Abstract
Rationale
Dysfunction of executive neuropsychological performance, mediated by the prefrontal cortex, has been the central focus of recent attention deficit/hyperkinetic disorder (AD-HKD) research. The role of other potential neuropsychological “risk factors”, such as recognition memory, remains understudied. Further, the impact of methylphenidate (MPH) on key neuropsychological processes in AD-HKD remains poorly understood.
Objectives
To compare the performance of boys with AD-HKD on a spatial working memory (SWM) task and on two non-working memory tasks [a simultaneous and delayed matching-to-sample task (DMtS) and a pattern-recognition task] with that of healthy boys, and to investigate the impact of acute and chronic MPH on performance of these tasks.
Methods
Baseline performance of 75 stimulant-naive boys with AD-HKD was compared with that of 70 healthy boys. The AD-HKD boys were then re-tested following the administration of acute and chronic challenges with MPH (0.3 mg/kg and 0.6 mg/kg) under randomised double-blind placebo controlled conditions.
Results
Compared with healthy boys, the AD-HKD boys demonstrated performance deficits on all neuropsychological tasks. A single dose of MPH restored performance on the DMtS task but had no impact on the SWM or pattern-recognition tasks. Chronic MPH administration did not alter performance on the SWM task but did improve performance on both the pattern-recognition and DMtS tasks. However, the acute restorative effect of MPH on DMtS diminished with repeated administration.
Conclusions
Our results suggest that current conceptualisations of the neuropsychological basis of AD-HKD and the proposed therapeutic mechanisms of MPH require broadening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disorders of attention and hyperactivity are common, but controversial, clinical constructs which present a major public health challenge (NIMH 2000). In view of continuing debate surrounding the nosology of these conditions, we have chosen to adopt the convention described by Schachar and Tannock (2002). Hence, we will refer to specific diagnostic terms, such as hyperkinetic disorder (HD) or attention deficit hyperactivity disorder (ADHD) when addressing a particular diagnostic entity and set of criteria. We will use the acronym deficit/hyperkinetic disorder (AD-HKD) when referring to characteristics that are believed to be shared by ADHD and HD. It is implausible that AD-HKD represents the clinical presentation of a single neuropsychological or neurophysiological abnormality (Castellanos and Tannock 2002; Todd 2000; Sonuga-Barke 2002). Whilst much AD-HKD research has sought to explain this disorder within a single “grand theory”, genetic (Nadder et al. 2002; Todd et al. 2001), neuropsychological (Solanto et al. 2001), pathophysiological (Rothenberger et al. 2000) and phenotypic (Biederman et al. 1992) studies have all identified a high degree of heterogeneity within the AD-HKD population, suggesting a multi-factorial aetiology, which is unlikely to be accounted for within any such model. More likely, AD-HKD is a constellation of behavioural features generated by several relatively independent pathophysiological risk factors. Putative risk factors, or “endophenotypes”, of assumed major effect, with direct experimental support, include deficits in executive neuropsychological functions such as inhibitory control (Barkley 1997) and working memory (Kempton et al. 1999). Executive neuropsychological functions are dependent on intact functioning of the prefrontal cortices and their projections to subcortical targets such as the caudate nucleus and nucleus accumbens (Fuster 1989). Imaging studies have consistently implicated these brain regions in the pathophysiology of AD-HKD (Giedd et al. 2001). Accurate performance of these tasks is dependent on intact dopaminergic and noradrenergic neurotransmission and can be modified by even small manipulations in catecholamine release (Mehta et al. 2001). Hence, the therapeutic effects of stimulant drugs such as methylphenidate (MPH) and dexamphetamine in AD-HKD are thought to arise from actions on these circuits (Volkow et al. 2001b). Delay aversion is another potential “endophenotype” with observed impulsivity representing a strategy to reduce the subjective experience of delay (Sonuga-Barke 2002).
Working memory and AD-HKD
Definitions of working memory are contentious and, at times, confusing. Whilst some authors consider working memory simply as the process of actively maintaining relevant information in mind for brief periods of time (Gleitman et al. 1999), a more comprehensive and influential view emphasises the importance of computational processing and states that working memory is best considered as the capacity to simultaneously store and manipulate information (Baddeley 2003, 1986). Indeed, Baddeley (1996) endorses Daneman and Carpenter’s definition of a working memory task as “one that simultaneously requires the storage and manipulation of information” (Daneman and Carpenter 1980), thus differentiating such tasks from those that require storage but no manipulation. Whilst deficits on “true” working memory tasks, with substantial executive demands, have been accepted as part of the pathophysiology of AD-HKD, relatively little attention has been paid to the possibility that these children, many of whom are, by definition, disorganised and forgetful, may also demonstrate specific performance deficits on non-working memory tasks which place much lower demands on executive functioning. Studies have reported that children with AD-HKD demonstrate deficits on free recall (Borcherding et al. 1988; Loge et al. 1990), paired associates learning (Conte et al. 1986; Chang et al. 1999), spatial recognition (Kempton et al. 1999) and delayed matching-to-sample (Chelonis et al. 2002; Kempton et al. 1999) tasks (DMtS); however, negative findings have also been reported particularly on memory tasks in which stimuli are clustered or recall strategies are presented (August 1987; Benezra and Douglas 1988; Voelker et al. 1989), and also on a pattern-recognition task (Kempton et al. 1999), suggesting that when executive demands are reduced, the tasks become manageable. Unfortunately, much of this work has been hampered by a range of methodological concerns, including the failure to use clearly defined, specific, sensitive measures, small sample sizes, the use of rating scales rather than clinical interviews in the assessment of subjects and the inclusion of children with AD-HKD who were either currently taking stimulant medication or who had been recently withdrawn from stimulant medication.
Thus, we compared the performance of 75 stimulant-naive boys meeting diagnostic criteria for ICD-10 HD and DSM-IV ADHD combined subtype, aged between 7 years and 15 years, with 70 age-matched healthy controls on three memory tasks selected from the Cambridge neuropsychological test automated battery (CANTAB) neuropsychological test battery (Fray and Robbins 1996). The CANTAB battery has been extensively validated in both child (Curtis et al. 2002; Luciana and Nelson 1998; Hughes et al. 1999; Williams et al. 2000) and adult (Robbins et al. 1994) populations. Tasks within the battery have been shown to be differentially sensitive to dysfunction in several brain regions, including frontal, temporal and amygdalo-hippocampal regions (Owen et al. 1995). Here, we report performance on three memory tasks selected from the battery—a spatial working memory (SWM) task and two “non-working” recognition memory tasks (pattern recognition and both simultaneous and delayed matching to sample). Performance on further tasks from the CANTAB battery, including stockings of Cambridge (Tower of London), intra-dimensional/extra-dimensional shift, spatial span, spatial recognition, paired associates learning and reaction time, will be reported separately. Successful performance on this SWM task has been shown to be associated with activations of the dorsolateral and ventrolateral PFC and posterior parietal cortex in functional neuroimaging studies in children (Nelson et al. 2000) and adults (Mehta et al. 2000b; Owen et al. 1996). Performance deficits on this SWM task have previously been reported in children (Kempton et al. 1999) and adults (Mehta et al. 2000a) with ADHD. Successful performance of the pattern recognition and DMtS “non-working” visual recognition memory tasks requires intact short-term visual memory processing, imposes minimal “executive” demands and is sensitive to both temporal lobe and amygdalo-hippocampal (but not frontal lobe) damage (Owen et al. 1995). Deficits on this DMtS task have been reported in children with ADHD (Kempton et al. 1999). Kempton and colleagues did not, however, find performance deficits on the pattern-recognition task.
In the present study, we wished to test subjects under drug-free baseline conditions and then to re-test the AD-HKD under randomised, double-blind, placebo-controlled conditions on the three tasks following acute and chronic challenges with MPH. The inclusion of a medication condition serves several purposes. From a clinical perspective, it provides an indication as to which aspects of neuropsychological performance may be enhanced or diminished by MPH. Knowledge of the effects of MPH, an indirect dopamine agonist, on neuropsychological performance also increases understanding of the complex pathophysiological processes that underpin AD-HKD. Improved performance following administration of MPH has been reported for the SWM task (Mehta et al. 2000a,b) and on a DMtS task (Chelonis et al. 2002).
On the basis of data published prior to our initiation of the present study, we made three predictions: (1) at baseline AD-HKD boys will display performance deficits on SWM, DMtS and pattern-recognition tasks; (2) acute MPH will improve performance on the SWM task but have no effect on the DMtS and pattern-recognition tasks; (3) the effects of chronic MPH on these tasks will be the same as those seen with acute MPH.
Materials and methods
This study was approved by the Tayside Committee on Medical Ethics. All volunteers provided written informed consent.
Subjects
Subjects in the AD-HKD group were recruited from a group of boys aged between 7 years and 15 years old who had been referred to the Tayside Child and Adolescent Psychiatry Service. We used a two-stage screening procedure. Eligible and consenting subjects scoring >1.5 standard deviations from the mean on both the Conners’ parent rating scale short version (CPRS-26) and the Conners’ teacher rating scale short version (CTRS-28) were interviewed by an experienced child and adolescent psychiatrist using the Kiddie-SADS present and lifetime (K-SADS-PL) (Kaufman et al. 1996) semi-structured diagnostic interview. Those meeting the diagnostic criteria for HD (F90)—as defined in the international classification of diseases version 10 (ICD 10 1992)—and ADHD combined subtype—as defined in the diagnostic and statistical manual version IV (DSM IV 1994)—and not meeting exclusion criteria, were invited to participate in the study. Exclusion criteria for subjects included a history of neurological impairment, previously determined learning disability (IQ<80), chronic physical illness, sensory or motor impairment, current or previous exposure to stimulant medication, and abuse of any illegal drugs. The presence of a range of commonly occurring co-morbid conditions, including oppositional defiant disorder, conduct disorder and anxiety disorder, did not result in exclusion from the study (Table 1). The intention was to ensure recruitment of a group of children representative of those seen in typical clinical practice within the National Health Service in the UK. All co-morbid diagnoses were considered secondary to the primary diagnosis of AD-HKD. Five children met criteria for multiple co-morbid diagnoses.
Subjects for the age-matched healthy control group were selected from local schools following a similar two-stage screen. Consenting pupils scoring <1 standard deviation from the mean on the CPRS-26 and the CTRS-28 and all subscales of the CBCL, with no current or past psychiatric diagnosis on the K-SADS-PL interview and not meeting exclusion criteria, were invited to participate in the study. Exclusion criteria were identical to that of the AD-HKD group
The British picture vocabulary scale (BPVS) (Dunn et al. 1997) [2nd edn] was used to estimate general intellectual ability for both the AD-HKD and control subjects. The BPVS assesses verbal intelligence and was chosen for its ease of administration and ability to be used with children aged between 3 years and 15 years. It is an individually administered, norm-referenced, wide-range test of receptive vocabulary for Standard English.
Neuropsychological testing
Delayed matching to sample
The DMtS task was selected from the CANTAB (Owen et al. 1995; Robbins et al. 1997). This task assesses a subject’s ability to remember the visual features of a complex, abstract, target stimulus. At the beginning of each trial, a pattern consisting of four quadrants, each differing in colour and form, appears in the centre of a touch-sensitive screen in a white box for a presentation period of 4.5 s. Subjects are asked to remember the pattern. In the “simultaneous condition”, four choice patterns then appear in red boxes located under the target pattern. The subject is required to respond by touching the choice pattern that corresponds exactly (in both colour and form) to the target pattern above. Only one of the choice patterns is identical to the target. Correct and incorrect responses are signalled by differing auditory tones and visual feedback in the form of green ticks or red crosses. If subjects’ make an incorrect response, they are required to continue to choose until the target stimulus has been chosen. The conditions for the delayed portion of the task are identical to those of the simultaneous condition with the exception that, after the initial presentation period, the target pattern disappears from the screen. The four choice patterns are then presented following one of three delays; 0, 4, and 12 s. Following three practice trials (one each of the simultaneous presentation, 0-s and 12-s delay), a total of 20 test trials are presented with each of the four conditions presented in a pseudorandom order. Data were analysed separately for the simultaneous and delay conditions.
Spatial working memory
This is a self-ordered searching task (Petrides and Milner 1982) that assesses working memory for spatial stimuli and requires a subject to use mnemonic information to work towards a goal. Subjects are required to “search through” a spatial array of coloured boxes presented on a screen to collect “blue tokens” hidden inside the boxes. Returning to a box where a token has already been found constitutes a “between search” error (BSE) and returning to a box already opened and shown to be empty earlier in the same search sequence constitutes a “within search” error (WSE). A strategy score is calculated based on how often a searching sequence was initiated from the same box during a trial (Fray and Robbins 1996).
Pattern recognition
This test measures a subject’s ability to recognise a previously presented abstract pattern from two adjacent stimuli. The primary measure in this task is the number of correct patterns chosen across two trials of 12 patterns in each set.
Procedure
The study was conducted in three stages: baseline, acute challenge and chronic challenge.
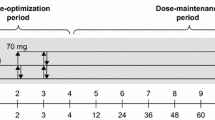
We first compared the baseline performance of the drug-naive AD-HKD group prior to exposure to MPH and control group on each of the tasks. The control group were not re-tested and exited the study at this point. The AD-HKD group were randomised under double-blind conditions into three treatment groups. Two weeks after the initial baseline test session, the AD-HKD boys were given a single oral dose acute challenge with MPH at between 0800 hours and 0900 hours, at one of three doses (group 1=placebo, group 2=0.3 mg/kg, group 3=0.6 mg/kg) and re-tested on the neuropsychological tasks using the first parallel test version 90 min later. For the chronic challenge, MPH administration was continued for a further three periods of 28 days immediately following the acute challenge. This phase of the study was also conducted under randomised, double-blind, placebo-controlled, conditions in a crossover design, with each subject taking MPH twice daily (at 0800 hours and 1200 hours) at each of the three doses (placebo, 0.3 mg/kg and 0.6 mg/kg per dose), starting with the dose given at the acute challenge and rotating around the other two doses (Fig. 1). Subjects were re-tested using the second, third and fourth parallel task batteries 90 min after taking their morning medication at the end of each 28-day block.
Data analysis
All baseline comparisons between AD-HKD and control boys were analysed using ANCOVA with BPVS percentile rank scores as a covariate. Performance at the simultaneous condition of the DMtS task was analysed separately using univariate ANCOVA, whilst performance at delay conditions of this task and BSE on the SWM task were analysed using repeated-measures ANCOVA (0, 4, 12-s delays for DMtS; 3, 4, 6, 8 boxes for BSE in SWM).
Acute challenge data were analysed using repeated-measures ANOVA for both simultaneous and delay conditions on the DMtS task and all measures on the SWM task. Percentage correct scores on the pattern recognition task failed to meet normality and homogeneity of variance assumptions and hence were analysed using the non-parametric Wilcoxon Sign test for repeated measures.
For chronic data, a mixed-design ANOVA was used with repeated measures on treatment taken (placebo, 0.3 mg/kg or 0.6 mg/kg MPH) and the order in which it was taken (1st, 2nd or 3rd). No effects of order in which the drug was taken were found for any task. Following ANCOVA/ANOVA, further exploration of the data was conducted by determination of simple effects or interactions.
Alpha for the primary outcome measures of the three tasks were adjusted using the Bonferroni method in order to keep the alpha-level overall at 0.05. As a result, alpha for each task was lowered to 0.017.
Results
Subject characteristics
The AD-HKD group comprised 75 boys (mean age 10.8 years) and the healthy control group comprised 70 boys (mean age 10.7 years). There was no significant age difference between the AD-HKD and the healthy control group (t 1,113<1). The AD-HKD group had significantly lower BPVS scores than controls (F=27.2, P<0.001); thus, BPVS scores were used as a covariate in the baseline analyses. As would be expected, the AD-HKD group scored significantly higher than the healthy control group with respect to ADHD index scores on the parent (F=1571.4, P<0.001) and teacher (F=103.9, P<0.001) Conners’ rating scales.
With respect to the acute and chronic challenge analyses, there were no significant differences between the three AD-HKD treatment groups (placebo, 0.3 mg/kg and 0.6 mg/kg) with respect to age (F<1), BPVS percentile rank (F<1), parent-rated ADHD composite score (Conners’ scale) (F<1), and teacher-rated ADHD composite score (Conner’s scale) (F<1). There were also no significant differences between these groups with respect to the incidence of most co-morbid diagnoses conduct disorder (F<1), oppositional defiant disorder (F<1), social phobia (F<1), generalised anxiety disorder (F=2.1, P>0.05), and tic disorder (F<1). However, there was a significant difference between the treatment groups with respect to separation anxiety disorder (F=3.4, P<0.04). All three boys diagnosed with this co-morbid condition were in one treatment group (those taking placebo at the acute and first chronic session). Separation anxiety disorder is not considered to be associated with neuropsychological impairment (Table 2).
Neuropsychological performance
Baseline
Delayed matching to sample
At baseline (Table 3), the AD-HKD group demonstrated deficits, relative to controls, at both the simultaneous (F 1,142=8.7, P<0.004, d=0.52) and delay (F 1,142=26.4, p<0.001, d=0.90) conditions (Fig. 2a). There was a significant interaction between performance accuracy and duration of task delay (F=4.7, P<0.01). AD-HKD subjects made fewer correct responses with increasing delay, showing greatest performance deficits at the 12-s delay condition (F=4.6, P<0.03), whilst control boys performed equally across all delays. To investigate the relationship between performance under both simultaneous and delay conditions, performance under the delay conditions was re-analysed with accuracy at the simultaneous condition as a second covariate. No significant effect of the simultaneous condition as a covariate was found. There remained a significant interaction between performance accuracy and duration of task delay (F 2,282=3.6, P<0.03)] and boys with AD-HKD still made fewer correct responses with increasing delay, showing greatest performance deficits at the 12-s delay condition (F=5.6, P<0.02).
Delayed matching to sample. a Percentage correct responses under simultaneous and delay conditions at baseline. AD-HKD group (closed circles) made fewer correct responses under both simultaneous and delay conditions than control group (open circles). There was a significant interaction between performance accuracy and duration of task delay. AD-HKD group made fewer correct responses with increasing delay, showing greatest performance deficits at the 12-s delay condition, whilst control group performed equally well across all delays. b Acute responses to oral methylphenidate (MPH). Acute oral MPH had no effect, at either dose, on performance accuracy under simultaneous test conditions. Planned contrasts revealed that MPH 0.6 mg/kg (closed squares) significantly enhanced performance accuracy across each of the delay conditions when compared with placebo (closed triangles). Indeed, task performance accuracy following MPH 0.6 mg/kg was restored to those levels observed in healthy controls (open circles). However, the AD-HKD group continued to show impaired functioning across each of the delay conditions when administered either placebo (closed triangles) or 0.3 mg/kg MPH (open triangles). c Chronic responses to oral methylphenidate (MPH). Chronic administration of MPH at 0.3 mg/kg (open triangles) and 0.6 mg/kg (closed squares) enhanced accuracy of responding under both simultaneous and delay conditions when compared with placebo (closed triangles). Although MPH continued to enhance visual memory performance in the AD-HKD group when administered chronically, this effect was smaller than that observed following acute challenge. Performance was improved, but not normalised. The AD-HKD group continued to display significant impairment in functioning under delay conditions compared with controls (open circles) despite MPH 0.3 mg/kg and 0.6 mg/kg
Incorrect responses were not associated with shorter response latencies for either group at the simultaneous condition (F<1). Under delay conditions, incorrect responses were associated with significantly shorter response latencies in the AD-HKD group (F 1,74=9.5, P<0.003). There were, however, no differences between response latencies across the three delay conditions for either group. Regression analysis revealed that latencies for incorrect responding did not predict accuracy of responding at the simultaneous, 4-s or 12-s delay conditions for AD-HKD boys. Shorter latencies were associated with increased error at the 0-s delay condition ([F=9.1, P<0.004]), but this contributed only a small proportion of the total variance for incorrect responses (r 2=0.145).
Spatial working memory
AD-HKD boys made more BSE on the SWM task (F 1,142=19.43, P<0.001, d=0.75). There was a significant interaction between group and difficulty level (F 1.6,223=15.1, P<0.001), and post-hoc tests revealed that AD-HKD boys made more errors at the eight-box stage than at three-box stages (P<0.001), four-box stages (P<0.001) or six-box stages (P<0.02). AD-HKD boys also had higher strategy scores indicating a lower use of strategy (F 2,142=16.52, P<0.001, d=0.70). There was no group difference in the number of WSE made. Strategy score was significantly correlated with total BSE for both AD-HKD (r=0.513, P<0.001) and control (r=0.588, P<0.001) boys.
Pattern recognition
AD-HKD boys made fewer correct responses on the pattern-recognition task (z=−5.267, P<0.001, d=0.89). There was no significant difference between the groups in latencies for correct responses. AD-HKD boys had shorter response latencies for incorrect choices (F 1,121=5.8, P<0.02). However, regression analysis revealed that the latencies for incorrect responses did not predict overall accuracy of responding for the AD-HKD boys.
Acute challenge
Delayed matching to sample
Acute oral MPH had no effect, at either dose, on performance accuracy under simultaneous test conditions (Table 4). MPH at a dose of 0.6 mg/kg, restored performance accuracy in AD-HKD boys, across each of the delay conditions, to the levels observed in controls (F 1,88=1.2, P>0.05) (Fig. 2b). However, the AD-HKD group continued to show impaired functioning across each of the delay conditions under both placebo (F 1,91=29.9, P<0.001) and 0.3 mg/kg MPH (F 1,90=10.8, P<0.001). Enhanced performance under MPH 0.6 mg/kg was not accompanied by significant changes in latencies to make correct responses.
Spatial working memory
Acute MPH did not affect performance on any of the key measures from the SWM task. There was no significant effect of treatment group on BSE. A significant effect of session (F 1,70=19.0, P<0.001) revealed that, overall, boys showed a reduction in BSE at the acute challenge session. There was no significant session × treatment group interaction (F 2,70 <1); however, showing that reduction of errors at the acute challenge session cannot be attributed to MPH. There were no other significant interactions between task difficulty, treatment group and session. MPH had no effect on WSE or strategy score.
Pattern recognition
MPH did not affect performance or latencies on the pattern-recognition task. There was no significant effect of treatment group on percentage of correct responses (F 2,70=1.1, P=0.34). Whilst subjects demonstrated improved responding at the acute challenge session (F 1,70=18.9, P<0.001), there was no significant treatment group × session interaction.
Chronic treatment
Delayed matching to sample
Chronic administration (Table 5) of MPH at both doses enhanced accuracy of responding under both simultaneous (F 2,114=9.8, P<0.001) and delay (F 2,116=15.4, P<0.001) conditions (Fig. 2c). This effect was smaller than that observed following acute challenge, with performance improved, but not normalised. The AD-HKD group continued to display significant impairment in functioning under delay conditions compared with controls, despite MPH 0.3 mg/kg (F 1,135=10.4, P<0.002) and 0.6 mg/kg (F 1,132=6.2, P<0.01). Chronic MPH treatment slowed response latencies for correct choices at both 0.3 mg/kg (P<0.03) and 0.6 mg/kg (P<0.01). However, a positive correlation between response latencies and accuracy of responding was only observed for children taking the 0.3-mg/kg dose at the 4-s (r=0.344, P<0.004) and 12-s (r=0.347, P<0.005) delays. More detailed evaluation of this relationship using linear regression analysis revealed that the predictive association was modest (4 s, r 2=0.119; 12 s, r 2=0.120).
Spatial working memory
Chronic MPH did not affect performance on the SWM task. There was no significant effect of treatment group on BSE, although effects narrowly failed to reach significance (F 2,116=2.85, P=0.067). There was a significant effect of difficulty level on BSE (F 3,174=279.6, P<0.001), but no significant task difficulty × treatment group interaction revealing that treatment groups performed similarly according to difficulty level. Likewise, there was no significant effect of treatment group on WSE, although this narrowly failed to reach significance (F 2,116=2.9, P=0.06), or on strategy score.
Pattern recognition
Chronic administration of MPH improved accuracy of responding on the pattern-recognition task (F 2,116=6.02, P<0.001) at both the 0.3-mg/kg (P<0.02) and 0.6-mg/kg (P<0.003) doses relative to placebo. There were no significant effects of MPH on latencies for correct or incorrect responding on the pattern-recognition task.
Discussion
Stimulant-naive boys with AD-HKD showed profound deficits in visual memory performance on a simultaneous and DMtS task and on a pattern-recognition task both known to be sensitive to temporal and amygdalo-hippocampal dysfunction, but on which patients with frontal lobe excisions show relatively intact performance (Owen et al. 1995). They also showed deficits on a SWM task known to be associated with activations of the dorsolateral and ventrolateral PFC and posterior parietal cortex, in functional neuroimaging studies, in children (Nelson et al. 2000). These performance deficits are not readily explained by existing neuropsychological models of AD-HKD. Acute MPH administration restored the deficit observed on the DMtS task but did not alter performance on the pattern-recognition or SWM tasks. Chronic MPH also improved, but did not normalise, performance on the DMtS task, improved performance on the pattern-recognition task but, again, did not alter performance on the SWM task. A facilitatory effect of MPH on inhibitory control does not explain the acute effects and can only offer a partial explanation for the chronic effects. There was evidence of a reduced effect of MPH on the DMtS task with chronic administration, perhaps reflecting the development of tolerance.
Limitations of the study
There are several limitations of the current study. The present sample comprises a group of children and young people meeting the rigorous ICD 10 criteria for HD. As such, these results may not be generalisable to those with DSM IV ADHD who fail to meet ICD 10 criteria. This may explain some of the differences between the current results and some previous studies and it will be important for future studies to include a range of subjects so that similarities and differences between the diagnostic systems can be fully explored. In order to include subjects representative of those referred to UK clinical services, we did not exclude subjects with co-morbid diagnoses. As expected, oppositional defiant disorder and conduct disorder were the most common co-morbidities. There is a debate in the literature as to whether or not these disorders are themselves associated with deficits in neuropsychological functioning (Pennington and Ozonoff 1996; Morgan and Lilienfeld 2000). Further studies are required to investigate the moderating effects of co-morbidity on baseline neuropsychological performance and the neuropsychopharmacological effects of MPH. Finally, intellectual functioning in the present study was measured using the BPVS, a standardised measure of verbal abilities, which in UK samples correlates highly with measures of general intelligence (Dunn et al. 1997), and which was used as a covariate in the baseline analyses to ensure that group differences were not a result of the lower verbal abilities of the AD-HKD subjects. Unfortunately measures of non-verbal or full-scale IQ were not available for these subjects and no comment can be made with respect to these aspects of functioning.
Neuropsychological performance of drug naive boys with AD-HKD
Our results support extension of the range of neuropsychological deficits ascribed to AD-HKD to include non-executive visual memory functioning. The performance deficits demonstrated on the SWM task were expected. Similar deficits have been demonstrated in previous studies (Nigg et al. 2002), two of which (Barnett et al. 2001; Kempton et al. 1999) used the same task as in the present study. However, unlike these two studies, the significant positive correlation between BSE and strategy scores, in both the AD-HKD and control groups, suggests that poor use of strategy may contribute to the poor task performance. These differences may be explained by the much larger sample size in the present study (n=15 Kempton et al. versus n=75 present study) and differences in the diagnostic status of the samples (DSM IV in Kempton et al. versus ICD 10 in present study).
With respect to the DMtS task, our results support, to an extent, those reported in previous studies. Kempton et al. (1999) used an identical task and reported delay-independent performance deficits in un-medicated ADHD subjects. Chelonis et al. (2002), using a different DMtS task, reported delay-dependent deficits in ADHD subjects withdrawn from stimulants for at least 18 h. Our data support and extend these findings to drug-naive subjects with AD-HKD. The finding in both the present study and that of Chelonis et al. (2002) of no deficit at a 0-s delay suggests that these recognition memory deficits result from difficulties in retention or recall rather than encoding or attending to information at presentation. The deficits observed on the pattern-recognition task in the present study were not predicted. Previous studies have reported no group differences on this (Kempton et al. 1999) and other recognition memory tasks (Douglas 1988). The differences between the present and previous studies may again be related to differences in sample size, rigor of diagnostic assessment, diagnostic classificatory system used and medication status of subjects.
Are these deficits in non-working recognition memory adequately explained by current theories of AD-HKD? Working memory deficits would not impact upon tasks with no requirement to manipulate information on line. Inhibition theories such as that described by Barkley (1997) would predict that AD-HKD-related performance deficits on these tasks should be associated with shorter response latencies, with “impulsive” responding pre-empting accurate solution of the discrimination. Hence, incorrect response latencies should be the shortest. No such association was found at the simultaneous condition of the DMtS. Whilst incorrect response latencies were shorter at the delay condition of the DMtS, this association was delay independent, making it unlikely that the reduced performance accuracy at longer delay intervals was attributable to impulsive responding. Furthermore, regression analysis demonstrated that, for AD-HKD boys, shorter response latencies made only a small contribution to the total variance at the 0-s delay condition and did not predict accuracy of responding at the 4-s or the 12-s delay conditions. Similarly, the shorter incorrect response latencies on the pattern-recognition task did not predict poor performance on this task.
Further, our data do not support the proposition that the performance deficits seen on the DMtS are due to classically defined “delay aversion” (Sonuga-Barke et al. 1992). The simultaneous and DMtS task imposes a range of fixed delays, presented in a pseudorandom order, such that, within each trial, subjects do not know whether the pattern to be remembered will disappear and, if so, for how long. The “delay aversion” hypothesis would predict that when children with AD-HKD have no control over the inter-trial delay and cannot respond in a manner that might reduce the subjective experience of delay, they would show no impairment of performance. Sonuga-Barke has recently argued that delay aversion can provide a motivational route into cognitive deficits in as much as it limits the opportunities to acquire the experience of working under delay conditions and so developing the necessary skills for effective performance (Sonuga-Barke 2002). It is not possible to conclusively discount this explanation from the current data, and further studies explicitly investigating the relationship between visual memory performance and delay aversion are required.
Our data also suggest that the conceptualisation of AD-HKD as a “frontal” disorder of monoaminergic neurocircuitry may be overly restrictive. Whilst performance on this SWM task has been shown to be associated with activations of the dorsolateral and ventrolateral PFC and posterior parietal cortex, both the pattern-recognition and DMtS tasks appear to have different neuroanatomical substrates. Performance on this version of the pattern-recognition task has been demonstrated to be sensitive to temporal and amygdalo-hippocampal damage, but not to frontal lobe damage (Owen et al. 1995). Animal data suggest that a comparable version of the DMtS task is also particularly sensitive temporal and amygdalo-hippocampal damage (Mishkin 1982; Bachevalier and Mishkin 1986). Further, patients with frontal, temporal and amygdalo-hippocampal excisions performed accurately on the simultaneous condition of the DMtS task, whilst temporal and amygdalo-hippocampal, but not frontal, patients were impaired when a delay was introduced (Owen et al. 1995). Similar patterns of delay-dependent impairment on this DMtS task have previously been described in patient groups with medial temporal lobe damage or disease notably senile dementia of Alzheimer’s type (SDAT) (Sahakian et al. 1988), elderly depressives (Abas et al. 1990) and healthy males exposed to the muscarinic antagonist scopolamine (Robbins et al. 1997). Other patient groups have shown delay-independent deficits on this task, for example, patients with Parkinson’s disease (Sahakian et al. 1988). Our data suggest a potential role for the temporal lobes, the amgydala and/or hippocampus in AD-HKD. This supports recent magnetic resonance imaging studies, one of which described reduced white and grey matter volumes in temporal, parietal and occipital areas in addition to frontal areas (Castellanos et al. 2002) and the other reported reduced brain volume in the anterior temporal lobe and increased grey matter in the posterior temporal lobe and inferior parietal lobe (Sowell et al. 2003). Further, the striking similarities between the delay-dependent DMtS deficits found in the AD-HKD group and those reported for patients with SDAT and healthy adult males following administration of scopolamine raise the possibility of altered cholinergic neurotransmission in children with AD-HKD. This is of interest given that both nicotinic agonists (Wilens et al. 1999) and donepezil, an acetylcholinesterase inhibitor which improves memory function in SDAT (Rogers et al. 1998), have been demonstrated to exert beneficial effects in AD-HKD (Wilens et al. 2000).
Intriguingly, in addition to demonstrating a striking delay-dependent deficit on the DMtS task, boys with AD-HKD were also impaired at the simultaneous condition. Such impairment has previously been reported in patients with Parkinson’s disease (Sahakian et al. 1988). Patients with frontal, temporal or amygdalo-hippocampal damage (Owen et al. 1995), SDAT (Sahakian et al. 1988), elderly depressives (Abas et al. 1990) and healthy males exposed to scopolamine (Robbins et al. 1997) did not show such impairment. A previous small study that reported delay-independent impairment on the DMtS task in children with ADHD found no impairment at the simultaneous condition (Kempton et al. 1999). The performance deficit in AD-HKD boys during the simultaneous matching component of the task did not account for poor performance during the delayed matching components. Hence, there may be two discrete deficits that can be identified. Much less is known about the mediating neural substrates of simultaneous matching components of this task. The Parkinson’s disease-related deficits in simultaneous matching and the chronic MPH amelioration of the AD-HKD-related deficit may point towards a dopaminergic substrate and frontostriatal circuitry. Further studies will be required to address these observations.
The effects of methylphenidate on neuropsychological performance
It is currently hypothesised that the pharmacological actions of MPH are mediated by its ability to inhibit the reuptake of dopamine and noradrenaline through blockade of the dopamine transporter (DAT). However, the precise effects of MPH in any particular brain region depends on the balance between tonic and phasic catecholamine release at baseline, the distribution of DATs and pre-synaptic autoreceptors within that region and the interaction between catecholaminergic neurotransmission and other neurotransmitter systems (Mehta et al. 2001). Our findings that MPH did not alter performance on the SWM task contrast strikingly with those of Kempton et al. (1999) and Mehta et al. (2000a,2000b). It is again possible that these differences are related to methodological differences. Both previous studies reported results on much smaller samples diagnosed using DSM IV criteria, and used less rigorous medication strategies than the present study; this non-replication of previous findings is important and raises the possibility of differential impacts of MPH between differently diagnosed samples on this important area of functioning. Interestingly, a recent re-analysis of the influential Multimodal Treatment of ADHD Study (MTA Cooperative Group 1999) has found that diagnostic status (ICD-10 HD versus DSM IV ADHD) is a moderator of treatment response (E. Taylor, personal communication). Also principle differences in monoamine metabolism between mild and severe forms of AD-HKD have been reported (Uzbekov and Misionzhnik 2003).
Whilst we have some understanding of how MPH may act on catecholamine systems in the prefrontal cortex and striatum (Volkow et al. 2001a), there has been limited study of the potential actions of MPH within other brain structures. Our results raise the possibility that the effects of MPH on aspects of visual memory function in AD-HKD may involve interaction between catecholaminergic and cholinergic neurotransmission. Whilst a single dose of MPH, at a dose of 0.6 mg/kg, did not affect performance on the pattern-recognition task, it restored performance accuracy on the DMtS delay conditions to the levels observed in controls. These observations support and extend the work of Chelonis et al. (2002) who also reported normalisation of DMtS performance following administration of stimulant medication. Chronic MPH treatment resulted in less pronounced effects than were observed after the acute challenge, with performance being improved but not normalised. This suggests the possibility that, at least with respect to this task, tolerance develops after chronic MPH administration. Whilst acute tolerance has been demonstrated with clinical doses of oral MPH (Swanson et al. 1999), the MPH literature to date has suggested that long-term tolerance does not occur in clinical cases (Greenhill et al. 2001). There are, however, some suggestions from the literature that long-term tolerance may occur. For example, increases in the mean daily MPH dose required to optimally control ADHD symptoms were reported over the 14 months of the Multimodal Treatment of ADHD study (Vitiello et al. 2001).
Unlike the study of Chelonis et al. (2002) enhanced performance on DMtS in the current study following acute 0.6 mg/kg MPH was not accompanied by significant changes in latencies to make correct responses. Therefore, enhanced accuracy of responding in the AD-HKD group in this condition was not a consequence of either increased deliberation time or reduced impulsivity. Chronic MPH treatment with either dose did not alter response latencies for the pattern-recognition task but did slow response latencies for correct choices on DMtS. This observation supports a presumed therapeutic mechanism of action whereby chronic MPH may enhance inhibitory control (Barkley 1997). However, positive correlations between response latencies and accuracy of responding were only observed for children taking the lower dose and only at the 4-s and 12-s delays. Linear regression analysis revealed that this predictive association was modest, suggesting that the therapeutic effects of MPH are, at best, only partially attributable to an enhancement of inhibitory control.
The dissociation with respect to the impact of MPH on the working memory and non-working memory tasks suggests that, at least in those children with the more refined ICD 10 HD phenotype, whilst treatment with MPH may result in significant improvement in behavioural symptoms and in some aspects of neuropsychological functioning, it does not normalise all aspects of functioning in all patients.
Conclusions
Our data highlights the heterogeneity of AD-HKD, challenges single-cause theories of AD-HKD and supports a multi-pathway model whereby AD-HKD is the phenotypic consequence of several endophenotypic risk factors (Castellanos and Tannock 2002). We propose that deficits in non-working visual memory may constitute a novel independent endophenotype for AD-HKD. In contrast to previous pathophysiological explanations of AD-HKD, this impairment is consistent with medial temporal lobe, but not frontal lobe dysfunction, and may implicate cholinergic neurotransmission. Further, we have demonstrated that, whilst none of the observed deficits resulted from previous exposure to stimulant medication, the deficit in DMtS performance was restored by acute administration of MPH and, on both DMtS and pattern recognition, was improved by chronic administration.
References
Abas MA, Sahakian BJ, Levy R (1990) Neuropsychological deficits and CT scan changes in elderly depressives. Psychol Med 20:507–520
August GJ (1987) Production deficiencies in free recall: a comparison of hyperactive, learning-disabled, and normal children. J Abnorm Child Psychol 15:429–440
Bachevalier J, Mishkin M (1986) Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res 20:249–261
Baddeley A (1986) Working memory. Oxford University Press, Oxford
Baddeley A (1996) The fractionation of working memory. Proc Natl Acad Sci U S A 93:13468–13472
Baddeley A (2003) Working memory and language: an overview. J Commun Disord 36:189–208
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94
Barnett R, Maruff P, Vance A, Luk ESL, Costin J, Wood C, Pantelis C (2001) Abnormal executive function in attention deficit hyperactivity disorder: the effect of stimulant medication and age on spatial working memory. Psychol Med 31:1107–1115
Benezra E, Douglas VI (1988) Short-term serial recall in ADDH, normal, and reading-disabled boys. J Abnorm Child Psychol 16:511–525
Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R (1992) Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 49:728–738
Borcherding B, Thompson K, Kruesi M, Bartko J, Rapoport JL, Weingartner H (1988) Automatic and effortful processing in attention deficit/hyperactivity disorder. J Abnorm Child Psychol 16:333–345
Castellanos FX, Tannock R (2002) Neuroscience of attention-deficit hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 3:617–628
Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL (2002) Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288:1740–1748
Chang HT, Klorman R, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM, Stuebing KK, Brumaghim JT, Shaywitz BA (1999) Paired-associate learning in attention-deficit/hyperactivity disorder as a function of hyperactivity–impulsivity and oppositional defiant disorder. J Abnorm Child Psychol 27:237–245
Chelonis JJ, Edwards MC, Schulz EG, Baldwin R, Blake DJ, Wenger A, Paule MG (2002) Stimulant medication improves recognition memory in children diagnosed with attention-deficit/hyperactivity disorder. Exp Clin Psychopharmacol 10:400–407
Conte R, Kinsbourne M, Swanson J, Zirk H, Samuels M (1986) Presentation rate effects on paired associate learning by attention deficit disordered children. Child Dev 57:681–687
Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA (2002) Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain 125:1646–1659
Daneman M, Carpenter PA (1980) Individual differences in working memory and reading. J Verbal Learn Verbal Behav 19:450–466
Douglas VI (1988) Cognitive deficits in children with attention deficit disorder with hyperactivity. In: Bloomingdale LM, Sergeant J (eds) Attention deficit disorder; criteria, cognition, intervention. Pergamon, Oxford, pp 65–81
DSM IV (1994) Diagnostic and statistical manual of mental disorders, 4th edn. Washington, DC
Dunn L, Dunn L, Whetton C, Burley J (1997) British Picture Vocabulary Scale, 2nd edn. NFER-Nelson, London
Fray PJ, Robbins TW (1996) CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol 18:499–504
Fuster JM (1989) The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe, 2nd edn. Raven Press, New York
Giedd JN, Blumenthal J, Molloy E, Castellanos FX (2001) Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci 931:33–49
Gleitman H, Fridlund A, Reisberg D (1999) Memory. Psychology. W.W. Norton, New York, pp 260–299
Greenhill LL, Perel JM, Rudolph G, Feldman B, Curran S, Puig-Antich J, Gardner R (2001) Correlations between motor persistence and plasma levels in methylphenidate-treated boys with ADHD. Int J Neuropsychopharmacol 4:207–215
Hughes C, Plumet MH, Leboyer M (1999) Towards a cognitive phenotype for autism: increased prevalence of executive dysfunction and superior spatial span amongst siblings of children with autism. J Child Psychol Psychiatry 40:705–718
ICD 10 (1992) The International Classification of Diseases, 10th revision edn. World Health Organisation, Geneva
Kaufman J, Birmaher B, Brent D, Rao U, Ryan N (1996) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL), Version 1.0. Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh (unpublished work)
Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C (1999) Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med 29:527–538
Loge DV, Staton RD, Beatty WW (1990) Performance of children with ADHD on tests sensitive to frontal lobe dysfunction. J Am Acad Child Adolesc Psychiatry 29:540–545
Luciana M, Nelson CA (1998) The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia 36:273–293
Mehta MA, Calloway P, Sahakian BJ (2000a) Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. J Psychopharmacol 14:299–302
Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW (2000b) Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20:RC65:1–6
Mehta MA, Sahakian BJ, Robbins TW (2001) Stimulant drugs and ADHD: basic and clinical neuroscience. In: Solanto MV, Arnsten AF, Castellanos FX (eds) Oxford University Press, New York, pp 303–331
Mishkin M (1982) A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci 298:83–95
Morgan AB, Lilienfeld SO (2000) A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev 20:113–136
MTA Cooperative Group (1999) A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry 56:1073–1086
Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ (2002) Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychol Med 32:39–53
Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL (2000) Functional neuroanatomy of spatial working memory in children. Dev Psychol 36:109–116
Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD (2002) Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry 41:59–66
NIMH (2000) National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry 39:182–193
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW (1995) Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33:1–24
Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW (1996) Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain 119:1597–1615
Pennington BF, Ozonoff S (1996) Executive functions and developmental psychopathology. J Child Psychol Psychiatry 37:57–87
Petrides M, Milner B (1982) Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 20:249–262
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P (1994) Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5:266–281
Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K (1997) Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology 134:95–106
Rogers SL, Doody RS, Mohs RC, Friedhoff LT (1998) Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 158:1021–1031
Rothenberger A, Banaschewski T, Heinrich H, Moll GH, Schmidt MH, van’t Klooster B (2000) Comorbidity in ADHD-children: effects of coexisting conduct disorder or tic disorder on event-related brain potentials in an auditory selective-attention task. Eur Arch Psychiatry Clin Neurosci 250:101–110
Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW (1988) A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 111(Pt 3):695–718
Schachar R, Tannock R (2002) Syndromes of hyperactivity and attention deficit. In: Rutter M, Taylor E (eds) 418. Blackwell, Oxford, pp 399
Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E (2001) The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol 29:215–228
Sonuga-Barke EJ (2002) Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res 130:29–36
Sonuga-Barke EJ, Taylor E, Sembi S, Smith J (1992) Hyperactivity and delay aversion. I. The effect of delay on choice. J Child Psychol Psychiatry 33:387–398
Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS (2003) Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet 362:1699–1707
Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S (1999) Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther 66:295–305
Todd RD (2000) Genetics of attention deficit/hyperactivity disorder: are we ready for molecular genetic studies? Am J Med Genet 96:241–243
Todd RD, Rasmussen ER, Neuman RJ, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Heath A (2001) Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. Am J Psychiatry 158:1891–1898
Uzbekov MG, Misionzhnik EY (2003) Changes in urinary monoamine excretion in hyperkinetic children. Hum Psychopharmacol 18:493–497
Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein OG, Elliott GR, Hechtman L, Jensen PS, Hinshaw SP, March JS, Newcorn JH, Swanson JM, Cantwell DP (2001) Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry 40:188–196
Voelker SL, Carter RA, Sprague DJ, Gdowski CL, Lachar D (1989) Developmental trends in memory and metamemory in children with attention deficit disorder. J Pediatr Psychol 14:75–88
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001a) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:RC121
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001b) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:RC121
Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D (1999) A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry 156:1931–1937
Wilens TE, Biederman J, Wong J, Spencer TJ, Prince JB (2000) Adjunctive donepezil in attention deficit hyperactivity disorder youth: case series. J Child Adolesc Psychopharmacol 10:217–222
Williams D, Stott CM, Goodyer IM, Sahakian BJ (2000) Specific language impairment with or without hyperactivity: neuropsychological evidence for frontostriatal dysfunction. Dev Med Child Neurol 42:368–375
Acknowledgements
This work was supported by a local trust through a TENOVUS-Scotland initiative. We thank M. Thrower, K. Antonson, A. Brown, J. Esperon, K. Walker and S. Trudu for assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhodes, S.M., Coghill, D.R. & Matthews, K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology 175, 319–330 (2004). https://doi.org/10.1007/s00213-004-1833-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1833-7