Abstract
Rationale
According to recent theories of addiction, nicotine deprivation may influence biases in the orienting and maintenance of attention on smoking-related cues.
Objectives
We examined the effect of nicotine deprivation on different aspects of attentional biases for smoking-related cues.
Methods
Smokers’ eye movements to smoking-related and control pictures were assessed during a visual probe task under deprived and non-deprived conditions.
Results
When deprived, smokers maintained their gaze for longer on smoking-related than control cues, relative to when non-deprived. Deprivation also increased craving and pleasantness ratings of smoking cues. Across both deprived and non-deprived conditions, smokers were more likely to shift their gaze towards smoking cues and were faster to respond to probes replacing smoking cues, relative to non-smoking cues, but these attentional bias measures were not significantly affected by the deprivation manipulation.
Conclusions
Results suggest a selective effect of deprivation on the maintenance of attention on smoking-related cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to recent theories of drug dependence, biases in selective attention play an important role in the development and maintenance of drug-taking behaviour. Robinson and Berridge (1993, 2001) proposed that attentional biases for drug-related cues are mediated by an incentive-salience mechanism. Through a process of salience attribution, stimuli that are associated with drug taking are perceived as highly attractive, ‘grab attention’, cannot be ignored and elicit approach behaviours. These processes operate automatically, outside awareness, and high levels of incentive salience are associated with the subjective experience of craving. According to Tiffany’s (1990) schema model, drug-taking behaviour is largely controlled by habit. However, when drug-taking behaviour is impeded or obstructed, the person experiences an increase in drug urges and craving, which is accompanied by an attentional bias for drug-related cues, which, in turn, directs processing resources away from ongoing tasks and activities, towards the goal of drug consumption. Thus, these theories predict that increased craving and urge to smoke should be associated with an enhanced attentional bias for smoking-related cues in smokers.
Consistent with these theories, recent research has indicated that smokers have an attentional bias for smoking-related stimuli, in comparison with non-smokers (Ehrman et al. 2002; Bradley et al. 2003). Moreover, this attentional bias in smokers is enhanced by abstinence from smoking and appears to be closely associated with increased craving (Gross et al. 1993; Sayette and Hufford 1994; Waters and Feyerabend 2000; Zack et al. 2001; Mogg and Bradley 2002; Hogarth et al. 2003a). Such studies have used a variety of different paradigms to assess attentional bias, including the modified Stroop and visual probe tasks. In the former task, deprived smokers are typically slower to name the colours of smoking-related words than controls words, consistent with an attentional bias for smoking cues (Waters and Feyerabend 2000). In the visual probe task, on each trial, a pair of pictures is presented simultaneously, and, immediately after the pictures disappear, a small probe appears in the location of one of them. Participants respond as quickly as possible to the probe. The rationale behind the task is that people respond faster to stimuli that appear in an attended, rather than unattended, region of a visual display (Posner et al. 1980). Using this task, Bradley et al. (2003) found that, in comparison with non-smokers, smokers were faster to respond to probes replacing smoking-related pictures than control pictures, which is consistent with an attentional bias for smoking-related cues (see also Ehrman et al. 2002; Hogarth et al. 2003b).
However, a limitation of much recent research into attentional biases in drug dependence is an over-simplified view of selective attention. Research in cognitive science suggests that the attentional system is not unitary. For example, important distinctions have been made between the mechanisms involved in the initial orienting versus maintenance of attention (Allport 1989). It has also been suggested that separate neural subsystems underlie these different cognitive operations of attentional shifting and maintenance, and that the maintenance of attention, rather than initial orienting, is more likely to be influenced by motivational variables (LaBerge1995).
It is unclear whether attentional biases for smoking-related cues in smokers are pervasive throughout all aspects of attentional processes, or whether they only operate in specific cognitive operations, such as the initial orienting of attention towards drug cues, or in the maintenance of attention on such cues. The methodology of the modified Stroop task is not well-suited to reveal these different components of selective attention. In the visual probe task, the exposure time of the pictures can be varied, which provides a snap-shot view of attentional processes at the offset of the picture display. For example, brief exposure durations of the stimulus pictures (e.g. 500 ms or less) may be more likely to reflect initial orienting of attention, whereas longer exposure times (e.g. 2000 ms or more) may be more sensitive to the maintenance of attention. Recent studies have indicated an attentional bias for briefly presented smoking-related cues (500 ms), which was particularly evident in smokers who had made repeated previous attempts to quit (Bradley et al. 2003), although this attentional bias was not affected by deprivation (Mogg and Bradley 2002). However, to the best of our knowledge, no study has so far investigated the effect of nicotine deprivation on different aspects of selective attention, namely, initial orienting of attention and the maintenance of attention to smoking cues. Hence, this was the main aim of the present study. A key advantage of using an experimental manipulation is that it allows investigation of whether nicotine deprivation causes changes in attentional bias measures. Such a relationship cannot be equivocally established by non-experimental methods, such as by examining the relationship between naturally occurring variation in time since last cigarette and cognitive bias indices.
To address the above issues, we examined biases in attention, as indexed by eye movements (EMs), to smoking-related and control pictures in smokers during a visual probe task under deprived and non-deprived conditions. EM measures have several advantages over other methods of measuring attentional biases. For example, they are directly observable and are ecologically valid measures of attentional allocation. They are also rapid, normally automatic, and typically closely follow, and are guided by, shifts in covert selective attention (Jonides 1981; Kowler 1995). In the present study, we measured the direction of the initial fixation when smoking-related and control pictures were presented simultaneously on the computer screen, which should reflect the initial orienting of attention. We also investigated the overall amount of time that gaze was directed to the smoking-related and control pictures over the course of picture presentation. This “dwell time” measure should reflect the maintenance of attention. Hence, eye-movement monitoring enables us to measure the initial orienting and maintenance of attention to pairs of stimuli within a given trial, which is an advantage over other measures of attentional processing.
Another aim of the study was to investigate whether the predicted attentional bias induced by the deprivation manipulation would be accompanied by changes in measures of subjective craving and perceived pleasantness of the smoking-related stimuli. According to the incentive-sensitisation model of addiction, attentional biases for drug-related cues should be closely associated with the perceived ‘attractiveness’ of those cues, and also with increased craving in response to those cues, because a common mechanism underlies these cognitive and motivational responses to drug cues, namely, a dopamine-based incentive-sensitisation system (Robinson and Berridge 1993, 2001).
The current study used a repeated-measures design to manipulate deprivation, with smokers being assessed twice: (a) after a minimum of 10 h of smoking abstinence and (b) after smoking normally in the day prior to testing and having smoked a cigarette immediately before the session. We wanted to ensure that participants were nicotine sated prior to the non-deprived session in order to minimise craving levels, which are known to increase significantly within an hour of smoking a cigarette (Schuh and Stitzer, 1995). To summarise, our main hypotheses were that, relative to the non-deprived condition, deprived smokers would show enhanced attentional biases in their initial orienting to smoking-related pictures (as reflected by the direction of the initial shift in gaze in response to the pictures) and in the maintenance of attention on smoking-related pictures (as reflected by gaze “dwell times” on smoking-related pictures). We also examined whether the predicted increase in attentional biases, resulting from deprivation, would be accompanied by corresponding increases in measures of craving, and the perceived pleasantness of the smoking cues.
Materials and methods
Participants
Participants were recruited from the students and staff at the University of Southampton via poster advertisements and through an online experiment booking system. Participants were recruited if they smoked at least ten cigarettes per day and if they normally smoked their first cigarette of the day before 1100 hours. Additional selection criteria for all participants were that they spoke fluent English and had visual acuity within normal limits. There were 23 participants (13 male), with a mean age of 21.96 years (SD = 3.16).
Materials
The pictorial stimuli in the computer tasks were the same as those used in previous research in our laboratory (Mogg et al. 2003). The picture set consisted of 20 colour photographs of smoking-related scenes (e.g. woman holding cigarette to mouth, cigarette beside ashtray). Each was paired with a photograph of another scene matched as closely as possible for content, but lacking any smoking-related cues (e.g. woman applying lipstick, pen beside bowl). An additional 20 picture pairs (unrelated to smoking) were prepared for use as fillers, and three pairs for practice and buffer trials. The pictures were digitised and converted to an indexed 256 colour palette. All tasks were presented on a 450-Mhz Pentium III PC, with 15” VGA monitor, attached to a MEL version-2 response box and standard keyboard. The computer tasks were presented using MEL version 2.01 software (Schneider, 1995). Participants’ horizontal EMs were recorded whilst they completed the visual probe task using a computerised eye tracking system (Pan/Tilt optics system, Model 504, Applied Science Laboratories, Bedford, Massachusetts). The EM software was run on a 333-Mhz Pentium Celeron PC.
Procedure
Participants attended the laboratory on two occasions, with an interval of at least 1 week between the sessions. Before the non-deprived session, participants were instructed to smoke normally and to smoke a cigarette immediately before coming to the laboratory. Before the ‘deprived’ session participants were required to abstain from smoking for at least 10 h before coming to the laboratory. The order of sessions was counterbalanced between participants. Testing took place in a dimly lit, sound-proofed room.
At the start of the first session, participants provided informed consent, completed the brief form of the Questionnaire of Smoking Urges (QSU; Cox et al. 2001), and then provided a sample of expired carbon monoxide (CO) on a smokerlyzer (Bedfont Scientific Ltd, Bedford, UK). Their visual acuity was also checked to ensure it was within normal limits. Participants were then seated at a desk, at a distance of 111 cm from the computer screen. The Eye Tracker camera was positioned 50 cm in front of the participant, below their right eye. The eye-tracking equipment was calibrated by presenting the numbers 1–9 on the screen in a 3×3 array (with number 1 at the top left of the screen, and 9 at the bottom right), and participants were instructed to look at each number in turn, whilst their position of gaze was recorded for each number.
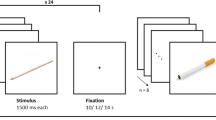
In the visual probe task, each trial started with a central fixation cross shown for 1000 ms, which was replaced by the display of a pair of pictures, side by side, for 2000 ms. Immediately after the offset of the picture pair, a probe was presented in the position of one of the preceding pictures, until the participant gave a manual response. The probe was a pair of dots (either “:” or “..”). Participants were instructed to press one of two response buttons to indicate the identity of the probe. They were also instructed to look at the fixation cross at the start of each trial, to sit completely still throughout the task and to refrain from moving their head during each trial. There was an inter-trial interval of 2000 ms. Eye-movement data were recorded during each trial, starting immediately before the onset of the fixation cross and terminating immediately after the participant had made a response.
There were 14 practice trials, followed by 2 buffer trials and 120 trials in the main task (80 critical trials and 40 filler trials). During the critical trials, each of the 20 smoking-control picture pairs was presented four times. Each smoking-related picture appeared twice on the left side of the screen, and twice on the right. The probe appeared in the location of either the smoking-related or the control picture with equal frequency and there was an equal number of trials with each probe type. The 20 filler picture pairs were presented twice each. Critical and filler trials were presented in a new random order for each participant. Each picture was 95-mm high by 130-mm wide when displayed on the screen, and the distance between their inner edges was 30 mm (visual angle of 1.0° between the fixation position and the inner edge of each picture). The distance between the two probe positions was 105 mm (visual angle of 5.4°).
Immediately after the visual probe task, participants were asked to indicate ‘how strong your urge to smoke is right now’ on an anchored rating scale which ranged from 0 (not at all) to 10 (extremely). Next, they completed the pleasantness rating and craving rating tasks, as well as a novel stimulus–response compatibility taskFootnote 1; the order of these tasks was counterbalanced across participants.
The pleasantness rating task consisted of two practice trials, which used filler pictures, and 40 test trials in which each smoking-related and control picture from the visual probe task was presented, one at a time, in a new random order for each participant. Participants were asked to rate how pleasant or unpleasant they found each picture. Each picture (73×100 mm) was presented for 2000 ms and, after a pause of 500 ms, a 7-point anchored rating scale was displayed on the screen until the participant’s response. The rating scale ranged from −3 (very unpleasant) to +3 (very pleasant), and participants were asked to press one of seven keys, which were correspondingly labelled from −3 to +3, to indicate their response. The inter-trial interval was 500 ms. The craving rating task was similar to the pleasantness rating task, except that participants were asked to indicate how much craving they felt in response to each picture, and the rating scale displayed after each picture ranged from 1 (no craving) to 7 (very strong craving), with the response keys correspondingly labelled from 1 to 7.
After the computer tasks, participants completed the brief QSU, shortened state and trait versions of the tension-anxiety, depression and vigour scales of the Profile of Mood States (POMS, with 6 items per scale, McNair et al. 1981), the Fagerstrom Test for Nicotine Dependence (FTND, Heatherton et al. 1991), and questionnaires about smoking habits and history. After completion of the questionnaires, participants were thanked for their time and instructed to return for the second session.
The second session was the same as the first session, except for the following procedural changes: the visual acuity check and consent forms were not required and, after the computer tasks, participants completed only the brief QSU, POMS and a short open-ended questionnaire asking if their smoking habits had changed or if they had tried to quit smoking since the last session. They were then thanked for their time, debriefed and paid 15 pounds sterling.
Preparation of eye-movement data
Data were analysed using the Eyenal Data Analysis Program (Applied Science Group 2000). The direction of gaze, measured in degrees, was measured once every 17 ms. If EMs were stable within 1° of visual angle for 100 ms or more, this was classified as a fixation to that position, the duration of which was recorded. Fixations were classified as being directed at the left or right pictures if they were 1° wide of the central position on the horizontal plane (this visual angle corresponds to the distance between the fixation cross position and the inner edge of each picture). Our dependent measures were the direction of the initial fixation during critical trials in which smoking-related and control pictures were presented, and the amount of time spent fixating on smoking and control pictures during critical trials (“dwell time”).
EM data were only analysed from critical trials in which smoking-related and control pictures were presented. For the analysis of initial EMs, fixations on either picture were identified if (i) participants were fixated in the central region before picture onset, (ii) EMs occurred at least 100 ms after picture onset, and before picture offset, and (iii) participants fixated on one of the pictures, rather than the central position, during picture presentation. Data from one participant were excluded from the analysis of initial EMs, because they had detectable initial fixations on only 8% of critical trials during the deprived session, which was largely due to calibration difficulties. For the remaining participants, an initial fixation was made to either picture on 70% of trials, averaged across deprived and non-deprived sessions (they did not fixate on the fixation cross before picture onset on 27% of trials, and a fixation was not made to either picture on 3% of trials). These percentages were not significantly different in the deprived and non-deprived sessions.
In order to calculate EM “dwell time”, we calculated the amount of time that fixations were directed to the regions occupied by the smoking-related and matched control pictures during picture presentation. We also recorded dwell time to the central region (i.e. area between the pictures), plus the amount of time when no fixations were recorded. “Dwell time” on each region of interest was calculated by summing the duration of fixations made to each region on each trial. This was calculated regardless of the latency to the first fixation and whether or not participants were looking at the central region (occupied by the fixation cross) before picture onset. This produced a measure of the total amount of time a person looked at each picture for each trial. Due to technical problems with the EM recording equipment, two participants had no dwell time data (i.e. no fixations were recorded) on a high proportion of trials (39% and 49%), so their data was excluded from the dwell-time analyses. For the remaining participants, trials with excessive missing data (where the amount of time with no recorded fixations was more than 3 SDs above the sample mean)Footnote 2 were excluded from the analysis. After outlying data had been excluded, EM fixations accounted for 85% of the time when pictures were presented, with no fixations recorded during the remaining 15% of the time due to EMs (saccades), eye blinks, and failures of the EM recording equipment to record data. Of the recorded fixation time, 80% was to one of the regions containing the pictures, with the remaining 20% to the central position. These percentages were not significantly different in the deprived and non-deprived sessions.
Results
Questionnaire and CO measures
On average, participants smoked 16.6 cigarettes per day (SD=8.2) and had been smoking for 4.1 years (SD=2.1). The average score on the FTND was 3.8 (SD=1.7). The mean self-reported time since the last cigarette was 816 min (13.6 h; SD=2.3 h) before the start of the deprived session, and approximately 5 min before the non-deprived session.
Paired t-tests were used to assess the effect of the deprivation manipulation on CO levels, urge to smoke and mood measures (see Table 1 for summary statistics and test results). In the deprived condition, participants had significantly lower levels of expired CO, higher QSU scores (at beginning and end of the session), higher ratings of urge to smoke (mid-session) and higher state anxiety levels (end of session).
EM results
Direction of initial fixation
A direction bias score was calculated for each participant by expressing the number of trials when gaze was directed initially at the smoking-related picture as a percentage of the total number of trials in which a fixation was made on either the smoking-related or control picture. Scores greater than 50% reflect a bias in orienting towards smoking-related pictures, relative to control pictures (50% indicates no bias). Smokers directed their gaze at smoking scenes on 55.3% of trials (SD=8.8) in the deprived condition, and on 55.7% of trials (SD=7.3) in the non-deprived condition and there was no significant difference between the two conditions (t 21=0.16, ns). To examine whether smokers preferentially direct their gaze at smoking-related, rather than control, pictures, their bias scores were compared with 50%. The percentage of fixations directed at smoking pictures was significantly greater than 50% in both deprived (t 21=2.81, P=0.01) and non-deprived conditions (t21=3.62, P<0.01), consistent with a bias in initial orienting to smoking cues.
Dwell time
The mean amount of time that subjects spent fixating on smoking and control pictures over the course of each trial is shown in Fig. 1. A repeated-measures analysis of variance (ANOVA) with picture type (2: smoking, control) and deprivation (2: deprived, non-deprived) as within-subject variables showed a significant main effect of picture type (F 1,20=23.88, P<0.001), which was qualified by a significant picture type×deprivation interaction (F 1,20=5.39, P<0.05). Post-hoc contrasts, examining the effect of deprivation on dwell time for each picture type separately, were non-significant. Separate analyses for each deprivation condition, showed that participants looked at smoking pictures for 260 ms longer than control pictures during the deprived condition (t 20=4.77, P<0.01), and they looked 172 ms longer at smoking than control pictures during the non-deprived condition (t 20=4.22, P<0.01). The picture type×deprivation condition interaction indicates that the difference in dwell time between these deprivation conditions (i.e. 260 ms versus 172 ms) was significant.
Visual probe task: manual RT
RT data from filler trials, and from trials with errors (2.6% of data) were discarded. To eliminate outliers, RTs were discarded if they were more than 2000 ms and then more than 3 SDs above each participant’s mean (1.3% of data). Mean RTs to probes replacing smoking and control pictures were 635 ms (SD=116) and 647 ms (SD=128), respectively, in the deprived condition, and 625 ms (SD=114) and 639 ms (SD=116) in the non-deprived condition. A 2×2 ANOVA with probe position (probe in same versus different location to smoking picture) and deprivation (deprived, non-deprived) as within-subject variables showed only a significant main effect of probe position (F 1,22=5.33, P<0.05). This reflects faster RTs to probes replacing smoking than control pictures, consistent with an attentional bias for the smoking pictures. However, this was not significantly influenced by the deprivation manipulation (F<1).
Rating tasks
Pleasantness ratings
Mean pleasantness ratings of the smoking-related and control pictures are shown in the upper panel of Fig. 2 for each condition. A 2×2 ANOVA of the ratings, with picture type (smoking-related, control) and deprivation (deprived, non-deprived) as within-subject variables showed a significant main effect of picture type (F 1,22=4.98, P<0.05), and a significant deprivation×picture type interaction (F 1,22=8.60, P<0.01). The smoking-related pictures were rated as more pleasant than control pictures in the deprived condition (t22=2.91, P<0.05), whereas the ratings of the two picture types did not differ significantly in the non-deprived condition (t22=1.05, P>0.1).
Craving ratings
The mean craving ratings evoked by the pictures are shown in the lower panel of Fig. 2 for both deprived and non-deprived conditions. A 2×2 ANOVA was carried out with deprivation (deprived, non-deprived) and picture type (smoking-related, control) as within-subjects variables. There was a significant main effect of deprivation (F 1,22=16.03, P<0.01), reflecting higher craving ratings in the deprived than non-deprived conditions, and a significant main effect of picture type (F 1,22=165.9, P<0.001), as craving ratings were higher in response to smoking than control pictures. There was also a significant picture type×deprivation interaction (F 1,22=15.50, P<0.01). Post-hoc contrasts showed that smoking pictures elicited more craving than control pictures in the deprived condition (5.0 vs 2.5), (t 22=12.41, P<0.01), and also in the non-deprived condition (4.2 vs 2.2), (t 22=11.08, P<0.01). In addition, craving ratings were higher in the deprived than non-deprived conditions for both smoking pictures (t 22=4.48, P<0.01) and control pictures (t 22=2.15, P<0.05). The significant interaction indicates that the difference in craving elicited by smoking pictures, relative to control pictures, was significantly greater in the deprived than non-deprived conditions.
Supplementary analyses with session order
The main analyses were repeated after including order (1. deprived condition in first session versus 2. non-deprived condition in first session) as a between-subjects variable. There was a significant deprivation×order interaction for the probe RT data (F 1,21=9.93, P<0.05), which reflects slower overall mean RTs in the deprived than non-deprived sessions in order 1 (675 vs 640 ms; t11=2.68, P<0.05), with no significant difference in RT between sessions in order 2 (603 vs 623, P>0.1). However, this result is not directly relevant to our hypothesis because it does not interact with picture content.
Discussion
The deprivation manipulation had a significant effect on the duration of gaze on smoking-related cues. That is, when nicotine-deprived, smokers maintained their gaze longer on smoking than control pictures, and this bias was reduced during the non-deprived session. However, nicotine deprivation did not significantly affect the other two main measures of attentional bias; namely, the direction of initial shifts in gaze, and the probe RT bias index from the visual probe task. The results also confirmed that the deprivation manipulation was effective in increasing subjective craving for cigarettes. Moreover, deprivation increased the perceived pleasantness of smoking-related pictures, as well as the degree of craving that was elicited by those pictures.
Although only the gaze dwell-time measure of attentional bias was influenced by nicotine deprivation, the results from the three main attentional measures (i.e., direction and duration of gaze, and probe RT) were compatible with an attentional bias for smoking cues in smokers. That is, smokers preferentially shifted their gaze towards smoking rather than control pictures, looked at them longer and responded more rapidly to probes replacing smoking-related than control pictures. Thus, while the results from the three different measures are consistent with an attentional bias for smoking-related cues in smokers, only the gaze dwell-time measure appears to be particularly sensitive to transient variation in nicotine deprivation and craving.
In interpreting these results, it is helpful to consider the extent to which they are in line with previous findings. In previous studies using the visual probe task, the probe RT measure has provided comparable evidence of an attentional bias for smoking-related cues in smokers, but this particular measure does not tend to be associated with transient variations in craving (Mogg and Bradley 2002; Mogg et al. 2003). In a previous EM study of smokers, which did not manipulate nicotine deprivation, we found that naturally occurring variation in craving correlated with longer initial fixations on smoking cues, but not with the direction of initial shifts in gaze (Mogg et al. 2003). Thus, the results from the present study, which experimentally manipulated deprivation levels, extend these previous findings and further indicate that different attentional bias measures vary in their sensitivity to fluctuations in an individual’s current motivational state. That is, the duration of gaze on smoking-related cues appears to be sensitive to changes in nicotine deprivation and craving, whereas other measures of attentional processing, such as initial shifts in gaze and the probe RT measure, seem to be less responsive.
Thus, the present findings raise the question of why some measures of attentional bias seem to be more affected than others by variation in levels of deprivation. As discussed earlier, different attentional bias measures may be tapping different underlying attentional processes, such as initial orienting versus maintenance of attention, which appear to be mediated by different cognitive mechanisms (LaBerge 1995). Thus, the duration of gaze may indicate the maintenance or engagement of attention, which may be a particularly sensitive indicator of the strength of activation of motivational states (LaBerge 1995). If so, such measures may be an invaluable tool in research into the fundamental mechanisms which are hypothesised to underlie addiction, such as incentive motivation.
The extent to which the probe RT measure used in the present study reflects initial orienting versus maintenance of attention is uncertain, because the picture pairs were presented for a relatively long duration to allow EM monitoring. The probe RT measure reflects the spatial location to which individuals are attending when the pictures disappear (i.e. when the probe appears). In the present study, each picture pair was shown for 2 s, which would allow attention to shift repeatedly between the pictures. Thus, the probe RT measure in the present study is likely to reflect a combination of attentional shifting and maintenance processes and so may be a relatively impure index of specific attentional component processes. If so, this may explain why the probe RT bias measure may be insensitive to changes in deprivation level, if, as suggested earlier, such variation in current motivational state exerts a selective effect on the maintenance of attention on smoking cues.
Thus, the present findings, taken together with those from previous studies, suggest that some attentional bias indices, such as initial shift in gaze or the probe RT measure, are associated with trait variables in drug dependence, such as addiction status (e.g. smokers versus non-smokers, Ehrman et al. 2002; Bradley et al. 2003); whereas, other attentional biases, for example, in the maintenance of gaze, appear to be more sensitive to state variables, such as current levels of deprivation. It would seem helpful for further research to clarify the relative effects of state and trait variables in addiction on specific aspects of cognition, since the present results clearly support the view that attentional biases in addiction are not mediated by a unitary mechanism and that the specific roles of initial orienting and maintenance of attention should be considered (cf. LaBerge 1995). It is also important to clarify the implications of these findings for predicting smoking behaviour outside the laboratory. For example, one area of research might examine whether delayed disengagement from smoking-related cues is associated with difficulty in resisting the urge to smoke in smokers who are attempting to quit.
The present results indicate that nicotine deprivation not only influenced the duration of gaze on smoking cues, but also enhanced the perceived pleasantness of the smoking-related pictures and the extent to which those pictures elicited subjective craving. Thus, these cognitive and motivational effects of smoking cues (holding attention, perceived attractiveness, evoked craving) may be mediated by a common underlying mechanism, which is influenced by deprivation, and which may reflect related components of processing of smoking-related stimuli, as suggested by Sayette et al. (2000). Models of drug dependence which emphasise the role of habit-based processes in addiction (Tiffany 1990) predict that deprivation should elicit craving and an accompanying attentional bias for smoking cues, but would not readily explain why deprivation should increase the perceived positive valence of smoking-related cues. Instead, the present findings seem more readily explained by incentive models of addiction, such as the incentive-sensitisation theory (Robinson and Berridge 1993), which proposes that smoking cues that have high incentive salience would be perceived as highly ‘attractive’, be ‘wanted’ and desired, and ‘grab attention’. The results of the present study suggest that these three indices (i.e., positive appraisal, craving and maintained attention on smoking cues) do indeed covary as deprivation increases, as expected from an incentive account of addiction. This would also seem compatible with motivational theories which argue that deprivation increases the incentive value of a reinforcer (Bindra 1974).
In summary, the present study indicates that nicotine deprivation affects some, but not all, aspects of attentional bias for smoking-related stimuli in smokers. Nicotine deprivation specifically enhanced the maintenance of attention of smoking-related cues, and this cognitive bias was accompanied by increased craving and by more positive evaluations of smoking-related stimuli. EM monitoring may have advantages over other methods of assessing attentional biases in addiction, with biases in the maintenance of gaze appearing to be particularly sensitive to variation in nicotine deprivation.
Notes
Results of the stimulus–response compatibility task will not be discussed as they were confounded by the order in which participants completed deprived and non-deprived sessions. Details of this task and the results are available on request from the authors.
The use of a 3 SD cut-off was applied to the dwell time and manual RT data, which is consistent with cut-offs used in previous research (Mogg et al. 2003).
References
Allport A (1989) Visual attention. In: Posner MI (ed) Foundations of cognitive science. MIT Press, Cambridge, pp 631–682
Bindra D (1974) A motivational view of learning, performance, and behavior modification. Psychol Rev 81:199–213
Bradley BP, Mogg K, Wright T, Field M (2003) Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychol Addict Behav 17:66–72
Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16
Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP (2002) Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend 67:185–191
Gross TM, Jarvik ME, Rosenblatt MR (1993) Nicotine abstinence produces context-specific stroop interference. Psychopharmacology 110:333–336
Heatherton TF, Koslowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127
Hogarth L, Dickinson A, Duka T (2003a) Discriminative stimuli that control instrumental tobacco-seeking by human smokers also command selective attention. Psychopharmacology 168:435–445
Hogarth LC, Mogg K, Bradley BP, Duka T, Dickinson T (2003b) Attentional orienting towards smoking-related stimuli. Behav Pharmacol 14:153–160
Jonides J (1981) Voluntary versus automatic control over the mind’s eye movements. In: Long J, Baddeley A (eds) Attention and performance IX. Elrbaum, Hillsdale, pp 187–203
Kowler E (1995) Eye movements. In: Kosslyn SM, Osheron DM (eds) Visual cognition. Harvard University Press, Cambridge, pp 215–265
LaBerge D (1995) Attentional processing. Harvard University Press, Cambridge
McNair DM, Lorr M, Droppleman LF (1981) Manual for the profile of mood states. Educational and Industrial Testing Service, San Diego
Mogg K, Bradley BP (2002) Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol 16:385–392
Mogg K, Bradley BP, Field M, De Houwer J (2003) Biases in eye movements to smoking-related cues in smokers: relationship to affective and motivational valence. Addiction 98:825–836
Posner MI, Snyder CR, Davidson BJ (1980) Attention and the detection of signals. J Exp Psychol Gen 109:160–174
Robinson KC, Berridge TC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Robinson KC, Berridge TC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Sayette MA, Hufford MR (1994) Effects of cue exposure and deprivation on cognitive resources in smokers. J Abnorm Psychol 103:812–818
Sayette M, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG (2000) The measurement of drug craving. Addiction 95[Suppl 2]:S189–S210
Schneider W (1995) MEL professional user’s guide. Psychology Software Tools, Pittsburgh
Schuh KJ, Stitzer ML (1995) Desire to smoke during spaced smoking intervals. Psychopharmacology 120:289–295
Tiffany ST (1990) A cognitive model of drug urges and drug-use behaviour: role of automatic and non-automatic processes. Psychol Rev 97:147–168
Waters AJ, Feyerabend C (2000) Determinants and effects of attentional bias in smokers. Psychol Addict Behav 14:111–120
Zack M, Belsito L, Scher R, Eissenberg T, Corrigall WA (2001) Effects of abstinence and smoking on information processing in adolescent smokers. Psychopharmacology 153:249–257
Acknowledgements
This work was supported by a grant from the Wellcome Trust to Brendan Bradley and Karin Mogg (ref. 057076). Karin Mogg holds a Wellcome Senior Research Fellowship in Basic Biomedical Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Field, M., Mogg, K. & Bradley, B.P. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology 173, 116–123 (2004). https://doi.org/10.1007/s00213-003-1689-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1689-2