day 10 [F(1,28)=0.96, P=0.34].
3α,5α-THP
Early stimulation significantly lowered plasma 3α,5α-THP levels on postnatal day 10. Rats exposed to ES had significantly lower plasma 3α,5α-THP levels compared with controls on postnatal day 10 [F(1,28)=15.86, P=0.0004; see Fig. 1, right]. There were no statistically significant effects of ES on hippocampal 3α,5α-THP levels when measured on postnatal day 10 [F(1,28)=2.06, P=0.16].
Effects in adulthood
Corticosterone
There were no significant effects of ES on postnatal days 9 and 10 to alter plasma corticosterone levels on postnatal day 90 [F(1,16)=0.59, P=0.45; see Table 1].
Progesterone
There was no significant effect of ES on plasma progesterone levels on postnatal day 90 [F(1,16)=1.99, P=0.17]. However, the ES group had apparently lower plasma progesterone levels (14±3 ng/ml) on postnatal day 90 than did controls (23±6 ng/ml). There were also no differences between groups in hippocampal progesterone levels on postnatal day 90 [F(1,16)=0.89, P=0.36].
3α,5α-THP
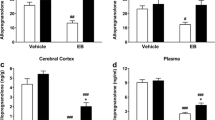
Although there were no effects of ES on plasma 3α,5α-THP levels on postnatal day 90 [F(1,16)=0.008, P=0.99; Fig. 2, left], there were significant decreases in hippocampal 3α,5α-THP levels of rats exposed to ES [F(1,16)=6.99, P=0.02; see Fig. 2, right].
Experiment 2
Postnatal day 10
Corticosterone
There were no significant effects of ES on corticosterone levels on postnatal day 10 [F(1,15)=0.95, P=0.34; see Table 2].
Progesterone
Plasma progesterone levels on postnatal day 10 were altered by ES. There was a tendency for rats exposed to ES to have lower plasma progesterone levels compared with controls [F(1,15)=4.04, P=0.06; see Fig. 3, left]. There were no effects of ES to alter levels of progesterone in the hippocampus on postnatal day 10 [F(1,15)=0.29, P=0.59].
3α,5α-THP
Exposure to ES altered plasma levels of 3α,5α-THP on postnatal day 10. Rats exposed to ES had significantly lower plasma 3α,5α-THP than did controls [F(1,15)=7.88, P=0.01; see Fig. 3, right]. There were no significant effects of ES on levels of 3α,5α-THP in the hippocampus on postnatal day 10 [F(1,15)=0.63, P=0.44].
Effects in adulthood
Corticosterone
There were no significant effects of ES on corticosterone levels at postnatal day 60 [F(1,15)=0.11, P=0.74; see Table 2].
Progesterone
There were no effects of ES to alter plasma progesterone levels on postnatal day 60 [F(1,15)=0.01, P=0.92]. There were also no effects of ES to alter levels of progesterone in the hippocampus on postnatal day 60 [F(1,15)=0.01, P=0.99].
3α,5α-THP
Plasma 3α,5α-THP on postnatal day 60 was altered by ES. Rats exposed to ES had significantly lower plasma 3α,5α-THP levels than did controls [F(1,15)=6.95, P=0.02; see Fig. 4, left]. Levels of 3α,5α-THP in the hippocampus were significantly altered on postnatal day 60 by ES. Rats exposed to ES had significantly lower 3α,5α-THP levels than did controls [F(1,15)=12.11, P=0.01; see Fig. 4, right].
Discussion
Results of the present studies supported our hypothesis that progestin levels would be altered immediately following ES, and that these alterations would persist into adulthood. On postnatal day 10, there was a pattern for plasma corticosterone to be increased in ES, compared with control rats. Plasma progesterone and 3α,5α-THP were decreased in rats exposed to ES compared with controls on postnatal day 10. As adults, rats exposed to ES had significantly lower hippocampal 3α,5α-THP levels than did controls. Together, these data suggest that acute ES can have pervasive effects on neuroendocrine parameters.
Results of the present studies confirm and extend prior reports that ES can alter 3α,5α-THP secretion. Previous data from our laboratory have shown that maternal separation increases whole brain 3α,5α-THP, perinatally and up to postnatal day 30 (Kehoe et al. 2000; McCormick et al. 2002). Here, we show that ES produces lower levels of progesterone and 3α,5α-THP in plasma on postnatal day 10. While we previously saw differences in whole brain levels of 3α,5α-THP produced by maternal separation, here, there were no differences in the levels of 3α,5α-THP in the hippocampus of rat pups on postnatal day 10 that had been exposed to ES. However, whole brain neuroendocrine measures do not reveal if there were increases or decreases in specific areas. Indeed, endogenous levels of 3α,5α-THP vary significantly between central nervous system sites (Frye and Bayon 1999). Furthermore, in the previous report, rats were exposed only to maternal separation, which typically increases response to stress, whereas the ES paradigm that we used here also involves handling which can decrease response to stress. Findings from the present studies also extend our findings with maternal separation to reveal decreases in levels of 3α,5α-THP in the hippocampus of adult rats exposed to ES. Thus, our present data extend these prior reports to suggest that ES can have effects on 3α,5α-THP secretion that may be site specific and pervasive.
The present data support previous reports that early stressors can produce proximate increases in corticosterone. On postnatal day 10, plasma corticosterone tended to be increased in rats exposed to ES compared with controls. Previous reports have shown that socially isolated rats show increased plasma corticosterone levels in response to acute restraint stress (Kehoe et al. 2000; McCormick et al. 2002). Daily injections of an endotoxin on postnatal days 3 and 5 produce elevated ACTH and corticosterone levels in adulthood (Nilsson et al. 2002). Thus, the present data are consistent with other models demonstrating that acute postnatal stimulation increases HPA reactivity.
The present findings that on postnatal day 10, circulating 3α,5α-THP was decreased and corticosterone was increased, imply that reductions in 3α,5α-THP secretion may underlie elevations in corticosterone. Although we cannot attribute causal effects of altered 3α,5α-THP levels on corticosterone levels from the present data, previous reports show that the administration of 3α,5α-THP significantly attenuates elevation of plasma ACTH and serum corticosterone following exposure to emotional stress (Patchev et al. 1996). The reduction in 3α,5α-THP observed in the present study is consistent with the idea of reduced GABAA receptor function altering stress-responsiveness. Previous reports have demonstrated that perinatal stress results in decreases in GABAA receptor α1 subunit expression (Hsu et al. 2003), which can reduce 3α,5α-THP’s ability to elicit a GABA response (Brussaard et al. 1997). Thus, effects of ES on 3α,5α-THP and GABAA receptors in the hippocampus may have salient effects on later stress-responsiveness.
3α,5α-THP may be an important endogenous homeostatic modulator. In adults, 3α,5α-THP is increased following exposure to acute cold-water swim, shock, ether, and/or carbon dioxide (Barbaccia et al. 2001; Paul and Purdy 1992; Serra et al. 2000). These effects of stress on 3α,5α-THP secretion reduce HPA responses. This attenuation of the HPA may enable organisms to return to homeostasis more quickly (Barbaccia et al. 2001; Frye 2001; Mensah-Nyagan et al. 2001; Patchev and Almeida 1996; Paul and Purdy 1992). Our data that 3α,5α-THP in the hippocampus is decreased long after handling (postnatal days 60 and 90) are congruent with prior results, suggesting that stress responses and/or neuroendocrine responses in adulthood are altered by early stressors. Although in the present study one might expect that corticosterone would have been increased concomitant with decreased 3α,5α-THP, it is important to note that rats were not stressed in adulthood prior to tissue collection, which may have produced only basal levels, thereby minimizing potential differences in adulthood. Examination of stress-induced elevations in corticosterone and 3α,5α-THP may have been necessary to observe other patterns of responses. Despite this limitation, our present data suggest that an underlying factor for disturbances in behavioral and HPA responses to stress may be decreased 3α,5α-THP levels in the hippocampus produced by ES.
Results of the present studies are intriguing; however, there are limitations that require careful consideration of the data. First, as discussed above, adult rats were not stressed prior to tissue collection. As such, it is not possible to definitively ascertain whether the observed reductions in 3α,5α-THP were due to altered HPA activity. Second, most of the measures replicated from experiment 1 to experiment 2, despite differences in research sites, personnel, rat strains, light cycle, and animal husbandry. However, in experiment 1, there were no differences in plasma 3α,5α-THP in adulthood; whereas in experiment 2, there were significant decreases in plasma 3α,5α-THP. Although it is possible that this discrepancy may be due to the lack of some of the ES groups in experiment 2, this seems unlikely given that the more salient ES were omitted. Finally, while it is intriguing that ES may have some effects on HPA-responsiveness by altering neurosteroid production, it is not possible from the present data to determine the direction of effects or which of these factors influences the other.
In summary, the present data suggest that ES can alter pregnane neurosteroids in the hippocampus, and that this effect can persist into adulthood. These are compelling data that suggest that progestins in the hippocampus may underlie stress-responsiveness. These are intriguing data that further our knowledge about the involvement of progestins in the neurobiology of stress and lay the groundwork for future research to elucidate putative substrates for progestins effects on stress. Notably, early experiences that result in enhanced stress-reactivity increase risk for illness in adulthood, including various affective disorders, diabetes, autoimmune disorders, and coronary heart disease (Chrousos and Gold 1992; Heim et al. 1997; Higley et al. 1991; McEwen and Stellar 1993; Seckl and Meaney 1994). Thus, understanding the mechanisms by which early stressors may influence neuroendocrine responses that mediate subsequent responses to stress is important.
References
Ader R (1965) Effects of early experience and differential housing on behavior susceptibility to gastric erosions in the rat. J Comp Physiol Psychol 60:233–238
Ader R (1970) The effects of early experience on the adrenocortical response to different magnitudes of stimulation. Physiol Behav 5:837–839
Barbaccia ML, Serra M, Purdy RH, Biggio G (2001) Stress and neuroactive steroids. Int Rev Neurobiol 46:243–272
Bitran D, Hilvers RJ, Kellogg CK (1991) Anxiolytic effects of 3a-hydroxy-5a[b]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res 561:157–161
Bitran D, Dugan M, Renda P, Ellis R, Foley M (1999) Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res 850:217–224
Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE (1997) Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron 19:1103–1114
Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22:219–229
Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252
Denenberg VH, Zarrow MX, Kalberer WD, Farooq A (1963) Maternal behaviour in the rabbit: effects of environmental variation. Nature 197:161–162
Finn DA, Gee KW (1994) The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther 271:164–170
Finn DA, Roberts AJ, Long S, Tanchuck M, Phillips TJ (2003) Neurosteroid consumption has anxiolytic effects in mice. Pharmacol Biochem Behav 76:451–462
Frisone DF, Frye CA, Zimmerberg B (2002) Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behav Brain Res 128:153–160
Frye CA (2001) The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev 37:201–222
Frye CA, Bayon LE (1999) Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J Neuroendocrinol 11:839–847
Frye CA, Vongher JM (1999) 3α,5α-THP in the midbrain ventral tegmental area of rats and hamsters is increased in exogenous hormonal states associated with estrous cyclicity and sexual receptivity. J Endocrinol Invest 22:455–464
Frye CA, McCormick CM, Coopersmith C, Erskine MS (1996) Effects of paced and non-paced mating stimulation on plasma progesterone, 3α-diol and corticosterone. Psychoneuroendocrinology 21:431–439
Frye CA, Bayon LE, Pursnani NK, Purdy RH (1998) The neurosteroids, progesterone and 3α,5β-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res 808:72–83
Frye CA, Petralia SM, Rhodes ME (2000) Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav 67:587–596
Heim C, Owens MJ, Plotsky PM, Nemeroff CB (1997) The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin-releasing factor. Ann N Y Acad Sci 821:194–207
Higley JD, Suomi SJ, Linnoila M (1991) CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 103:551–556
Hsu FC, Zhang GJ, Raol Y, Valentino RJ, Coulter DA, Brooks-Kayal AR (2003) Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A 100:12213–12218
Kehoe P, Mallinson K, McCormick CM, Frye CA (2000) Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Brain Res Dev Brain Res 124:133–136
Kellogg CK, Frye CA (1999) Endogenous levels of 5α-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res 115:17–24
Kellogg CK, Kenjars TP, Pleger GL, Frye CA (2005) Region-, age-, and sex-specific effects of fetal diazepam exposure on the postnatal development of neurosteroids. Brain Res Dev Brain Res (in press)
Levine S, Haltmeyer GC, Karas GG, Denenberg VH (1967) Physiological and behavioral effects of infantile stimulation. Physiol Behav 2:55–63
Macri S, Mason GJ, Wurbel H (2004) Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci 20:1017–1024
Madruga C, Xavier LL, Achaval M, Sanvitto GL, Lucion AB (2005) Early handling, but not maternal separation, decreases emotional responses in two paradigms of fear without changes in mesolimbic dopamine. Behav Brain Res (in press)
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007
Maldonado AM, Kirstein CL (2005) Cocaine-induced locomotor activity is increased by prior handling in adolescent but not adult female rats. Physiol Behav 86:568–572
McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA (2002) Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav 73:77–85
McEwen BS, Stellar E (1993) Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153:2093–2101
Meaney MJ, Aitken DH (1985) The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res 354:301–304
Meaney MJ, Viau V, Aitken DH, Bhatnagar S (1988) Stress-induced occupancy and translocation of hippocampal glucocorticoid receptors. Brain Res 445:198–203
Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM (1996) Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 18:49–72
Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H (2001) Regulation of neurosteroid biosynthesis in the frog diencephalon by GABA and endozepines. Horm Behav 40:218–225
Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A (2002) Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur J Endocrinol 146:251–260
Nunez JF, Ferre P, Escorihuela RM, Tobena A, Fernandez-Teruel A (1996) Effects of postnatal handling of rats on emotional, HPA-axis, and prolactin reactivity to novelty and conflict. Physiol Behav 60:1355–1359
Patchev VK, Almeida OF (1996) Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci 16:7077–7084
Patchev VK, Hassan AH, Holsboer DF, Almeida OF (1996) The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15:533–540
Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB J 6:2311–2322
Purdy RH, Morrow AL, Moore PH Jr, Paul SM (1991) Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A 88:4553–4557
Rhodes ME, Frye CA (2001) Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decrease anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci 1:287–296
Rodbard D, Hutt DM (1974) Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency (ed) Symposium on radioimmunoassay and related procedures in medicine Uniput, New York, pp 209–223
Seckl JR, Meaney MJ (1994) Early life events and later development of ischaemic heart disease. Lancet 342:1236
Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuvera F, Usala L, Purdy RH, Biggio G (2000) Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem 75:732–740
Smythe JW, McCormick CM, Rochford J, Meaney MJ (1994) The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Physiol Behav 55:971–974
Zimmerberg B, Kajunski EW (2004) Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav 78:465–471
Zimmerberg B, Rackow SH, George-Friedman KP (1999) Sex-dependent behavioral effects of the neurosteroid allopregnanolone (3α,5α-THP) in neonatal and adult rats after postnatal stress. Pharmacol Biochem Behav 64:717–724
Acknowledgements
This research was supported by grants to CAF from the National Institute of Mental Health (MH06769), and to ARB-K from the National Institute of Neurological Disorders and Stroke (NS38595).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frye, C.A., Rhodes, M.E., Raol, Y.H. et al. Early postnatal stimulation alters pregnane neurosteroids in the hippocampus. Psychopharmacology 186, 343–350 (2006). https://doi.org/10.1007/s00213-005-0253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0253-7