Abstract
Rationale
Most nicotine self-administration (NSA) studies in rats are performed under limited-access conditions. Few studies have examined the relationship between nicotine dependence and NSA.
Objectives
To determine how NSA access conditions affect NSA and the duration of nicotine dependence during abstinence, as reflected in somatic signs of withdrawal precipitated by administration of the nicotinic receptor antagonist mecamylamine.
Methods
The effects of different NSA access conditions (zero, 1 h/5 days, 1 h/7 days and 6 h/7 days per week) and non-contingent nicotine administration on NSA and somatic signs were examined.
Results
Daily NSA access (30 days) resulted in spontaneous and mecamylamine-induced somatic signs. Both daily access groups (1 h/day and 6 h/day, 7 days/week) exhibited spontaneous somatic signs on day 25 of NSA (17 h post-NSA) and sensitivity to mecamylamine up to 2 and 4 weeks of abstinence, respectively. In contrast, the 1 h/day, 5 days/week access group exhibited mecamylamine-induced somatic signs only up to 1 week of abstinence. NSA behavior was stable in rats with 1 h/day 5 days/week and 1 h/day 7 days/week access, but decreased from initially high rates in the 6 h/day 7 days/week access group, and decreased in rats receiving non-contingent nicotine. In contrast, extended cocaine self-administration access resulted in a gradual escalation in cocaine intake.
Conclusion
There was no escalation in nicotine intake with extended access conditions, unlike cocaine self-administration. Nevertheless, daily nicotine self-administration seven days per week, for either 1 or 6 h per day, was sufficient to induce long-lasting adaptations in nicotinic acetylcholine receptor activity reflected in spontaneous and antagonist-precipitated somatic signs of withdrawal, possibly reflecting aspects of nicotine dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine is self-administered intravenously by a broad range of species under a variety of conditions (Goldberg et al. 1981; Risner and Goldberg 1983; Donny et al. 1995; Shoaib et al. 1997; Picciotto et al. 1998; Paterson and Markou 2002). The majority of these studies have been carried out under limited-access conditions, usually 1 h/day, 5 days/week (e.g. Corrigall and Coen 1989). More recently, work has been conducted using 23-h access conditions (Valentine et al. 1997; Fu et al. 2001; Brower et al. 2002; LeSage et al. 2002; but see Cox et al. 1984) to mimic the access conditions of human smokers. Nevertheless, few studies compared nicotine intake across different access levels. In the present study, in addition to 1 h/day, 5 days/week commonly used in most self-administration studies, rats self-administered nicotine for 1 h/day, 7 days/week, and 6 h/day, 7 days/week. Rats allowed to self-administer cocaine for 6 h daily exhibit significant escalations in cocaine intake compared to rats allowed 1 h of daily access (Ahmed and Koob 1998, 1999). These increases in cocaine intake are postulated to reflect the development of cocaine dependence. A similar study showed that heroin self-administration also escalated under extended access conditions (Ahmed et al. 2000). Nevertheless, it is not known whether such an escalation also occurs with nicotine self-administration, and whether nicotine dependence develops when nicotine is self-administered. Thus, the main aim of the present study was to compare the effects of different access conditions on nicotine self-administration behavior, and the induction and maintenance of nicotine dependence in rats.
The present study examined spontaneous and mecamylamine-induced somatic signs of nicotine withdrawal, as an index of adaptations in the activity of the nicotinic cholinergic system and aspects of nicotine dependence, in rats allowed different nicotine self-administration access. Cessation of chronic non-contingent nicotine administration or administration of a nicotinic receptor antagonist, such as mecamylamine, to rats or mice chronically exposed to non-contingent nicotine (e.g. Hildebrand et al. 1997; Epping-Jordan et al. 1998; Carboni et al. 2000; Watkins et al. 2000; Malin 2001; Markou and Paterson 2001; Isola et al. 2002; however, see Semenova et al. 2003) resulted in the emergence of a withdrawal syndrome characterized by somatic signs such as eye-blinks, body-shakes, gasps and abdominal writhes (i.e. “somatic signs of nicotine withdrawal”). Similar signs have been reported in human smokers (Hughes et al. 1991; American Psychiatric Association 1994). Thus, the appearance of somatic signs of nicotine withdrawal in rats may reflect aspects of a nicotine-dependence state analogous to that observed in human smokers. Further, symptoms of nicotine withdrawal are associated with strong urges to smoke during cessation attempts (Doherty et al. 1995). The affective and somatic signs of nicotine withdrawal provide measures of different components of the nicotine withdrawal syndrome (Epping-Jordan et al. 1998; Watkins et al. 2000; Harrison et al. 2001; Semenova and Markou 2003). Cessation of non-contingent nicotine administration reliably produces both affective and somatic signs of nicotine withdrawal. Therefore, the somatic signs of withdrawal are a reliable index of nicotine dependence, although their presence may not necessarily indicate the presence of affective symptoms. The present study also examined how nicotine dependence induced via chronic subcutaneous non-contingent nicotine affected nicotine self-administration under limited access conditions during both nicotine exposure and after termination of non-contingent nicotine administration. Finally, during an extended period of abstinence from nicotine self-administration, subjects were tested for the continued presence of mecamylamine-induced somatic signs of nicotine withdrawal.
In addition, the effects of mecamylamine pretreatment on nicotine self-administration were determined to test the hypothesis that nicotine-dependent rats would differ from non-dependent rats in their response to mecamylamine. Nicotine-dependent humans show compensatory increases after mecamylamine administration (Stolerman et al. 1973; Nemeth-Coslett et al. 1986), while non-nicotine-dependent rats show decreases (Martin et al. 1990; Donny et al. 1995; Watkins et al. 1999).
In summary, the present study set out to determine the effects of different nicotine self-administration access conditions, and the effects of prolonged abstinence from nicotine self-administration, on nicotine dependence as indicated by somatic signs of nicotine withdrawal. Further, the effects of nicotine dependence induced via extended access to nicotine self-administration or chronic non-contingent nicotine infusion on nicotine self-administration behavior were assessed. Finally, the effects of extended access to cocaine self-administration under the same conditions were investigated as a positive control.
Materials and methods
Subjects
Male Wistar rats (Charles River, Raleigh, N.C., USA) weighing 300–350 g upon arrival in the laboratory were group housed (two per cage) in a temperature- and humidity-controlled vivarium on a 12-h reverse light-dark cycle with unrestricted access to water except during testing. Rats were maintained on either 12 or 20 g food/day (see below). All behavioral testing occurred during the dark phase of the light-dark cycle. Subjects were treated in accordance with the National Institutes of Health guidelines on animal care (National Institutes of Health 1996) and the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Drugs
(−)Nicotine hydrogen tartrate and mecamylamine were purchased from Sigma (St Louis, Mo., USA), and cocaine HCl was obtained from the National Institute on Drug Abuse (Washington D.C., USA). Nicotine (doses expressed as free base) was dissolved in saline, the pH was adjusted to 7.0 (±0.5) with sodium hydroxide, and the solution was sterilized via filtration. Mecamylamine and cocaine were dissolved in saline; doses are expressed as salt.
Apparatus
Intravenous nicotine self-administration and food-maintained responding took place in 12 Plexiglas experimental chambers (25×31×24 cm; Med Associates, St Albans, Vt., USA) each housed in a sound-attenuated box (San Diego Instruments, San Diego, Calif., USA) described previously (Markou and Paterson 2001).
Intravenous self-administration
Food training
Approximately 1 week after arrival in the laboratory, rats were food-restricted (5 g/day) for 48 h. During food training, animals received 12 g rat chow per day at least 1 h after the end of the food training session. Training schedules progressed from fixed ratio 1 time out 1 (FR1 TO1), FR1 TO10, FR2 TO20 to FR5 TO20 s, with sessions lasting approximately 30 min. After successful acquisition of food-maintained responding, rats were allowed free access to food until nicotine/cocaine self-administration was initiated.
Intravenous catheterization surgery
Rats were anesthetized with an isoflurane/oxygen mixture (1–3% isoflurane) and prepared with a catheter inserted into the right jugular vein. Catheters were constructed as previously described (Markou and Paterson 2001). Animals were given 7 days to recover from surgery, during which time they received the antibiotic ticarcillin clavulanate (20 mg/day). Catheter patency was maintained by infusing 0.2 ml heparinized saline (33.3 IU/ml) daily.
Acquisition of nicotine and cocaine self-administration
After catheter implantation and recovery, rats were allowed to respond for food for one session prior to initiating nicotine/cocaine self-administration (0.03 mg/kg per infusion of nicotine; 0.25 mg per infusion of cocaine). Rats received 20 g rat chow per day, at least 1 h after termination of the self-administration sessions. Responding on the active lever (previously paired with delivery of a food pellet) resulted in the delivery of the nicotine/cocaine solution in a volume of 0.1 ml over a 1-s (nicotine) or 4-s (cocaine) period (Razel syringe pump model A; Razel Scientific Instruments Inc., Stamford, Conn., USA). Different infusion rates were used for nicotine and cocaine to be consistent with methods used in the majority of previous self-administration studies with nicotine (Paterson and Markou 2002) and cocaine (e.g. Ahmed and Koob 1998). Responding on the inactive lever (introduced during the first self-administration session) had no consequence. The initiation of the drug infusion was paired with a cue light, which remained illuminated throughout the 20-s time-out period, during which responding was recorded but not reinforced. Rats were considered to have acquired stable drug self-administration when they pressed the active lever more than twice the number of times they pressed the inactive lever, and received a minimum of six infusions per 1-h session with less than 20% variation in the number of infusions earned per session.
Somatic signs of nicotine withdrawal
Somatic signs of nicotine withdrawal were observed under white light conditions in cylindrical Plexiglas chambers (diameter 15 cm) with sawdust bedding on the floor, in a different room from the self-administration room. Each subject was observed for 10 min by one of two observers blind to the subjects’ treatments, using a standardized scoring sheet modified from the somatic signs of opiate withdrawal scoring sheet (Malin et al. 1992). Initially, one observer instructed the other in the identification of somatic signs to ensure standardization of behavioral scoring; subsequently, both observers counted signs in control and nicotine-withdrawal subjects not used in the present experiment: there was more than 90% agreement in scoring. The following somatic signs were recorded: body shakes, chews, cheek tremors, escape attempts, eye-blinks, foot licks, gasps, writhes, genital licks, head shakes, ptosis, teeth chattering and yawns. Ptosis, if present, was recorded only once per minute.
Experiment 1: effects of different access conditions on nicotine self-administration and somatic signs of nicotine withdrawal
After successful acquisition of lever-pressing for food and recovery from catheter implantation, rats were assigned to five groups: zero access (n=6); 1 h/day, 5 days/week (n=6); 1 h/day 7 days/week (n=5); 6 h/day, 7 days/week (n=11). The 6 h/day 7 days/week group comprised of two sets of animals: one set (n=6) initially acquired self-administration over 8 days of 1-h sessions daily, and the second set (n=5) was immediately allowed to self-administer nicotine for 6 h/day, 7 days/week. The second set of animals was included to determine whether previous experience at a limited access level had affected subsequent self-administration under prolonged access conditions. The zero access control group was included to assess the effects of repeated mecamylamine (1 mg/kg) injections on somatic signs in the absence of nicotine exposure; this group was not allowed access to the experimental chambers after acquisition of food-maintained responding and preparation with catheters. Different access groups were counter-balanced for initial rates of nicotine self-administration and body weight. Rats were allowed a total of 30 days of access to nicotine self-administration prior to any additional experimental manipulations.
Prior to any access to nicotine self-administration, all animals (n=27) underwent a period of somatic signs observation to determine baseline numbers of signs. Animals were habituated to the somatic signs observation chambers on two occasions prior to the first observation session. Animals were observed for spontaneous somatic signs on day 25 of nicotine self-administration, 17 h after the termination of the previous session. Six days later, on day 31 of self-administration (17 h after termination of the previous session) all animals underwent observation for mecamylamine-induced somatic signs (1 mg/kg SC; 10 min pretreatment).
After completion of 31 days of nicotine self-administration, the effects of mecamylamine treatment on nicotine self-administration were determined. Mecamylamine (0, 0.5, 1.0 and 2.0 mg/kg SC; 15 min pretreatment) was administered according to a within-subjects Latin square design. Each mecamylamine dose was administered only after subjects had demonstrated a stable 2-day baseline (i.e. less than 15% variation in the number of infusions earned). Animals were assigned to two groups: the 1 h/day and 6 h/day, 7 days/week nicotine self-administration access groups (“nicotine dependent”, n=15), and the 1 h/day, 5 days/week access group (“non-nicotine-dependent”, n=5; see results below for definition of groups). One rat from the “nicotine dependent” group died due to infection prior to completion of the Latin square design. The zero access group received identical mecamylamine doses, in a random order, in their home cages.
After completion of the mecamylamine Latin square design (conducted over 16 sessions; all subjects were allowed a total of 46 sessions during the self-administration phase of the experiment), animals were subjected to enforced abstinence from nicotine (i.e. remained in their home cages). Mecamylamine-induced (1 mg/kg SC; 10 min pretreatment) somatic signs were observed on days 7, 14, 28 and 42 of abstinence. On day 10 of abstinence, saline was administered to assess potential conditioning effects resulting from repeated mecamylamine-induced observation sessions.
Experiment 2: effects of different access conditions on cocaine self-administration
After acquisition of lever-pressing for food and recovery from catheter implantation, two additional groups of drug-naive rats were formed, counter-balanced for body weights and initial rates of cocaine self-administration (determined during an 8-day baseline period of 1 h access per day). Then, the groups were allowed to self-administer cocaine for either 1 h (n=6) or 6 h (n=6) daily for 14 days (procedure adapted from Ahmed and Koob 1999; Ahmed et al. 2002).
Experiment 3: effect of continuous non-contingent nicotine administration on intravenous nicotine self-administration
After acquiring stable nicotine self-administration (0.03 mg/kg per infusion) under the FR5 TO20 s schedule during daily (7 days/week) 1 h sessions, additional rats were anesthetized with an isoflurane/oxygen mixture (1–3% isoflurane) and prepared with subcutaneous osmotic minipumps (Alzet model 2ML1) delivering either 0.0 (n=8), 0.25 (n=7) or 3.16 (n=8) mg/kg per day nicotine. Pumps were implanted subcutaneously on the flank of the animal, parallel to the spine with the flow moderator directed posteriorly, immediately after the self-administration session to allow maximum recovery time (approximately 22 h) before the next session. The wound was closed with surgical clips and a topical iodine preparation was applied. Groups were counter-balanced for baseline rates of nicotine self-administration and body weights. Rats self-administered nicotine daily for the next 14 days, and pumps were removed on day 7. One nicotine dose (3.16 mg/kg per day) administered was chosen because it results in nicotine dependence and withdrawal upon cessation of infusion (Skjei and Markou 2003; for review, see Malin 2001), thereby allowing the investigation of how nicotine dependence induced by non-contingent nicotine might affect self-administration behavior. The second nicotine dose (0.25 mg/kg per day) was selected because it results in a greater increase in acute nicotine-induced extracellular dopamine levels in the nucleus accumbens compared to saline infusion (Benwell et al. 1995).
Data analyses
Data were analyzed using appropriate one- and two-way mixed-design ANOVAs, with factors defined as within- or between-subjects according to the experimental design. All data were expressed as the number of drug infusions (active lever data), the number of lever presses (inactive lever data), or the total number of somatic signs observed. Analyses were followed with Newman-Keuls post-hoc comparison tests where appropriate (Winer 1971). The level of significance was set at 0.05.
Results
Experiment 1: effects of different access conditions on nicotine self-administration and somatic signs of nicotine withdrawal
Data from the two 6-h access conditions were analyzed using a two-way ANOVA that revealed no significant effect of initial access on nicotine self-administration or somatic signs. Therefore, the data from these two groups were combined for all subsequent analyses.
Self-administration data
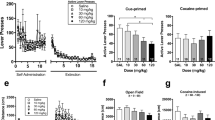
Nicotine self-administration during the entire session differed significantly as a function of access condition across days [Access×Days: F(58,522)=6.98, P<0.001; Access: F(2,58)=48.92, P<0.001; Days: F(29, 522)=4.74, P<0.001]. Pre-planned individual comparisons indicated that nicotine self-administration was significantly decreased in the 6 h/day, 7 days/week group from day 3 onwards compared to day 1 (Fig. 1A). Analysis of the data from the first 60 min of each session indicated that there were significant differences in self-administration behavior between the different access groups [Access: F(2,58)=22.64, P<0.01], and that self-administration behavior changed across days [F(29,522)=5.79, P<0.001]. Nevertheless, there was no significant Access×Days interaction effect. Pre-planned individual comparisons indicated that nicotine self-administration was significantly lower in the 6 h/day, 7 days/week group compared to the 1 h/day, 5 days/week group, on days 13 through 20, and from day 24 onwards, and significantly lower in the 1 h/day, 7 days/week group compared to the 1 h/day, 5 days/week group, on days 14 and 18 (Fig. 1B). Analysis of inactive lever data indicated no significant main or interaction effects (data not shown). One-way ANOVA performed on daily nicotine consumption [expressed as the mean daily intake over the last 3 days (days 28–30)] indicated significantly higher total intake in the 6 h/day, 7 days/week group compared to the other two treatment groups [Access: F(2,17)=27.12, P<0.0001]. The two 1-h access groups were not significantly different from each other (see Table 1).
Effects of different access conditions on nicotine self-administration. The graphs show the number of infusions (mean±SEM) earned for each group during 30 days of nicotine self-administration, during the entire session (A), and the first 60 min of the session (B). Open symbols indicate nicotine self-administration (mean±SEM) during the first 8 days of limited access (1 h/day). Asterisks (*P<0.05) indicate significant differences from day 1 within groups in A, and significant differences between groups in B
Somatic signs during nicotine self-administration
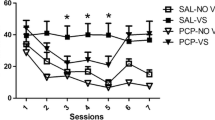
The different access groups differed in the number of somatic signs across time [Access×Time interaction: F(6,42)=6.36, P<0.01; Time: F(2,42)=23.25, P<0.01 and Access: F(3,21)=9.35, P<0.01]. Post-hoc comparisons indicated that spontaneous and mecamylamine-induced somatic signs were significantly increased in both the 1 h/day, 7 days/week and 6 h/day, 7 days/week groups compared with pre-nicotine baseline levels, and with the number of signs observed in the zero and 1 h/day, 5 days/week access groups. Further, both the 1 h/day, 7 days/week and 6 h/day, 7 days/week groups exhibited significantly increased numbers of signs between spontaneous and mecamylamine-induced observation sessions (Fig. 2A).
The effects of different nicotine self-administration access conditions on somatic signs. A Total number of somatic signs observed before and during 31 days of nicotine self-administration. B Total number of mecamylamine-induced somatic signs observed during 42 days of abstinence from nicotine self-administration. Asterisks (*P<0.05, **P<0.01) indicate significant differences from the pre-nicotine (A) or day 42 (B) observation sessions, within each group. Hache signs (#P<0.05) indicate significant differences between different nicotine self-administration access groups compared to the zero access group
Somatic signs during abstinence from nicotine self-administration
Groups differed in the persistence of mecamylamine-induced somatic signs during the abstinence phase [Access×Days: F(9,63)=9.36, P<0.01; Access: F(3,21)=4.99, P<0.01; Days: F(3,63)=19.43, P<0.01]. Post-hoc comparisons showed that the total numbers of signs were significantly elevated in the 1 h/day, 7 days/week and 6 h/day, 7 days/week groups on days 7 and 14 of abstinence. Only the 6 h/day, 7 days/week group exhibited increased number of signs on day 28 of abstinence. The 1 h/day, 5 days/week group showed a significantly increased number of signs only on day 7 of abstinence. The zero access group did not show any increases in the numbers of signs at any time-point (Fig. 2B). The saline challenge on day 10 of abstinence did not induce somatic signs: zero access: 5.8±0.74; 1 h/day, 5 days/week: 7.4±0.28; 1 h/day, 7 days/week: 9.6±0.76; 6 h/day, 7 days/week: 7.2±1.13. Comparison of the somatic signs data obtained on the pre-nicotine observation day, the saline challenge on day 10 of abstinence, and the mecamylamine challenge on day 42 of abstinence indicated no significant main or interaction effects.
Effects of mecamylamine on nicotine self-administration
Based on the previously demonstrated appearance of somatic signs of nicotine withdrawal in all groups except the 1 h/day, 5 days/week group during the first 31 days of nicotine self-administration, the self-administration data obtained after treatment with mecamylamine was collapsed into two groups: a “dependent” group (n=15; comprising the 6 h/day, 7 days/week and 1 h/day, 7 days/week groups) and a “non-dependent” group (n=5; comprising the 1 h/day, 5 days/week group). A two-way ANOVA indicated that mecamylamine decreased nicotine self-administration [F(3,54)=10.86, P<0.01] in both the dependent and non-dependent groups, as indicated by the lack of a significant main effect of Access or a significant interaction effect. Analysis of inactive lever data indicated no significant main or interaction effects (data not shown). Analysis of all pre-injection 2-day baselines indicated no significant effect of repeated testing on baseline levels of nicotine self-administration (data not shown).
Experiment 2: effects of different access conditions on cocaine self-administration
Cocaine self-administration during the entire session increased significantly in the 6 h/day, 7 days/week access group across days, but remained stable in the 1 h/day, 7 days/week access group [Access×Days: F(13,130)=4.95, P<0.05; Access: F(1,10)=386.4, P<0.001; Days: F(13,130)=4.11, P<0.05]. Post-hoc comparisons indicated that cocaine self-administration was significantly increased in the 6 h/day, 7 days/week group from day 2 onwards (Fig. 3A).
The effects of different access conditions on cocaine self-administration. The graphs show the number of infusions (mean±SEM) earned during 14 days of cocaine self-administration, during the entire session (A), and the first 60 min of the session (B). Open symbols indicate nicotine self-administration (mean±SEM) during the first 8 days of limited access (1 h/day). Asterisks (*P<0.05, **P<0.01) indicates significant differences from day 1 within each group. Hache sign (#P<0.05) indicates significant differences between groups
Analysis of the data from the first 60 min of each session indicated that cocaine intake increased significantly in the 6 h/day, 7 days/week access group compared to the 1 h/day, 7 days/week access group [Access×Days: [F(13,130)=3.75, P<0.05]. There were no significant main effects of Access or Days. Post-hoc comparisons indicated that cocaine self-administration was significantly higher in the 6 h/day, 7 days/week group compared to the 1 h/day, 7 days/week group on day 14. Compared to first hour intake on day 1, cocaine intake was significantly increased in the 6 h/day, 7 days/week group on days 2, 3 and 5, and from day 7 onwards (Fig. 3B). Analysis of inactive lever data indicated no significant main or interaction effects (data not shown).
Experiment 3: effect of continuous non-contingent nicotine administration on intravenous nicotine self-administration
Continuous nicotine delivery (3.16 mg/kg per day) via minipumps decreased nicotine self-administration [Pump: F(2,20)=5.42, P<0.05; days: F(14,280)=7.83, P<0.01]. There was a strong trend towards a significant Pump×Days interaction effect [F(28,280)=1.61, P=0.05]. Post-hoc comparisons indicated that nicotine self-administration was significantly decreased on days 1, 5, 6 and 7 of pump treatment, and also 1 day after pump removal in the group receiving 3.16 mg/kg nicotine per day, compared to nicotine self-administration prior to pump implantation (Fig. 4). Continuous infusion of 0.25 mg/kg per day did not affect nicotine self-administration.
Effect of continuous non-contingent nicotine administration via minipumps on intravenous nicotine self-administration. The graph shows the number of infusions (mean±SEM) earned during 14 days of nicotine self-administration. Open symbols indicate nicotine self-administration (mean±SEM) prior to pump implantation. Arrows indicate pump implantation and removal. Asterisks (*P<0.05, **P<0.01) indicate significant differences from baseline nicotine self-administration. Hache signs (#P<0.05) indicate significant differences between the nicotine (3.16 mg/kg per day) treatment group and the other two treatment groups
Discussion
Extended access to nicotine self-administration did not result in increased self-administration, in contrast to cocaine self-administration, but did result in the development of nicotine dependence as indicated by an increased number of somatic signs of nicotine withdrawal. Observation of somatic signs of withdrawal at 17 h after the previous session, on day 25, reflected spontaneous nicotine withdrawal immediately prior to the next self-administration session. Further, mecamylamine administration induced somatic signs of nicotine withdrawal for a maximum of 4 weeks after self-administration ceased. The results also indicated that mecamylamine treatment decreased nicotine self-administration in all subjects, consistent with previous studies (Martin et al. 1990; Donny et al. 1995; Watkins et al. 1999). Finally, nicotine self-administration under limited access conditions was decreased by non-contingent nicotine (3.16 mg/kg per day only) delivery via osmotic minipumps, while nicotine withdrawal induced by minipump removal may have decreased nicotine self-administration for 1 day.
Extended access to nicotine self-administration for 6 h/day, daily for 30 days, did not result in increased nicotine self-administration, unlike the effects of extended access to cocaine self-administration (present study; Ahmed and Koob 1998, 1999; Ahmed et al. 2002). Rats allowed to self-administer nicotine for 1 h/day, 5 days/week showed stable levels of responding throughout the 30-day period, as did rats allowed access for 1 h/day, 7 days/week. Animals allowed the greatest level of nicotine self-administration access (6 h/day, 7 days/week) significantly decreased their nicotine intake during the first hour of the session from initial levels of nicotine intake, and exhibited significantly lower nicotine intake over the first hour compared to rats responding for 1 h/day, 5 days/week. Perhaps the differences in pharmacological modes of action of cocaine (monoamine reuptake inhibition) versus nicotine (receptor agonist) underlie the observed differences between cocaine and nicotine self-administration in the present study. Specifically, the rapid desensitization of central nicotinic acetylcholine receptors (nAChRs) in response to self-administered nicotine in both humans and rats (Dani and Heinneman 1996; Buisson and Bertrand 2001) may limit the effects of increases in nicotine self-administration rates, while the effects of cocaine can be augmented by continued increases in self-administration rates. Interestingly, nicotine addicts remain at stable levels of drug intake for decades (Stolerman and Jarvis 1995), although they usually exhibit a progressive increase in consumption during the “acquisition” phase of the habit (McNeill et al. 1989). A parallel increase during acquisition was also observed in studies utilizing 23-h daily access in rats with no prior operant training (Valentine et al. 1997; Fu et al. 2001). In the present study, rats were trained to respond for food prior to nicotine self-administration. Thus, in the present study, the progression from initially high rates to stable, much lower levels of daily intake probably reflects the transition from food-maintained to nicotine-maintained responding. Regardless of previous experience, both humans (McNeill et al. 1989) and rats (Valentine et al. 1997; Fu et al. 2001) eventually display stable daily intakes. In contrast to nicotine addicts, cocaine addicts tend to exhibit uncontrolled and excessive drug use (American Psychiatric Association 1994), similar to the escalating cocaine self-administration observed in rats here.
Despite the lack of an increase in nicotine intake, nicotine dependence as reflected by somatic signs of nicotine withdrawal (Malin 2001) appeared and persisted for different periods of time during abstinence depending on previous nicotine self-administration access conditions. Somatic signs provide a measure of “physical” dependence, and are both centrally and peripherally mediated (Watkins et al. 2000). Work by Malin (2001) and our laboratory (Epping-Jordan et al. 1998; Watkins et al. 2000; Harrison et al. 2001; Cryan et al. 2003; Semenova and Markou 2003; Skjei and Markou 2003) indicated the validity of somatic signs as a model of nicotine dependence, including sensitivity to clinically effective therapeutic interventions. Interestingly, both groups of animals with access for 7 days/week showed similar levels of somatic signs during the self-administration phase of the experiment despite one group having 1 h daily access and a nicotine intake of 0.36±0.08 mg/kg per day and the other group having 6 h daily access and a nicotine intake of 0.88±0.19 mg/kg per day. In contrast, the 1 h/day, 5 days/week group did not exhibit somatic signs during the first 31 days of nicotine self-administration. The only difference between the two 1-h groups (5 days/week versus 7 days/week) was 2 days abstinence per week, since both groups displayed similar daily nicotine consumption (0.4±0.12 mg/kg per day in the 5 days/week group). This 2-day period was sufficient to substantially delay the appearance, and limit the persistence, of somatic signs, presumably due to some degree of reversal of nicotine-induced changes in nAChR number or function during the 2-day abstinence period. A previous study (Skjei and Markou 2003) indicated that the duration of nicotine withdrawal (somatic signs) induced by cessation of non-contingent nicotine administration increased as the duration of nicotine exposure increased, but that the dose of nicotine had only a small effect. Consistent with the present data and the Skjei and Markou (2003) study, nicotine consumption levels in human smokers are not directly related to the severity of withdrawal (Hughes et al. 1990).
The only observed difference between the two daily access groups (1 h/day versus 6 h/day) was the continued presence of mecamylamine-induced somatic signs of nicotine withdrawal 4 weeks after the initiation of abstinence in the 6-h group compared to 2 weeks in the 1-h group. Although the 5 days/week group did not show somatic signs during the nicotine self-administration period, they did exhibit mecamylamine-induced signs at 1 week of abstinence. The appearance of signs at this time-point may be due to progressive neurobiological changes, or mecamylamine challenge may reveal nicotine-induced neurobiological adaptations that are not evident under baseline conditions. The observed non-significant trend toward increased signs after mecamylamine challenge in the zero access group could be due to upregulation of tonically activated nAChRs attributable to repeated mecamylamine administration (Collins et al. 1994).
Up-regulation of nAChRs occurs in rats that receive non-contingent (Marks et al. 1983) or contingent nicotine (Donny et al. 2000), and in human smokers (Benwell et al. 1988). There is a complex relationship between non-contingent nicotine dose and frequency of administration, and up-regulation of nAChRs in rats (Rowell and Li 1997). It is possible that the 6 h/day group in the present study would have exhibited the greatest degree of nAChR up-regulation. Although the persistence of nicotine-induced nAChR up-regulation during nicotine abstinence is not known, the continued sensitivity to mecamylamine during abstinence in the present study may be related to the degree of previous nAChR up-regulation.
The observed differences between the different access groups in the development of somatic signs of nicotine withdrawal may have implications for nicotine self-administration studies. Because most nicotine self-administration studies are conducted using 1 h/day, 5 days/week access (e.g. Corrigall and Coen 1989; Donny et al. 1995; Watkins et al. 1999; Markou and Paterson 2001; Paterson and Markou 2002), the results from those studies are perhaps best interpreted in terms of the neurobiological mechanisms underlying the positive rewarding effects of acute nicotine. In contrast, studies utilizing extended nicotine self-administration access levels (present study; Cox et al. 1984; Valentine et al. 1997; Fu et al. 2001; Brower et al. 2002; LeSage et al. 2002) are perhaps more valuable in predicting the effects of potential pharmacotherapies in the human population because the experimental subjects exhibit nicotine withdrawal (present study), and the access conditions more closely mimic those of human smokers. Further, several models of nicotine dependence currently used are based on non-contingent nicotine infusions (e.g. Epping-Jordan et al. 1998; Malin 2001), although it was shown previously that non-contingent and contingent nicotine differ in their effects (Donny et al. 2000). Thus, the use of extended access procedures offers the opportunity to track the development of nicotine dependence and the underlying neurobiological changes via contingent rather than non-contingent nicotine delivery.
In contrast to the observed differences in spontaneous and mecamylamine-induced somatic signs of nicotine withdrawal in the different nicotine self-administration access groups, the groups did not differ in the effect of mecamylamine pretreatment on nicotine self-administration. Mecamylamine decreased nicotine self-administration in all groups (i.e. there was no compensatory increase in nicotine self-administration in “dependent” rats). This effect may have occurred because of the use of time-out periods in the self-administration sessions, or because the “non-dependent” rats were slowly developing sensitivity to mecamylamine-induced somatic signs during the period of testing. It is also possible that longer exposure to nicotine (i.e. increased number of hours per day and/or total number of days) may have resulted in differential effects of mecamylamine in the two groups. Finally, the mecamylamine doses employed for pretreatment may have been too high, thereby inducing severe withdrawal and reduced motor function. In conclusion, under the present conditions, the state of nicotine dependence in different groups of rats did not affect the subject’s self-administration behavior after mecamylamine pretreatment.
Continuous non-contingent nicotine (3.16 mg/kg per day) delivery via osmotic minipumps decreased nicotine self-administration rates, while delivery of a lower nicotine dose (0.25 mg/kg per day) had no effect. Nicotine withdrawal induced by removal of the minipumps may have resulted in the 50% decrease in responding for 1 day compared to pre-pump baseline and the control group. Nonetheless, this decrease may be attributable to the effects of surgery during the preceding 24 h. Three days after pump removal, corresponding to the recovery of brain reward function after cessation of chronic nicotine treatment (Harrison et al. 2001; Semenova and Markou 2003; Skjei and Markou 2003), nicotine self-administration behavior returned to pre-pump and control group levels. Similarly, non-contingent nicotine infusion decreased nicotine self-administration rates to approximately 50% of baseline under 23-h daily access conditions (LeSage et al. 2002). These data sets are similar to human data, where nicotine patches tend to decrease but not totally abolish cigarette smoking (Benowitz et al. 1998).
In summary, the results of the present study indicate the importance of nicotine self-administration access conditions in determining the development and persistence of nicotine dependence, as indicated by somatic signs of nicotine withdrawal during nicotine self-administration and abstinence. The present results suggest that extended access to nicotine self-administration may be useful in delineating and characterizing the neurobiological changes underlying the development of nicotine dependence via contingent nicotine administration. Daily nicotine self-administration 7 days per week, for either 1 or 6 h per day, is sufficient to induce nicotine dependence, as measured in the present study with both spontaneous and mecamylamine-precipitated somatic signs of nicotine withdrawal, that is not correlated with an escalation in nicotine intake.
References
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Ahmed SH, Koob GF (1999) Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology 146:303–312
Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421
Ahmed SH, Kenny PJ, Koob GF, Markou A (2002) Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature Neurosci 5:625–626
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Press, Washington D.C.
Benowitz NL, Zevin S, Jacob P 3rd (1998) Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther 287:958–962
Benwell MEM, Balfour DJK, Anderson JM (1988) Evidence that tobacco smoking increases the density of (−)-[3H]-nicotine binding sites in human brain. J Neurochem 50:1243–1247
Benwell MEM, Balfour DJK, Birrell CE (1995) Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol 114:454–460
Brower VG, Fu Y, Matta SG, Sharp BM (2002) Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res 930:12–20
Buisson B, Bertrand D (2001) Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci 21:1819–1829
Carboni E, Bortone L, Giua C, Di Chiara G (2000) Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend 58:93–102
Collins AC, Luo Y, Selvaag S, Marks MJ (1994) Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther 271:125–133
Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited access schedule. Psychopharmacology 99:473–478
Cox BM, Goldstein A, Nelson WT (1984) Nicotine self-administration in rats. Br J Pharmacol 83:49–55
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168:347–358
Dani JA, Heinemann S (1996) Molecular and cellular aspects of nicotine abuse. Neuron 16:905–908
Doherty K, Kinnunen T, Militello FS, Garvey AJ (1995) Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology 119:171–178
Donny EC, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology 122:390–394
Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF (2000) Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol 402:231–240
Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79
Fu Y, Matta SG, Brower VG, Sharp BM (2001) Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: an in vivo microdialysis study. J Neurosci 21:8979–8989
Goldberg SR, Spealman RD, Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214:573–575
Harrison AA, Liem Y, Markou A (2001) Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology 25:55–71
Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology 129:348–356
Hughes JR, Gust SW, Keenan RM, Fenwick JW (1990) Effect of dose on nicotine’s reinforcing, withdrawal-suppression and self-reported effects. J Pharmacol Exp Ther 252:1175–1183
Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW (1991) Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry 48:52–59
Isola R, Zhang H, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M (2002) Met-enkephalin and preproenkephalin mRNA changes in the striatum of the nicotine abstinence mouse. Neurosci Lett 325:67–71
LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR (2002) Continuous nicotine infusion reduces nicotine self-administration in rats with 23 h/day access to nicotine. Pharmacol Biochem Behav 72:279–289
Malin DH (2001) Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav 70:551–559
Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB (1992) Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav 43:777–784
Markou A, Paterson NE (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tobacco Res 3:361–373
Marks MJ, Burch JB, Collins AC (1983) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825
Martin TJ, Suchocki J, May EL, Martin BR (1990) Pharmacological evaluation of the antagonism of nicotine’s central effects by mecamylamine and pempidine. J Pharmacol Exp Ther 254:45–51
McNeill AD, Jarvis MJ, Stapleton JA, West RJ, Bryant A (1989) Nicotine intake in young smokers: longitudinal study of saliva cotinine concentrations. Am J Public Health 79:172–175
National Institutes of Health (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
Nemeth-Coslett R, Henningfield JE, O’Keefe MK, Griffiths RR (1986) Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology 88:420–425
Paterson NE, Markou A (2002) Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse 44:252–253
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Merlo Pich E, Fuxe K Changeux J-P (1998) Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Risner ME, Goldberg SR (1983) A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther 224:319–326
Rowell PP, Li M (1997) Dose-response relationship for nicotine-induced up-regulation of rat brain nicotine receptors. J Neurochem 68:1982–1989
Semenova S, Markou A (2003) Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry 54:1249–1264
Semenova S, Bespalov A, Markou A (2003) Decreased prepulse inhibition during nicotine withdrawal in DBA/2J mice is reversed by nicotine self-administration. Eur J Pharmacol 472:99–110
Shoaib M, Schindler CW, Goldberg SR (1997) Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology 129:35–43
Skjei KL, Markou A (2003) Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology 168:280–292
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive Psychopharmacology 117:2–10
Stolerman IP, Goldfarb T, Fink R, Jarvik ME (1973) Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia 28:247–259
Valentine JD, Hokanson JS, Matta SG, Sharp BM (1997) Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology 133:300–304
Watkins SS, Epping-Jordan MP, Koob GF, Markou A (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62:743–751
Watkins SS, Stinus L, Koob GF, Markou A (2000) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 292:1053–1064
Winer BJ (1971) Statistical principles in experimental design, 2nd edn. McGraw-Hill, New York
Acknowledgements
This is publication number 15655-NP from The Scripps Research Institute. The authors gratefully acknowledge technical support from Jessica Chevrette and Robert Lintz, and editorial assistance from Michael Arends. This study was supported by NIDA grant DA11946, NIDA/NIMH grant U01 MH69062, Tobacco-Related Disease Research Program Grant 12RT-0231 from the State of California, and a Novartis Research Grant to A.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paterson, N.E., Markou, A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology 173, 64–72 (2004). https://doi.org/10.1007/s00213-003-1692-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1692-7