Abstract
Rationale and objectives
Dopamine (DA) agonists reliably disrupt prepulse inhibition (PPI) of the startle reflex in animals but less so in humans despite cross-species similarities in the neural regulation of PPI. This study examines whether individual variation in baseline PPI may account for the inconsistencies in DA agonist-induced PPI disruption in humans.
Methods
Baseline PPI measures were obtained from 32 healthy adult men. Subjects were subsequently tested in three sessions after ingestion of placebo or active drug in a balanced double-blind design. Seventeen subjects were given 0.05 and 0.1 mg of pergolide (a direct DA agonist) and 15 subjects were given 100 and 200 mg of amantadine (an indirect DA agonist). In each treatment group, subjects were assigned to “high” and “low” PPI subgroups based on the median split of their baseline PPI.
Results
Amantadine and pergolide disrupted PPI in high- but not in low-PPI subjects. In contrast, low-PPI subjects showed a trend towards PPI facilitation especially with pergolide.
Conclusions
Our results suggest that baseline PPI is an important determinant of the effect of DA agonists on PPI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prepulse inhibition (PPI) of the acoustic startle response is a measure of inhibitory control of information processing in which a weak sensory stimulus (the prepulse) inhibits the startle response to a subsequent sudden intense stimulus (pulse). PPI is thought to reflect “sensorimotor gating”, which involves the ability to filter out irrelevant information in the early stages of processing so that attention can be focused on more salient features of the environment (Braff et al. 1978; Braff and Geyer 1990).
Functional imaging studies of healthy humans have shown PPI to be associated with increased activation in the frontal lobes extending to the striatum, hippocampus, thalamus and inferior parietal lobes (Hazlett et al. 2001; Kumari et al. 2003). In this circuitry, PPI is potently regulated by the dopaminergic neurotransmission, although important effects of N-methyl-d-aspartate (NMDA) receptor antagonism have also been reported (Geyer et al. 2001; Swerdlow et al. 1992; Koch and Schnitzler 1997).
In animal studies, administration of agents that facilitate dopamine (DA) neurotransmission reliably disrupts PPI (Mansbach et al. 1988; Swerdlow et al. 1998, 2001, 2002b, 2003; Geyer et al. 2001), whereas results in humans are conflicting. For example, the D2 agonist pergolide does not produce significant effects in humans (Swerdlow et al. 2002a), whereas in animals it exhibits PPI potentiation with weak prepulses at long intervals or intense prepulses at short intervals (<20 ms) and disruption with intense prepulses at long (>60 ms) intervals (Swerdlow et al. 2001).
A similar pattern appears to emerge with NMDA antagonists, such as ketamine and phencyclidine (PCP), which reliably disrupt PPI in animal studies (Geyer et al. 2001), but in humans they either produce PPI facilitation or no effect (Braff et al. 2001). Administration of amantadine, an indirectly acting dopaminergic agonist and uncompetitive NMDA receptor antagonist (Heimans et al. 1972), results in disruption of PPI at long intervals in rats (Swerdlow et al. 2002b), whereas in humans its administration has been shown to increase sensorimotor gating (Swerdlow et al. 2002a,b).
Between-subject PPI variability may account at least in part for these conflicting results (Hamm et al. 2001). Indeed, studies using within-subject designs have found disruption of PPI by dopaminergic agonists [Hutchison and Swift 1999 (amphetamine); Abduljawad et al. 1998, 1999 (bromocriptine)], whereas between-subject comparisons showed no such effect [Swerdlow et al. 2002a (amantadine, pergolide, bromocriptine, amphetamine); Swerdlow et al. 2002b (amantadine, bromocriptine)]. Individual differences at baseline PPI characteristics may be important determinants of the effect of DA agonists on PPI, as subjects with the highest PPI at baseline appear more sensitive to amphetamine-induced PPI reduction (Swerdlow et al. 2003).
Based on the observation of Swerdlow et al. (2003), the present study explored the possibility that healthy subjects with “high” or “low” PPI at baseline may differ in their sensitivity to the disruptive effects of two DA agents, amantadine and pergolide. We used a range of stimulus parameters designed to explore potential stimulus-dependent effects of the drugs.
Materials and methods
We examined the effects of amantadine and pergolide on PPI in two separate groups of healthy male volunteers. We restricted the sample to men to avoid additional PPI variability related to gender (Swerdlow et al. 1993, 1995, 1997; Weike et al. 2000) and menstrual cycle in women (Swerdlow et al. 1997). In line with Swerdlow et al. (2002a,b), we included subjects with initial startle reactivity of >50 digital units (2.44 μV per digital unit) to eliminate possible confounds from startle habituation (Blumenthal 1996, 1997) in calculating percentage PPI (%PPI) in individuals with low startle amplitudes. Additional inclusion criteria included right-handedness, general intellectual ability above 70, absence of personal history of head trauma, medical and neurological conditions or use of prescribed and recreational drugs, absence of personal or family (up to second-degree relatives) history of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I disorders, and hearing threshold of 1 kHz>20 dB. Amantadine and pergolide were administered in a placebo-controlled, within-subject design in two separate experiments using identical experimental procedures. The choice of type and dose of drugs was based on their tolerability and the uncertainty regarding their effect on PPI in human subjects (Swerdlow et al. 2002a, b).
Subjects
The study was approved by the Ethics Committee of the University of Crete. All participants gave their written informed consent before screening. All participants underwent physical and psychiatric assessment using the Mini-International Neuropsychiatric Interview (Sheehan et al. 1998) and a hearing test. General intellectual ability was estimated using Raven's Standard Progressive Matrices.
Amantadine experiment
Twenty-six healthy male medical students volunteered for this experiment. Five failed to meet inclusion criteria at screening, four withdrew consent and two discontinued after the first session. Fifteen subjects aged 21–28 years (mean±SD, 23.3±2.6) participated in the full study protocol. Eleven subjects were non-smokers and four smoked 10–30 cigarettes a day. All subjects were regular caffeine (2 cups of coffee per day on average) and occasional alcohol consumers.
Pergolide experiment
Twenty-one healthy male medical students volunteered for this experiment but four withdrew consent after screening. The 17 remaining, aged 21–29 years (mean±SD, 23.8±2.7 years), participated in the study. Twelve subjects were non-smokers and five smoked 10–20 cigarettes a day. All subjects were regular caffeine (2 cups of coffee per day on average) and occasional alcohol consumers.
After screening, subjects received a block of four pulse-alone and four prepulse–pulse stimulus trials in a pseudorandom order to calculate baseline startle and %PPI of the startle reflex. Pulses consisted of 40-ms, 115-dB white noise tones and prepulses consisted of 20-ms, 75-dB white noise tones presented at an 80-ms lead interval (onset to onset), over a 70-dB background noise. We chose weak prepulses (5 dB above background) because they are putatively better than intense prepulses in discriminating between subjects with different levels of startle inhibition and because they have the potential of revealing individual differences, as PPI becomes least robust and stable with the weak prepulses (Schwarzkopf et al. 1993).
In each treatment condition, subjects were divided into a low- and a high-PPI group based on a median split of their baseline PPI excluding one subject with baseline PPI value equal to the median. In each treatment condition, comparison of the high- and low-PPI groups on age, years of education, body weight, smoking status, %PPI, initial reactivity and startle did not reveal any statistical differences (Table 1). One to three days later, all subjects participated in three testing sessions 10 days apart, having been instructed to maintain their normal patterns of caffeine and nicotine consumption to avoid possible effects of caffeine (Swerdlow et al. 2000) and nicotine withdrawal (Kumari and Gray 1999) on PPI.
Design and drugs
Amantadine experiment
Amantadine hydrochloride (100 and 200 mg) and placebo were administered. Testing was carried out 3 h post-administration at the time of amantadine's peak effect (Hardman et al. 2001). Subjects were allocated to sessions and treatments according to a balanced, crossover, double-blind design.
Pergolide experiment
Pergolide mesylate (0.05 and 0.1 mg) and placebo were administered. Testing was carried out 2 h post-administration at the time of pergolide's peak effect (Factor 1999). Domperidone, a D2 antagonist, was available at 10 mg to counteract any adverse effects of pergolide (general malaise, nausea and vomiting) after they were present.
Startle response measurement
A commercially available electromyographic (EMG) startle system (EMG SR-LAB, San Diego Instruments, San Diego, CA, USA) was used to examine the eyeblink component of the acoustic startle response. This was used to deliver acoustic startle stimuli and record the EMG activity for 150 ms (sample interval=1 ms) starting from the onset of the startle stimulus, and the raw data were stored for later application of rejection criteria and averaging. Acoustic stimuli were administered binaurally through headphones (model TDH-39-P, Maico, Minneapolis, MN, USA). Electromyographic recordings were taken while subjects were seated comfortably in an armchair and instructed to relax but stay awake. The eyeblink component of the startle reflex was indexed by recording EMG activity of the orbicularis oculi muscle directly beneath the right eye, by positioning two miniature silver/silver chloride electrodes filled with Signa Gel electrolyte paste (Parker Laboratories, Inc., New Jersey, USA) with a ground electrode behind the right ear on the mastoid (R<10 kΩ). EMG activity was band-pass filtered (100–1,000 Hz) and a 50-Hz filter was used to eliminate the 50-Hz interference. Pulses consisted of 40-ms, 115-dB white noise tones and prepulses consisted of 20 ms of either 75- or 85-dB white noise bursts over a 70-dB background noise. Recording began with 3 min of acclimation when only background noise was present. The recording period consisted of 58 trials. There were two blocks of five pulse-alone trials, one at the beginning and one at the end, with three blocks of 16 trials in between. The startle responses to the initial and final pulse-alone blocks were used to estimate startle habituation as per Vollenweider et al. (1999) and were not included in the calculation of PPI. Each of the three 16-trial blocks consisted of four pulse-alone and 12 prepulse–pulse trials. Three lead intervals (onset to onset) were used (50, 80 and 140 ms). For each interval, there were two trials with 75-dB prepulse and two with 85-dB prepulse. All trials were presented in a pseudorandom order with the constraint that no two identical trials occurred in succession. We chose an extended range of intervals since preclinical research showed that pergolide and amantadine disrupt PPI at the 60- to 120-ms interval range [pergolide 60–100 ms (Swerdlow et al. 2001); amantadine 60–120 ms (Swerdlow et al. 2002b). The intertrial interval varied between 9 and 23 s (average 15 s). The entire test session lasted approximately 17 min. Before scoring and data analysis, all recordings were screened for spontaneous eyeblink activity. Trials were excluded if excessive EMG activity (>20 digital units=48.8 μV) was observed during the first 20 ms of recording or when onset latencies (defined by a shift of 20 digital units = 48.8 from the baseline value, occurring within 20–85 ms after the onset of the pulse stimulus) and peak latencies (the point of maximal amplitude) differed by more than 95 ms (Braff et al. 1992, 1999). About 3% of trials across all drug and placebo conditions were excluded using these criteria. No subjects had more than two (out of six) trials per trial type discarded at any one session. The maximum absolute amplitude of the raw EMG data occurring in the 20–150 time window of the non-rejected trials was scored offline and stored for averaging and data analysis (see below).
Psychological and physiological measures
Subjects completed the State Trait Anxiety Inventory (STAI-State) questionnaire (Spielberger 1983) and rated their subjective mood and feelings on a battery of 16-item 10-cm visual analogue scales (VAS) (Norris 1971). For each subject, the raw values (in centimetres) for each item were weighted by multiplication with their respective factor loading. The weighted values for each item were then allocated to “alertness”, “anxiety” and “discontentment” factors, based upon a principal component analysis (Bond and Lader 1974). The average of the weighted values for each factor was entered in the statistical analysis. The VAS and STAI-State questionnaire were completed twice during each session, at baseline and after 2 or 3 h post-treatment for the pergolide and amantadine experiments, respectively.
Temperature, systolic and diastolic blood pressures were also recorded twice during each session, at baseline and after 2 or 3 h post-treatment for the pergolide and amantadine experiments, respectively.
Statistical analysis
Data were analysed separately for each treatment condition (amantadine and pergolide). Within each treatment condition, (1) startle data were analysed first for the entire treatment group and then separately for the high- and low-PPI groups. Data from the five pulse-alone trial blocks from the beginning and the end of the session were averaged separately for each block and the respective means were subjected to a 3×2 (Treatment×Block) repeated-measures analysis of variance (ANOVA) to examine treatment effects on startle and startle habituation. The maximal amplitudes of the raw EMG responses from each trial were averaged across all trials of the same type. Percentage PPI (%PPI) was calculated using the formula [(Amplitudepulse-alone−Amplitudeprepulse–pulse)/Amplitudepulse-alone]×100. %PPI data were analysed by a 3×2×3 (Treatment×Prepulse×Lead Interval) repeated-measures ANOVA. Significant main effect of treatment was further examined by comparing active treatments to placebo using Dunnett's test. (2) Changes in the STAI-State questionnaire, the VAS data, systolic and diastolic blood pressure, and temperature between baseline and post-treatment were analysed using repeated-measures ANOVA.
Results
In order to confirm the validity of dividing subjects into the high- and low-PPI groups based on the results of the screen, we examined the subjects' PPI response to the low-intensity prepulse trials at 80-ms lead interval (75–80) from the placebo condition. Using the median split of the placebo data set, we identified exactly the same individuals as belonging to the high- and low-PPI groups as those identified from the screen results. Furthermore, pairwise t tests did not reveal significant differences between the mean PPI score in the screen or placebo conditions (amantadine t=−0.92, df=13, p=0.37; pergolide t=1.77, df=15, p=0.09).
Startle amplitude and habituation
Amantadine experiment
Startle amplitudes (mean±SEM) in the first and last block used to test the effects of treatment on startle amplitude and habituation were placebo (first 161.34±23.55, last 98.00±18.22), amantadine 100 mg (first 200.23±42.11, last 101.00±24.13) and amantadine 200 mg (first 163.34±28.26, last 131.80±31.78) for the high-PPI group; and placebo (first 142.91±24.72, last 84.97±23.81), amantadine 100 mg (first 143.49±30.94, last 105.63±31.39) and amantadine 200 mg (first 148.83±42.97, last 106.43±37.80) for the low-PPI group. There was no significant main effect of treatment or treatment by block interaction in either the high-PPI [F(2,12)=1.20, p>0.1 and F(2,12)=2.98, p=0.09, respectively] or the low-PPI [F<1 and F(2,12)=1.08, p>0.1, respectively] groups. Both groups showed significant startle habituation [high-PPI group F(1,6)=35.63, p<0.001; low-PPI group F(1,6)=33.55, p<0.001]. Data analysis from the entire group of 15 subjects using same factorial design produced similar results (i.e., no effect of treatment or treatment by block interaction but a significant block main effect).
Pergolide experiment
Startle amplitudes (mean±SEM) in the first and last block used to test the effects of treatment on startle amplitude and habituation were placebo (first 204.28±24.09, last 137.43±24.82), pergolide 0.05 mg (first 170.33±28.18, last 117.00±15.37) and pergolide 0.1 mg (first 171.18±21.95, last 123.05±11.41) for the high-PPI group; and placebo (first 138.93±31.69, last 108.80±34.64), pergolide 0.05 mg (first 141.65±30.57, last 92.90±31.18), and pergolide 0.1 mg (first 112.80±25.31, last 81.10±18.04) for the low-PPI group. There was no significant main effect of treatment or treatment×block interaction in either the high-PPI [F(2,14)=2.09, p>0.1 and F<1, respectively] or low-PPI [F(2,14)=3.65, p=0.053; and F(2,14)=1.43, p>0.1, respectively] group. Both groups showed significant startle habituation [high-PPI group F(1,7)=13.80, p<0.05; low-PPI group F(1,7)=10.87, p<0.05]. Data analysis from the entire group of 17 subjects using same factorial design revealed a significant reduction of startle response amplitude by pergolide [treatment main effect F(2,32)=3.39, p<0.05]. Post hoc tests showed that this effect was significant only for pergolide 0.1 mg. There was also robust habituation of the startle response [block main effect F(1,16)=27.07, p<0.001], which was dose independent [Treatment×Block interaction F(2,32)<1].
Prepulse inhibition
Amantadine experiment
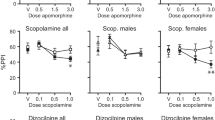
Figure 1 shows the %PPI of the high-PPI (Fig. 1a) and low-PPI (Fig. 1b) groups after administration of placebo or amantadine 100 and 200 mg in trials with 75- or 85-dB prepulses at 50-, 80- and 140-ms lead interval types. A Treatment×Prepulse×Lead Interval repeated-measures ANOVA was used to analyse the results separately for the high- and low-PPI groups and the total sample.
In the high-PPI group we found significant main effects of treatment [F(2,12)=4.31, p<0.05], prepulse intensity [F(1,6)=58.51, p<0.001] and interval [F(2,12)=5.90, p<0.02]. None of the interactions was significant. Post hoc analyses of the effect of treatment for each interval showed significant reductions of PPI with amantadine 200 mg at trials with 75-dB prepulse and 80-ms intervals and 85-dB prepulse and 50-ms intervals.
In the low-PPI group there was a significant main effect of prepulse intensity [F(1,6)=56.99, p<0.001] but not of treatment (F<1) or interval [F(2,12)=3.37, p=0.07] or any interactions (F's <1.3, all p values >0.1).
Analysis of PPI data from the entire group (n=15) revealed significant main effects of prepulse intensity [F(1,14)=134.94, p<0.001] and interval [F(2,28)=8.24, p=0.002] but not treatment [F(2,28)=1.9, p>0.1]. None of the interactions were significant (F's <2.4, all p values >0.1).
Pergolide experiment
Figure 2 shows the %PPI of the high-PPI (Fig. 2a) and low-PPI (Fig. 2b) groups after administration of placebo or pergolide 0.05 and 0.1 mg in trials with 75- or 85-dB prepulses at 50-, 80- and 140-ms lead interval types. A Treatment×Prepulse×lead interval repeated-measures ANOVA was used to analyse the results separately for the high- and low-PPI groups and the total sample.
In the high-PPI group we found significant main effects for treatment [F(2,14)=3.7, p=0.05], prepulse intensity [F(1,7)=161.79, p<0.001] and interval [F(2,14)=10.34, p<0.002]. The only significant interaction was Prepulse×Interval [F(2,14)=4.15, p<0.05]. Post hoc analyses showed that PPI was reduced in the 85-dB prepulse trials. This effect was present for both doses of pergolide at 80-ms lead intervals and by the higher dose only for 140-ms intervals.
In the low-PPI group we found a significant main effect for prepulse intensity [F(1,7)=7.37, p<0.05] but not for treatment or interval (F's <1.4, all p values >0.1). None of the interactions were significant (F's <2, all p values >0.1).
Analysis of PPI data from the entire group (n=17) revealed significant main effects for prepulse intensity [F(1,16)=34.28, p<0.001] and interval [F(2,32)=3.42, p<0.05] but not treatment (F<1). The only significant interaction was Prepulse×Interval [F(2,32)=5.03, p<0.02].
Physiological and psychological measures
The changes in physiological and psychological measures under the three treatment conditions in the amantadine and the pergolide groups are shown in Tables 2 and 3, respectively. In the amantadine group, there was no effect of treatment on any physiological or psychological measure (all p values >0.1). Both doses of pergolide reduced temperature and systolic and diastolic blood pressure in the sitting and standing positions. A statistically significant increase was seen with pergolide in the STAI-State questionnaire scores [F(2,32)=4.62, p<0.05] and a reduction in VAS-rated alertness [F(2,32)=8.17, p<0.001]. There were no differences between the high- and low-PPI groups in terms of pergolide-induced physiological and psychological measures. Seven subjects received domperidone (four in the high-PPI group and three in the low-PPI group) after administration of pergolide 0.1 mg, always at the end of the session.
Discussion
We found that in healthy men, baseline PPI level is an important determinant of the effects of amantadine and pergolide on PPI. Pergolide and amantadine significantly disrupted PPI in subjects with high but not low baseline PPI. When low- and high-PPI subjects were grouped together, the effects of amantadine and pergolide were no longer detectable.
Pergolide is a D1- and D2-like receptor agonist, a 5-HT2 receptor antagonist and has mild adrenergic effects (Langtry and Clissold 1990). Because its affinity is highest for D2-like receptors, activation of this receptor family is considered important for its pharmacological action. The potential of pergolide to increase central dopaminergic neurotransmission is further enhanced by its antagonism at 5-HT2 receptors (Nomikos et al. 1994). In clinical pharmacology, pergolide is given at a starting dose of 0.05 mg to minimise the potential of side effects from a sudden increase in central dopaminergic neurotransmission (British Medical Association and Royal Pharmaceutical Society of Great Britain 2005). This dose had no effect on PPI in our study, confirming that its effect on dopaminergic systems is indeed low. We also found that pergolide 0.1 mg induced PPI disruption only in those subjects with high baseline PPI, suggesting the possibility that the effect of pharmacological manipulations of the central dopaminergic system may depend on individual variability of baseline levels of this system, as we will discuss below.
Amantadine has an amphetamine-like effect presynaptically in that it releases stored catecholamines (Heimans et al. 1972). Re-uptake inhibition of DA has also been proposed but this mechanism is only observed in very high doses, outside the range employed here (Heimans et al. 1972). However, the main effect of amantadine on dopaminergic function is thought to be indirect through its non-competitive antagonism on NMDA receptors (Stoof et al. 1992). Although it is not possible for us to discern the effect of amantadine on each neurotransmitter system implicated by its pharmacology, the doses employed here (100–200 mg) are similar to those found to be clinically useful in patients with Parkinson's disease, suggesting that the “net” effect of this compound is increased DA availability. The effect of amantadine on PPI was most reliably seen at the higher dose of 200 mg in the high-PPI group, again suggesting the possibility of an interaction with individual baseline levels of dopaminergic function.
There is a large body of evidence from animal studies supporting a close link between PPI and prefrontal cortical (PFC) DA activity. Reductions in DA activity in the PFC after local injection of selective D2 or D1 antagonists or 6-hydroxydopamine lesions result in significant PPI reduction (Bubser and Koch 1994; Ellenbroek et al. 1996; Zavitsanou et al. 1999). Conversely, increased PFC DA activity after local apomorphine infusions also disrupts PPI (Broersen et al. 1999; Lacroix et al. 2000). The role of PFC in PPI in humans is supported by functional imaging studies of PPI, which report increased PFC activity (Hazlett et al. 2001; Kumari et al. 2003). Experimental evidence indicates that the impact of DA on prefrontal function follows an inverted U-shaped dose–response curve, such that the optimal response is achieved within a narrow range of DA activity, with too little or too much DA having a relatively negative effect (Williams and Goldman-Rakic 1995). High- and low-PPI individuals may occupy different positions on this putative inverted U-shaped curve (Fig. 3). It is tempting to speculate that high-PPI subjects may have a higher baseline DA activity and further DA increases induced by dopaminergic agonists may “push” the group downwards the slope of the putative inverted U-shaped curve leading to PPI reductions. Our findings of pergolide- and amantadine-induced PPI disruption in the high-PPI group and no effect in the low-PPI group resonate with observations by Mattay et al. (2003). They found that healthy subjects who performed relatively well on PFC-related cognitive tasks got worse after DA agonist administration. This formulation of an interaction between baseline PPI and the effect dopaminergic agents on PPI requires further investigation in different paradigms. One such possibility is to examine the effect of catechol-O-methyltransferase (COMT) polymorphism [val(108/158)met] on PPI both at baseline and after dopaminergic agonist administration since it is known to influence baseline PFC DA activity.
Our results could not be attributed to the physiological or psychological effects of amantadine and pergolide. There were no such effects with amantadine, and in the pergolide group there were no significant differences in these measures between high- and low-PPI subjects. It is unlikely that PPI calculation was affected by differences in startle amplitude between the high- and low-PPI groups since they were both specifically preselected for high startle reactivity (>50 digital units=122 μV) and did not differ in initial startle reactivity at baseline (Table 1) or in startle amplitude at testing. It could be argued that the higher the baseline PPI, the more range there is for stimulant-induced changes, whereas in subjects with low baseline PPI, stimulant-induced disruption may not be detected because of “floor” effects. Although this possibility cannot be totally excluded with regard to the effect of amantadine 200 mg, it is unlikely that it can account for our findings on pergolide, which disrupts PPI in the high group and appears to affect the low group in the opposite way. Although the latter effect is not significant, it is at odds with the operation of a floor effect. It is possible that with higher pergolide doses and/or greater numbers of subjects with low baseline PPI a significant pergolide-induced PPI facilitation may be detected in this group.
In this study, the effect of baseline PPI levels was independently replicated in two different samples and with two different dopaminergic agents. The importance of baseline PPI levels for another dopaminergic agent, amphetamine, has also been highlighted by Swerdlow et al. (2003) who reported that individuals with the highest baseline PPI levels were most sensitive to the PPI-disruptive effects of amphetamine. Therefore, it could be argued that previous inconsistent or negative results regarding the effect of dopaminergic agents on PPI reflect the inclusion of subjects with different baseline PPI levels. Although the findings of this study will need to be independently replicated they do suggest that future studies on pharmacological manipulations of the PPI may need to consider screening participants for baseline PPI as well as startle reactivity. We also propose that the existing literature on drug-induced PPI changes in humans may be reappraised with more emphasis given to results from studies using within-subject designs. If baseline PPI measures are to be more regularly incorporated in future studies, it is important to have normative data across different acoustic stimuli and lead intervals. This would allow the categorisation of individuals in terms of baseline PPI levels in an epidemiologically meaningful way. Hamm et al. (2001) have contributed towards this goal by providing weighted averages for PPI obtained in control samples from 12 studies. However, since their meta-analysis was based on studies that had excluded outliers, the weighted averages may not be truly representative of the distribution of PPI values in the normal population.
References
Abduljawad KAJ, Langley RW, Bradshaw CM, Szabadi E (1998) Effects of bromocriptine and haloperidol on prepulse inhibition of the acoustic startle response in man. J Psychopharmacol 12:239–245
Abduljawad KAJ, Langley RW, Bradshaw CM, Szabadi E (1999) Effects of bromocriptine and haloperidol on prepulse inhibition: comparison of the acoustic startle eyeblink response and the N1/P2 auditory-evoked response in man. J Psychopharmacol 13:3–9
Blumenthal TD (1996) Inhibition of the human startle response is affected by both prepulse intensity and eliciting stimulus intensity. Biol Psychol 44:85–104
Blumenthal TD (1997) Prepulse inhibition decreases as startle reactivity habituates. Psychophysiology 34:446–450
Bond AJ, Lader MH (1974) The use of analogue scales in rating subjective feelings. Br J Med Psychol 47:211–218
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 47:181–188
Braff DL, Stone C, Callaway E, Geyer MA, Glick I, Bali L (1978) Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 15:339–343
Braff DL, Grillon C, Geyer MA (1992) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49:206–215
Braff DL, Swerdlow NR, Geyer MA (1999) Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry 156:596–602
Braff DL, Geyer MA, Swerdlow NR (2001) Human studies of prepulse inhibition of startle: normal subjects, patients groups, and pharmacological studies. Psychopharmacology 156:234–258
British Medical Association and Royal Pharmaceutical Society of Great Britain (2005) British National Formulary, 49th edn. British Medical Association and Royal Pharmaceutical Society of Great Britain, London
Broersen LM, Feldon J, Weike I (1999) Dissociative effects of apomorphine infusions into the medial prefrontal cortex of rats on latent inhibition, prepulse inhibition and amphetamine-induced locomotion. Neuroscience 94:39–46
Bubser M, Koch M (1994) Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions in the medial prefrontal cortex. Psychopharmacology 113:487–492
Ellenbroek BA, Budde S, Cools AR (1996) Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75:535–542
Factor SA (1999) Dopamine agonists. Med Clin North Am 83:415–443
Geyer MA, Krebs-Thompson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154
Hamm AO, Weike AI, Schuoo HT (2001) The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacology 156:259–265
Hardman JG, Limbird LE, Goodman Gilman A (2001) The pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York
Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MB, Wei TC, Byne W, Haznedar MM (2001) Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry 50:281–291
Heimans RL, Rand MJ, Fennesy MR (1972) Effects of amantadine on uptake and release of dopamine by a particulate fraction of rat basal ganglia. J Pharm Pharmacol 24:875–879
Hutchison KE, Swift R (1999) Effect of d-amphetamine on prepulse inhibition of the startle reflex in humans. Psychopharmacology 143:394–400
Koch M, Schnitzler HU (1997) The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res 89:35–49
Kumari V, Gray JA (1999) Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology 141:11–15
Kumari V, Gray JA, Geyer MA, Ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T (2003) Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res 122:99–113
Lacroix L, Broersen LM, Feldon J, Weiner I (2000) Effects of local infusions of dopaminergic drugs into the medial prefrontal cortex of rats on latent inhibition, prepulse inhibition amphetamine-induced activity. Behav Brain Res 107:111–121
Langtry HD, Clissold SP (1990) Pergolide. A review of its pharmacological properties and therapeutic potential in Parkinson's disease. Drugs 39:491–506
Mansbach RS, Geyer MA, Braff DL (1988) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology 94:507–514
Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF (2003) Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 100:6186–6191
Nomikos GG, Iurlo M, Andersson JL, Kimura K, Svensson TH (1994) Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopamine release in the rat medial prefrontal cortex. Psychopharmacology 115:147–156
Norris H (1971) The action of sedation on brain-stem oculomotor systems in man. Neuropharmacology 10:181–191
Schwarzkopf S, McCoy L, Smith D, Boutros N (1993) Test–retest reliability of prepulse inhibition of the acoustic startle response. Biol Psychiatry 34:896–900
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33
Spielberger CD (1983) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto
Stoof JC, Booij J, Drukarch B (1992) Amantadine as N-methyl-d-aspartic acid receptor antagonist. New possibilities for therapeutic application? Clin Neurol Neurosurg 94(Suppl):S4–S6
Swerdlow NR, Caine SB, Braff DL, Geyer MA (1992) Neural substrates of sensorimotor gating of the startle reflex: preclinical findings and their implications. J Psychopharmacol 6:176–190
Swerdlow NR, Auerbach P, Monroe SM, Harston H, Geyer MA, Braff DL (1993) Men are more inhibited than women by weak prepulses. Biol Psychiatry 34:253–260
Swerdlow NR, Filion D, Geyer MA, Braff DL (1995) “Normal” personality correlates of sensorimotor, cognitive and visuospatial gating. Biol Psychiatry 37:286–299
Swerdlow NR, Hartman PL, Auerbach PP (1997) Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry 4:452–460
Swerdlow NR, Taaid N, Oostwegel JL, Randolph E, Geyer MA (1998) Towards a cross-species pharmacology of sensorimotor gating: effects of amantadine, bromocriptine, pergolide and ropinirole on prepulse inhibition of acoustic startle in rats. Behav Pharmacol 9:389–396
Swerdlow NR, Eastvold A, Gerbranda T, Uyan KM, Hartman P, Doan Q, Auerbach P (2000) Effects of caffeine on sensorimotor gating of the startle reflex in normal control subjects: impact of caffeine intake and withdrawal. Psychopharmacology 151:368–378
Swerdlow NR, Platten A, Shoemaker J, Pitcher L, Auerbach P (2001) Effects of pergolide on sensorimotor gating of the startle reflex in rats. Psychopharmacology 158:230–240
Swerdlow NR, Eastvold A, Karban B, Ploum Y, Stephany N, Geyer MA, Cadenhead K, Auerbach PP (2002a) Dopamine agonist effects on startle and sensorimotor gating in normal male subjects: time course studies. Psychopharmacology 161:189–201
Swerdlow NR, Stephany N, Shoemaker JM, Ross L, Wasserman LC, Talledo J, Auerbach PP (2002b) Effects of amantadine and bromocriptine on startle and sensorimotor gating: parametric studies and cross-species comparisons. Psychopharmacology 164:82–92
Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP (2003) Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology 28:640–650
Vollenweider FX, Remensberger S, Hell D, Geyer MA (1999) Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology 143:365–372
Weike AI, Bauer U, Hamm AO (2000) Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry 47:61–70
Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575
Zavitsanou K, Cranney J, Richardson R (1999) Dopamine agonists in the orbital prefrontal cortex can reduce prepulse inhibition of the acoustic startle reflex in the rat. Pharmacol Biochem Behav 63:55–61
Acknowledgements
The authors have no conflict of interest or any involvement, financial or otherwise, that might have biased the present work. This project was supported by University of Crete Research Funds Account (E.L.K.E. 1348). The studies reported in this original investigation comply with the current laws of Greece, where they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitsios, P., Giakoumaki, S.G. & Frangou, S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology 182, 144–152 (2005). https://doi.org/10.1007/s00213-005-0056-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0056-x